Abstract
The interplay of selective and homeostatic processes dominates the behavior of B lineage subsets following B cell antigen receptor (BCR) expression, and extends to determinants of immune response quality and the persistence of immunologic memory. A key concept emerging from these considerations is that primary events acting upstream of mature B lymphocyte pools can profoundly impact downstream populations as the system attempts homeostatic adjustments. Since advancing age is accompanied by profound changes in B cell generation and homeostasis, establishing the relative contributions of primary lesions versus compensatory homeostatic processes is critical to understanding these perturbations. Exploration of this problem requires an understanding of: 1) the identity, dynamics, and progenitor/successor relationships of marrow and peripheral B cell subsets; 2) the nature and interactions of selective and homeostatic processes acting in these subsets; and 3) how these change with age. Our data show that BLyS and its receptors mediate peripheral B cell homeostasis, and that the size, dynamics and behavior of all B cell subsets influenced by B Lymphocyte Stimulator change with age. These findings suggest that homeostatic processes mediated through B Lymphocyte Stimulator are altered with age, and that these perturbations may primarily reflect compensatory homeostatic adjustments to upstream reductions in B cell generation.
Introduction: Lymphocyte homeostasis and the aging immune system
The relationship between homeostasis and selection in the maintenance of primary B cells has only recently been appreciated, and suggests that events acting upstream of mature lymphocyte pools impact downstream populations as the system makes homeostatic adjustments (reviewed in (Cancro and Sprent 2005)). Advancing age is accompanied by substantial changes in all B cell compartments and, consequently, humoral immune function. These changes include shifts in the magnitude of all B cell compartments (Figure 1), specificity repertoire changes, modified peripheral B cell dynamics, and weakened humoral responses. Accordingly, insights about how age-related shifts in homeostatic pressures impact functional B cell populations is key to a mechanistic understanding of age-associated changes in B cell compartments.
Figure 1.
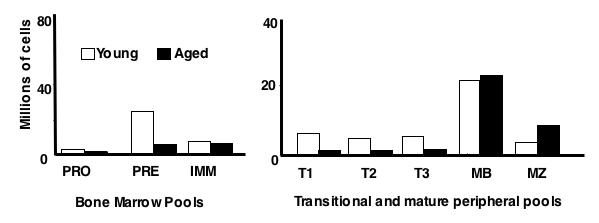
Numbers of cells in each B cell subset in young and aged mice. These numbers represent samples of >18 mice in each case with c.v.≤ 10%.
Homeostatic demands control B cell survival and selection
B lymphocytes are continuously generated throughout adult life, arising in the marrow and transiting a series of developmental stages during which they undergo rearrangement and expression of antigen receptor genes. Successive B cell developmental stages are thus defined according to surface markers and Ig gene rearrangement status and are summarized in Table 1. In the pro-B stage, cells rearrange their Ig heavy chain genes. This is followed by surface Ig heavy chain expression with surrogate light chain, delineating onset of the pre-B cell stage. Early pre-B cells undergo a proliferative burst, followed by Ig light chain gene rearrangement. Successful light chain rearrangement and surface expression of a complete B cell antigen receptor (Ig heavy-light chain pair) identifies the immature marrow B cell, which may complete maturation after migrating to the periphery. These late peripheral B cell maturation intermediates are termed “transitional” stages, and are divided into subsets T1, T2, and T3. Cells that successfully complete transitional differentiation enter the mature peripheral B cell pools. Mature follicular B cells, also referred to as B-2 or “conventional” B cells, encompass the majority (>80%) of B lymphocytes in peripheral tissues, and are to be the progenitors of both primary antibody forming cells and memory B cells. In addition to follicular B cells, several additional subsets of mature B cells exist. Marginal Zone B cells are phenotypically, functionally, and anatomically distinct from follicular B cells and play a major role in responses to T cell independent (TI) antigens. A final subset, the B1 B cells, is the first to appear in ontogeny and is maintained throughout life by self-renewal.
Table 1.
Magnitude and kinetics of developing and primary B cell subsets
| B cell subset | Pool Size (millions of cells) |
Turnover Rate (%/day) |
Production rate (106/day) |
|
|---|---|---|---|---|
|
Bone Marrow Progenitors |
pro-B | 5 | 30 | 1.5 |
| pre-B | 50 | 30 | 15 | |
| Immature | 35 | 30 | 10-15 | |
| Transitional | T1 | 1.5 | 30 | |
| T2 | 2.0 | 30 | ∼1.5 overall |
|
| T3 | 1.0 | 30 | ||
|
Mature Primary |
Follicular | ∼45 | <2 | 0.5 |
|
Marginal Zone |
5-7 | ∼4 | varies | |
| B1 | 1.5 | ? | ? | |
Over 95% of immature and transitional cells die before joining mature pools, reflecting extensive specificity-mediated negative and positive selection (Fulcher and Basten 1994; Hayakawa, Asano et al. 1999; Wang and Clarke 2004). The remaining cells mature and enter the follicular (follicular) B cell pool, with a smaller subset joining the marginal zone (marginal zone) population. Homeostatic mechanisms controlling B cell numbers are superimposed on these BCR-mediated selection processes. In general, newly formed and mature B lymphocytes compete for trophic resources, so that when these resources become limiting, steady state B cell numbers are achieved and maintained by means of equal production and loss rates.
BLyS (also termed BAFF), a recently characterized Tumor Necrosis Factor (TNF) family member, is the limiting resource for which transitional, follicular and marginal zone B cells compete (reviewed in (Cancro 2004; Miller, Stadanlick et al. 2006)). BLyS can be produced by a broad variety of cell types either constitutively or following activation stimuli. These include macrophages, dendritic cells, some lymphocytes, and unidentified, radioresistant stromal elements. Although BLyS binds three receptors - BR3 (also termed BAFFr), TACI and BCMA – the interaction of BLyS with BR3 mediates homeostatic survival among transitional, follicular, and marginal zone B cells (Harless, Lentz et al. 2001; Schiemann, Gommerman et al. 2001; Yan, Brady et al. 2001). Through this competition mechanism, steady state numbers of mature B cell numbers are governed by controlling the proportion of transitional cells that complete maturation, as well as the lifespan of mature follicular and marginal zone B cells (Harless, Lentz et al. 2001).
The link between BCR specificity and relative competitive ability implies that the emerging cohort of specificities, in concert with BLyS availability, can set thresholds for negative and positive selection in the transitional compartment. This has been tested in experiments using the HEL/anti-HEL double transgenic model (Lesley, Xu et al. 2004; Thien, Phan et al. 2004), and in our recent collaborative studies using the 3H9 transgenic model of dsDNA autoreactivity (Hondowicz, et al, submitted). Together, these studies show that the likelihood of autoreactive clonotypes being eliminated at the transitional stage is determined by the availability of BLyS and the degree of competition from other, concomitantly emerging clonotypes. Moreover, these data suggest that decreased B cell generation rates – a feature of the aging immune system - might afford admission of autoreactive cells to peripheral pools (Miller, Stadanlick et al. 2006).
B lineage progenitors decline with age, suggesting new homeostatic demands
Age related changes in bone marrow B cell commitment and development include declines in the frequency of precursors, lowered expression of key genes, reductions in pro- and pre-B cell compartments, and attenuated responsiveness to differentiation factors (Frasca, Nguyen et al. 2004; Labrie, Sah et al. 2004; Miller, Izon et al. 2002; Riley, Kruger et al. 1991; Sherwood, Xu et al. 2000; Stephan, Reilly et al. 1998; Stephan, Sanders et al. 1996; Szabo, Shen et al. 2003). Overall, these findings suggest reductions in the generation and throughput of B cell progenitors.
In vivo labeling analyses of marrow B lineage subsets reveal features consistent with compensatory homeostatic responses to reduced B lineage commitment (Johnson, Owen et al. 2002; Kline, Hayden et al. 1999; Labrie, Sah et al. 2004). These studies have shown that pro- to pre B cell transit success is reduced, yielding a 4-fold reduction in pre-B cell numbers, as well as a decrease in the generation rate of immature B cells. Interestingly, these studies also show that the proportional success rate of pre-B cell differentiation to the immature pool is increased, so a larger fraction of pre-B cells enters the immature pool in aged mice. Moreover, the residency time within immature BM pools is extended. Together, these two features partially compensate for the reduced absolute generation rate; indeed, they yield an immature pool that is only reduced about two-fold compared to that of young adults. Given the generally reduced throughput of newly formed B cells, many of these changes might reflect compensatory homeostatic processes that yield altered turnover kinetics and shifting requisites for either negative or positive selection.
Peripheral B cell pools display altered homeostatic properties with age
The behavior of transitional pools mirrors the marrow immature subset: residency time is extended; yielding a reduction in magnitude somewhat less than would be predicted by the upstream drops in pro- and pre-B cell production rates. Moreover, the turnover rate of the follicular pool slows as much as two-fold (Kline, Hayden et al. 1999), and the marginal zone pool enlarges. These changes in the dynamics of peripheral subsets are provocative, given recent insight into the relationship between homeostatic and selective events during transitional differentiation.
Within this context, BCR mediated selection at the marrow/periphery interface may shift with age as a homeostatic response to reduced marrow output. For example, because thresholds for selection at transitional stages can be varied based on the available space and competition, reduced throughput and increased residency time may yield an explanation for the regular appearance of polyreactive and autoreactive antibodies with age (Eaton-Bassiri, Mandik-Nayak et al. 2000). A second prediction is that features associated with homeostatic competition in the transitional pools may vary with age. Since BLyS and its receptors are closely tied to these processes, we have begun to analyze BLyS receptor levels of peripheral B cell populations in aged versus young mice. Initial results suggest that the usual selection for cells expressing relatively high levels of TACI during transitional differentiation is blunted in aged individuals, such that clonotypes expressing low TACI levels persist and appear to join the mature follicular pool (Quinn, Scholz et al. 2005). While these ideas and findings are consistent with a shift in specificity-based selection leading to increased frequencies of autoreactive specificities with age, rigorous assessments will require analysis of the emerging repertoire as parameters of throughput and space are varied.
We have also observed that follicular and transitional cells from aged mice respond more robustly to BLyS in vitro (Fig. 2). When similar numbers of pooled transitional or follicular cells from young vs. aged mice are cultured in the presence of varying amounts of BLyS, more aged B cells persist as BLyS becomes limiting. This result suggests that enhanced BLyS responsiveness may contribute mechanistically to the increased lifespan and decreased turnover rates of the aged B cell pool. The basis for this enhanced responsiveness to BLyS remains unclear. It may reflect the selection of emerging B cells capable of surviving with limiting trophic resources, since Stefan et al have shown that aged marrow stroma generates lower levels of key survival cytokines (Stephan, Reilly et al. 1998). Alternatively, it might reflect the outcome of life-long selection for follicular B cells with the strongest ability to capture and process BLyS-BR3 signals. Regardless of exact mechanism, it is tempting to speculate that – even under physiologically normal BLyS levels – aged B cells that are BLyS hyper-responsive will mimic the behavior of young B cells given increased BLyS: relaxed negative selection thresholds and enlarged marginal zone pools.
Figure 2.
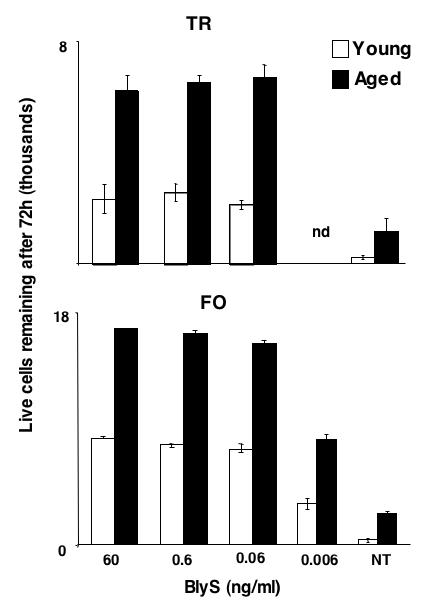
Similar numbers of transitional and follicular cells were obtained from C57BL/6 mice and cultured in vitro for 72 hours in the presence of varying amounts of BLyS, and then viability assessed by TOPRO-3 staining and FACS analysis. Cultures were performed in triplicate. At all doses, the survival of B cells from aged (18 month old) mice differs from those from young (3 month old) mice based upon student's T test (p ≤ 0.01). Bars show means ± S.D. of triplicate cultures from pooled cells of three mice; and these data are representative of three independent experiments.
Acknowledgements
This work was supported in part by USPHS grant AG-R01-16841.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cancro MP. The BLyS family of ligands and receptors: an archetype for niche-specific homeostatic regulation. Immunol Rev. 2004;202:237–249. doi: 10.1111/j.0105-2896.2004.00212.x. [DOI] [PubMed] [Google Scholar]
- Cancro MP, Sprent J. Counting on homeostasis: governing the size and composition of lymphocyte pools. Semin Immunol. 2005;17:173. [Google Scholar]
- Eaton-Bassiri AS, Mandik-Nayak L, Seo SJ, Madaio MP, Cancro MP, Erikson J. Alterations in splenic architecture and the localization of anti-double-stranded DNA B cells in aged mice. Int Immunol. 2000;12:915–926. doi: 10.1093/intimm/12.6.915. [DOI] [PubMed] [Google Scholar]
- Frasca D, Nguyen D, Riley RL, Blomberg BB. Effects of aging on DNA-binding activity of the E47 transcription factor in splenic B cells. Mech Ageing Dev. 2004;125:111–112. doi: 10.1016/j.mad.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Fulcher DA, Basten A. Reduced life span of anergic self-reactive B cells in a double transgenic model. Journal of Experimental Medicine. 1994;179:125–134. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harless SM, Lentz VM, Sah AP, Hsu BL, Clise-Dwyer K, Hilbert DM, Hayes CE, Cancro MP. Competition for BLyS-mediated signaling through Bcmd/BR3 regulates peripheral B lymphocyte numbers. Curr Biol. 2001;11:1986–1989. doi: 10.1016/s0960-9822(01)00598-x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, Hardy RR. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Owen K, Witte PL. Aging and developmental transitions in the B cell lineage. Int Immunol. 2002;14:1313–1323. doi: 10.1093/intimm/dxf092. [DOI] [PubMed] [Google Scholar]
- Kline GH, Hayden TA, Klinman NR. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J Immunol. 1999;162:3342–3349. [PubMed] [Google Scholar]
- Labrie JE, 3rd, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004;200:411–423. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- Miller JP, Izon D, DeMuth W, Gerstein R, Bhandoola A, Allman D. The earliest step in B lineage differentiation from common lymphoid progenitors is critically dependent upon interleukin 7. J Exp Med. 2002;196:705–711. doi: 10.1084/jem.20020784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JP, Stadanlick JE, Cancro MP. Space, selection, and surveillance: setting boundaries with BLyS. J Immunol. 2006;176:6405–6410. doi: 10.4049/jimmunol.176.11.6405. [DOI] [PubMed] [Google Scholar]
- Quinn WJ, 3rd, Scholz JL, Cancro MP. Dwindling competition with constant demand: can homeostatic adjustments explain age-associated changes in peripheral B cell selection? Semin Immunol. 2005;17:362–369. doi: 10.1016/j.smim.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Riley RL, Kruger MG, Elia J. B cell precursors are decreased in senescent BALB/c mice, but retain normal mitotic activity in vivo and in vitro. Clin Immunol Immunopathol. 1991;59:301–313. doi: 10.1016/0090-1229(91)90026-7. [DOI] [PubMed] [Google Scholar]
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- Sherwood EM, Xu W, King AM, Blomberg BB, Riley RL. The reduced expression of surrogate light chains in B cell precursors from senescent BALB/c mice is associated with decreased E2A proteins. Mech Ageing Dev. 2000;118:45–59. doi: 10.1016/s0047-6374(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Stephan RP, Reilly CR, Witte PL. Impaired ability of bone marrow stromal cells to support B-lymphopoiesis with age. Blood. 1998;91:75–88. [PubMed] [Google Scholar]
- Stephan RP, Sanders VM, Witte PL. Stage-specific alterations in murine B lymphopoiesis with age. Int Immunol. 1996;8:509–518. doi: 10.1093/intimm/8.4.509. [DOI] [PubMed] [Google Scholar]
- Szabo P, Shen S, Telford W, Weksler ME. Impaired rearrangement of IgH V to DJ segments in bone marrow Pro-B cells from old mice. Cell Immunol. 2003;222:78–87. doi: 10.1016/s0008-8749(03)00084-4. [DOI] [PubMed] [Google Scholar]
- Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Wang H, Clarke SH. Positive selection focuses the VH12 B-cell repertoire towards a single B1 specificity with survival function. Immunol Rev. 2004;197:51–59. doi: 10.1111/j.0105-2896.2004.0098.x. [DOI] [PubMed] [Google Scholar]
- Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S, Cancro M, Grewal IS, Dixit VM. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]


