Abstract
This study tests the hypothesis that central sensitization initiated by nociceptive input can be maintained by repeated brief innocuous peripheral inputs. Capsaicin was injected intradermally into the hind paw of adult rats. Three different types of daily cutaneous mechanical stimulations (vibration, soft brush, or pressure) were applied to the capsaicin-injected paw for a period of 2 weeks. Daily stimulation consisted of a 10-second stimulation repeated every 30 seconds for 30 minutes. Foot withdrawal thresholds to von Frey stimuli applied to the paw were measured once a day for 4 weeks. The capsaicin-only group (control rats without daily stimulation) showed hyperalgesia lasting for 3 days. In contrast, hyperalgesia persisted for 2 weeks in the group that received vibration stimulation. Neither the soft brush nor the pressure group showed a significant difference in mechanical threshold from the control group. The vibration-induced prolonged hyperalgesia was significantly reduced by systemic injection of ifenprodil, an NMDA-receptor antagonist, but it was not influenced by either an AMPA receptor blocker or a free radical scavenger. Furthermore, a dorsal column lesion did not interfere with the prolongation of hyperalgesia. Data suggest that vibration-induced prolongation of hyperalgesia is mediated by spinal NMDA receptors, and a similar mechanism may underlie some forms of chronic pain with no obvious causes, such as complex regional pain syndrome type 1 (CRPS-1).
Keywords: Capsaicin, CRPS-1, Hyperalgesia, Persistent Pain, Vibration Stimulation
INTRODUCTION
Intradermal capsaicin injection produces primary and secondary hyperalgesia (Treede et al., 1992). Primary hyperalgesia, occurring at the capsaicin-injected site, is explained by peripheral nociceptor sensitization (Baumann et al., 1991; Simone et al., 1989; Steen et al., 1992). On the other hand, secondary hyperalgesia, which occurs at cutaneous areas away from the injection site, is known to be due to central sensitization of spinal dorsal horn neurons. This central sensitization results in increased responses to peripheral inputs and enhanced excitability of dorsal horn cells (LaMotte et al., 1992; Raja et al., 1988; Willis, 2002). It is well known that intradermal injection of capsaicin induces central sensitization by activating nociceptive C fibers (Weng and Dougherty, 2005; Wu et al., 2001).
An interesting aspect of secondary mechanical hyperalgesia is that although it requires noxious C-afferent input to be developed, it can be modified by innocuous tactile input. For example, repeated light touch stimuli delivered at 5-min intervals to a paw inflamed by an injection of complete Freund’s adjuvant produced progressive tactile hypersensitivity lasting several hours (Ma and Woolf, 1996), suggesting that innocuous tactile input enhances secondary hyperalgesia. The secondary hyperalgesia caused by intradermal injection of capsaicin was prolonged with repeated applications of innocuous brush stimuli (Kim et al., 2001). These studies strongly suggest that secondary hyperalgesia can be extended by innocuous stimulation and may play a role in certain conditions of neuropathic pain that persist without further nociceptive input.
The present study was designed to confirm and extend the above studies. Its purpose is to determine: (1) whether innocuous afferent input can maintain mechanical hyperalgesia for a long period of time (weeks); (2) the most effective type of innocuous stimuli for prolongation of the hyperalgesia; (3) whether the maintained hyperalgesia is mediated by NMDA receptors; and (4) whether the ascending dorsal column pathway is involved in the prolonged hyperalgesia.
Some of our data have previously been presented in abstract form (Kim et al., 2004b).
MATERIALS AND METHODS
All procedures and experimental protocols were performed in accordance with the guidelines of the International Association for the Study of Pain (Zimmermann, 1983), the Institutional Animal Care and Use Committee at the University of Texas Medical Branch, and the National Institutes of Health.
Experimental animals and protocols
Adult male Sprague-Dawley rats (200–300 g, Harlan Sprague-Dawley Co., Prattville, AL) were used for the experiments. The rats were housed in groups of three to four in plastic cages with soft bedding under a reversed 12-hour light/dark cycle (dark cycle: 8:00 a.m.–8:00 p.m.). Rats were housed in the same room at a constant ambient temperature with free access to food and water. All rats were housed for 4 to 7 days under these conditions prior to any experimental manipulations. Rats were randomly assigned to various treatment groups. Experiments were conducted in three stages, as follows:
Experiment 1
The effect of three different types of non-noxious mechanical stimulations on capsaicin-induced hyperalgesia was evaluated. Capsaicin was injected once at day 0. Mechanical stimulations were applied for 10 seconds, repeated every 30 seconds for 30 minutes between 10 a.m. and noon once a day from the day of injection (day 0) to day 13 after capsaicin injection (14 days). Behavioral tests were performed just before capsaicin injection at day 0 and just before application of daily mechanical stimulations during a 2-week stimulation period, then in the morning for a 2-week post-stimulation period (Fig. 1B). Each mechanical threshold test was followed by a sedation test for the entire 4-week period. The following treatment groups were used:
Fig. 1.
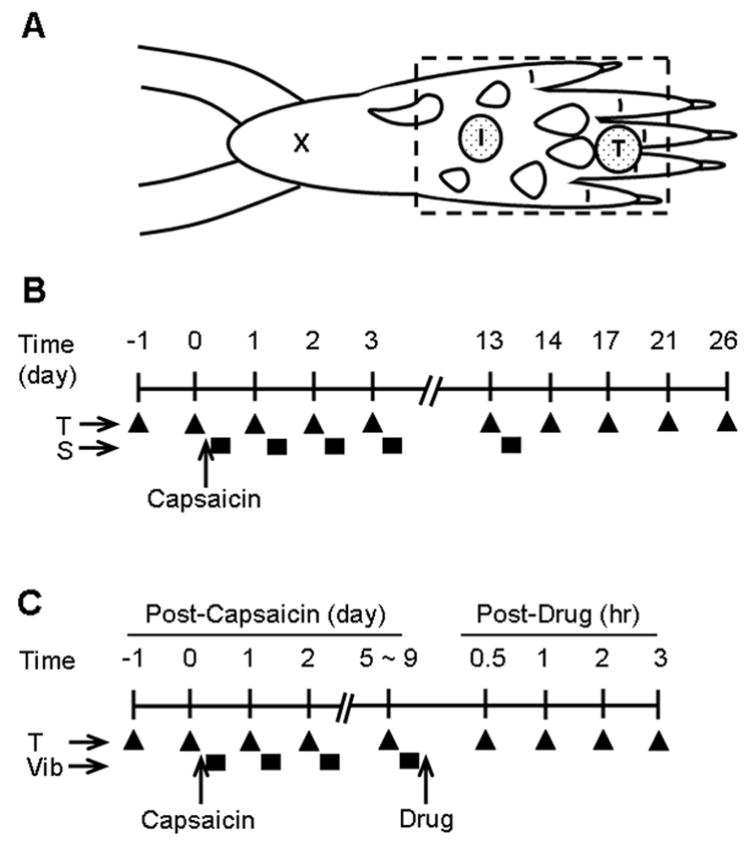
Diagrammatic representations of experimental paradigms for the capsaicin injection, behavioral test (T), innocuous mechanical stimulation (S), and drug treatment. (A) Illustration of the sites of capsaicin injection (I), behavioral testing site (T), and the region where conditioning stimuli were applied (dashed rectangle). The “x” is the initial needle insertion site for the capsaicin injection. (B) the experimental schedule for Experiment 1. “T” indicates the timing of behavioral testing (measurement of mechanical thresholds, filled triangle) in relation to the capsaicin injection (0.5%, 20 μl, i.d.) and repeated innocuous mechanical stimulation (S, filled rectangle boxes). (C) The experimental schedule for Experiment 2. “T” indicates the timing of behavioral testing, and “Vib” indicates that of vibration stimulation (filled rectangle boxes).
Control group (capsaicin only, n = 8). Capsaicin (100 μg, Sigma, St Louis, MO) was dissolved in 20 μl of olive oil and injected intradermally into the middle of the plantar surface of the left hind paw of a rat under halothane anesthesia (2% induction, 1% maintenance) once at day 0. A hypodermic needle (27 gauge) was inserted near the heel (marked “X” in Fig. 1A) and advanced to the target injection site (marked “I” in Fig. 1A). A successful injection produced a small bleb with a diameter less than 5 mm. Faint redness and minor swelling could be visualized in the bleb area lasting less then 2 minutes. There was no obvious sign of tissue injury beyond the boundary of the bleb. The needle insertion site was pressed with a finger for 1 minute after withdrawal of the needle in order to prevent leakage of the capsaicin. Anesthesia was continued for the next 30 minutes without additional stimulation in this group (control group). A behavioral test for secondary hyperalgesia was conducted by measuring mechanical thresholds at the proximal part of the third or fourth toe (marked “T” in Fig. 1A), approximately 12 mm from the capsaicin injection site, which is remote from the apparent tissue injury site (see above).
Brush group (capsaicin + soft brush, n = 8). Capsaicin was injected in the same way as in the control group. After capsaicin injection, brush stimulation was applied to the whole plantar surface of the left hind paw (a rectangular area outlined with a dashed line in Fig. 1A), including the capsaicin injection site as well as the behavioral testing site, with a soft paintbrush (Winsor & Newton brush series 340, size 4) while the animal was under halothane anesthesia. The injected hind paw was placed in a fixed position using “play dough,” and the plantar surface of the paw, including the capsaicin-injected site, was brushed. The brush stimulation was applied bidirectionally for 10 seconds (at a rate of two strokes per second), repeated every 30 seconds for 30 minutes. When the same brush was applied to the forearm of the experimenter, a definite tactile sensation was elicited. Immediately after the stimulation, anesthesia was discontinued, and the animals were returned to their cages. Stimulation was applied under halothane anesthesia once a day, between 10 a.m. and noon, from post-capsaicin day 0 to day 13 (see Fig. 1B for the stimulation schedule).
Pressure group (capsaicin + pressure, n = 7 rats). Treatment procedures were the same as in the brush group, except for the stimulation modality. To apply the pressure stimulus, a square metal plate (2 × 2 cm, weighing 100 g) was placed on the paw, over the capsaicin injection site, for 10 seconds every 30 seconds for 30 minutes under halothane anesthesia (Fig. 1B).
Vibration group (capsaicin + vibration, n = 8 rats). The treatment procedures were the same as in the brush group, except for the stimulation modality (Fig. 1B). To apply the vibration stimulus, a hand-held, commercially available mini-vibrator (Hitachi HV-1) with a round tip was applied on the plantar surface in an area with a diameter of about 15 mm, including the capsaicin injection site, under halothane anesthesia. The vibrator is specified to produce stimulation with 38 g/cm2 strength at 100 Hz of frequency. When the unit was applied to the experimenter’s forearm, a definite vibratory sensation was elicited.
Experiment 2
To explore the mechanisms involved in prolongation of hyperalgesia by vibration stimulation, we examined the effects of three compounds: (1) the ionotrophic glutamate receptor antagonist, ifenprodil hemitartrate (an NMDA-receptor antagonist; Tocris, Ellisville, MO; dissolved in 1% Tween 80 in saline); (2) the AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX;; Sigma, St Louis, MO; dissolved in saline); and (3) the reactive oxygen species scavenger phenyl-N-tert-butylnitrone (PBN; Sigma, St Louis, MO; dissolved in saline). Drug tests were performed while the rats were in a state of prolonged hyperalgesia maintained by daily vibration stimulation (Fig. 1C). Sedation tests were conducted following each behavioral test at 0.5, 1, 2, and 3 hours after drug injection to determine whether the tested compounds produced sedation. This experiment was divided into two parts to test drugs using two different vehicles (Tween 80 and saline).
Part 1: Ifenprodil test
Drug tests were performed on the 5th and 7th days after capsaicin injection using the randomized Latin square design. Beginning the 5th day after capsaicin injection, rats were randomly divided into two groups of three. Rats in each group received an intraperitoneal injection of ifenprodil dissolved in vehicle (1% Tween 80 in saline, volume of 1 ml) or vehicle alone, and behavioral tests were conducted at 0.5, 1, 2, and 3 hours after the injection. On the 7th day after capsaicin injection, rats were injected with the compounds that were not given previously, so that all rats received both treatments (ifenprodil and vehicle) in a random order.
Part 2: CNQX and PBN test
Drug tests were performed the 7th, 8th, and 9th days after capsaicin injection using the randomized Latin square design. Beginning on the 7th day after capsaicin injection, rats were randomly divided into three groups of two. Rats in each group received an intraperitoneal injection of CNQX, PBN, or vehicle (saline, volume of 1 ml), and behavioral tests were conducted at 1, 2, and 3 hours after the injection. On the 8th and 9th days after capsaicin injection, rats received the treatments that were not given previously, so that all rats received all three treatments (CNQX, PBN, and vehicle) in a random order.
Experiment 3
The purpose of this experiment was to examine the possible contribution of the ascending dorsal column pathway to the prolongation of capsaicin-induced hyperalgesia by vibration stimulation. Dorsal column lesion was performed at T11 on six rats 2 days before capsaicin injection, and a sham operation was conducted on another five rats. Capsaicin was injected intradermally, and daily vibration stimulation was applied as in Experiment 2 for 4 days. The mechanical threshold for foot withdrawal was measured each day just before the application of vibration stimulation.
Behavioral test for secondary hyperalgesia
Behavioral testing was performed in a blinded fashion so that the investigator conducting the tests was not aware of the nature of manipulations done to the animals. As an indicator for mechanical sensitivity, the 50% foot withdrawal threshold to mechanical stimuli applied to the paw (mechanical threshold) was measured using the “up-down” method (Chaplan et al., 1994), following the procedures described in previous studies (Baik et al., 2003; Kim et al., 2004a). The rat was placed in a transparent plastic box on a metal wire mesh floor. A series of eight filaments with von Frey values increasing at approximately equal increments (3.65, 3.87, 4.10, 4.31, 4.52, 4.74, 4.92, and 5.16) were used to determine the threshold stiffness required for 50% paw withdrawal. Since von Frey values are logarithmically related to bending forces in grams, these chosen values are equivalent to 0.45, 0.74, 1.26, 2.04, 3.31, 5.50, 8.32, and 14.45 g, respectively. Starting with filament 4.31, von Frey filaments were applied perpendicularly to the plantar surface of the hind foot with sufficient force to bend the filament slightly for 2–3 seconds. All behavioral testing was performed at the proximal part of the third or fourth toe (marked “T” in Fig. 1A), about 1.2 cm away from the capsaicin injection site (marked “I” in Fig. 1A), in order to test for secondary hyperalgesia.
Whenever a positive response to a stimulus occurred, the next weaker von Frey filament was applied. Whenever a negative response occurred, the next stronger one was applied. The test was continued until the responses to six stimuli had been obtained after the first change in response or until the test reached either end of the spectrum of the von Frey set. The 50% threshold value was calculated using Dixon’s formula (Dixon, 1980): 50% threshold = 10(X+kd)/104, where X is the value of the final von Frey filament used (in logarithmic units), k is the tabular value for the pattern of positive/negative responses, and d is the mean difference between stimuli in logarithmic units (0.22). In cases in which continuous positive or negative responses were observed all the way out to the end of the stimulus spectrum, values of 3.54 or 5.27 were assigned, respectively, by assuming a value of ±0.5 for k in these cases (Dixon, 1980). The outcomes of behavioral data were expressed as von Frey (VF) values (maximum range: 3.54–5.27) and plotted on a linear scale (plotting in gram values requires logarithmic plots). A few examples of conversion between the two values are: VF 4.0 = 1.0 g; VF 4.3 = 2.0 g; VF 4.7 = 5.0 g; and VF 5.0 = 10 g.
Tests for Sedation
To determine whether the tested compounds induced sedation, posture and righting reflexes were assessed following a method used in a previous study by Devor and Zalkind (2001).
Five-point scale for posture:
0, Normal posture, rearing and grooming;
1, Moderate atonia and ataxia. Weight support, but no rearing;
2, Weight support, but severe ataxia (e.g., may fall off table edge);
3, Muscle tone but no weight support and small purposive movements;
4, Flaccid atonia, fully immobilized with no attempts at movement.
Five-point scale for righting reflex:
0, Rat struggles when placed on its side, rapid forceful righting;
1, Moderate resistance to placement on side, rapid righting;
2, No resistance to placement on side, effortful but successful righting;
3, Unsuccessful attempt at righting; 4, Righting not attempted, no movements.
Rats were scored immediately after each mechanical sensitivity test in Experiments 1 and 2.
Dorsal column lesion
The dorsal column was transected unilaterally at the 11th thoracic spinal cord level while the animal was under halothane anesthesia (3% for induction and 2% for maintenance). The rat was placed in the prone position, and the skin where the incision would be made was disinfected three times with 70% ethanol and 10% povidone-iodine solution. A laminectomy was performed at the 10th thoracic vertebra. The dura mater was then opened with a pair of fine forceps, and the right dorsal column of the spinal cord was cut using a pair of fine scissors, aiming for a depth of 1 mm from the dorsal surface. Muscles and subcutaneous tissue were sutured with 3–0 silk. The skin was clipped and then disinfected with 10% povidone-iodine solution. For controls, a sham operation was performed in the same way as the dorsal column lesion with the exception of the cutting of the dorsal column. After the experiment, the 11th thoracic spinal cord was sectioned and stained with cresyl violet. The lesion of the spinal cord was confirmed, and the boundary of the lesioned area was drawn under a light microscope equipped with a drawing tube.
Data analysis
Behavioral data are presented as mean plus or minus the standard error of the mean (SEM), and statistical treatments were made using the SAS statistical program. The mechanical thresholds were expressed both in von Frey values on the left side and in grams on the right side of Figs. 2, 3, and 4. The data were analyzed by two-way repeated ANOVA with one or two repeated factors, followed by Duncan’s post-hoc test. A two-tailed P value of less than 0.05 was considered to be significant.
Fig. 2.
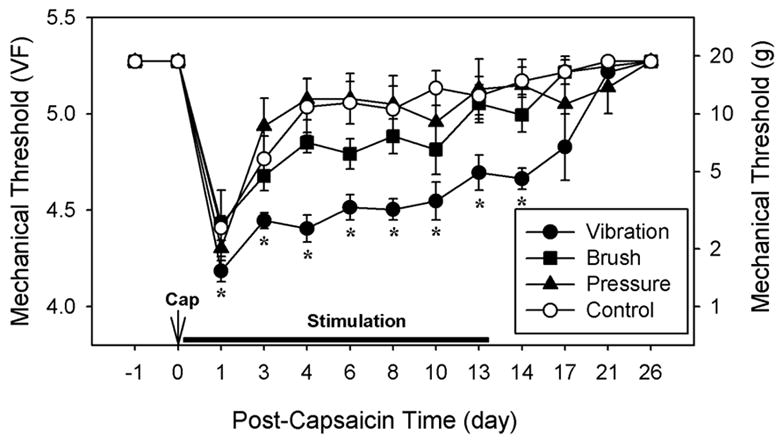
The effects of various innocuous mechanical conditioning stimuli on capsaicin-induced hyperalgesia. Rats in the vibration (n = 8), brush (n = 8), and pressure (n = 7) groups received daily corresponding stimulations (a 30-minute session each day) on the capsaicin-injected paw under halothane anesthesia for 14 days. The control group (n = 8) received daily anesthesia alone without stimulation. Mechanical thresholds were measured once a day just prior to stimulation. The data are expressed in von Frey values (VF) on the left and in grams on the right side of the graph. The vibration group maintained significantly lower thresholds than the control group throughout the entire stimulation period. Asterisks indicate values significantly different (P < 0.05) from the corresponding values in the control group by two-way repeated ANOVA with one repeated factor (time), followed by Duncan’s post-hoc tests.
Fig. 3.
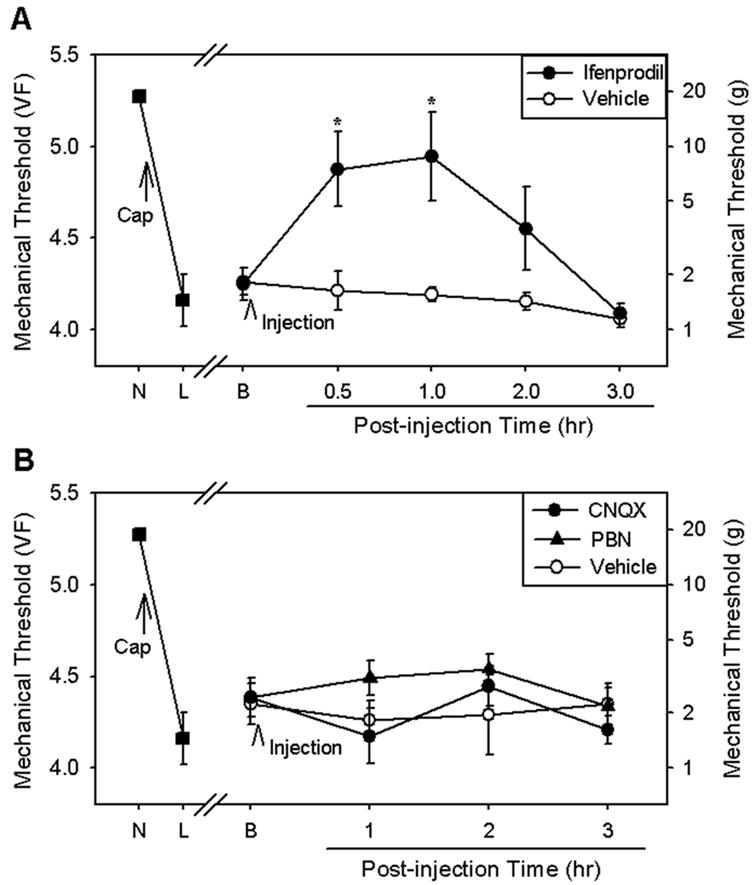
The effects of analgesic compounds on the vibration-induced prolongation of hyperalgesia. (A) In one group of rats (n = 6), capsaicin injection and daily vibration stimulation produced a significant reduction in mechanical thresholds (L) from normal levels prior to capsaicin injection (N). Intradermal capsaicin injection was followed by daily vibration stimulation for 7 days. Immediately after vibration stimulation on post-capsaicin day 5 or day 7, the threshold was measured (B), and an NMDA-receptor blocker, ifenprodil (30 mg/kg dissolved in 1% Tween 80 in saline), or vehicle (1% Tween 80 in saline) was given intraperitoneally using the randomized Latin square design (all rats received both vehicle and ifenprodil once on either day 5 or 7). Ifenprodil significantly increased mechanical thresholds for at least 1 hour, whereas vehicle had no effect. Asterisks indicate values significantly (P < 0.05) different from those of the vehicle control group according to two-way ANOVA with one repeated factor (time), followed by Duncan’s post hoc tests. (B) In another group of rats (n = 6), capsaicin injection and daily vibration stimulation significantly reduced mechanical thresholds (L) from normal levels prior to capsaicin injection (N). Intradermal capsaicin injection was followed by daily vibration stimulation for 9 days. Immediately after vibration stimulation on post-capsaicin day 7, 8, or 9, the threshold was measured (B), and CNQX (an AMPA-receptor antagonist, 15 mg/kg in saline), PBN (a ROS scavenger, 100 mg/kg in saline), or vehicle (saline) was given intraperitoneally using the randomized Latin square design (all rats received all three compounds once on either day 7, 8, or 9). None of the compounds produced a significant increase in mechanical thresholds according to analysis by two-way ANOVA with one repeated factor (time).
Fig. 4.
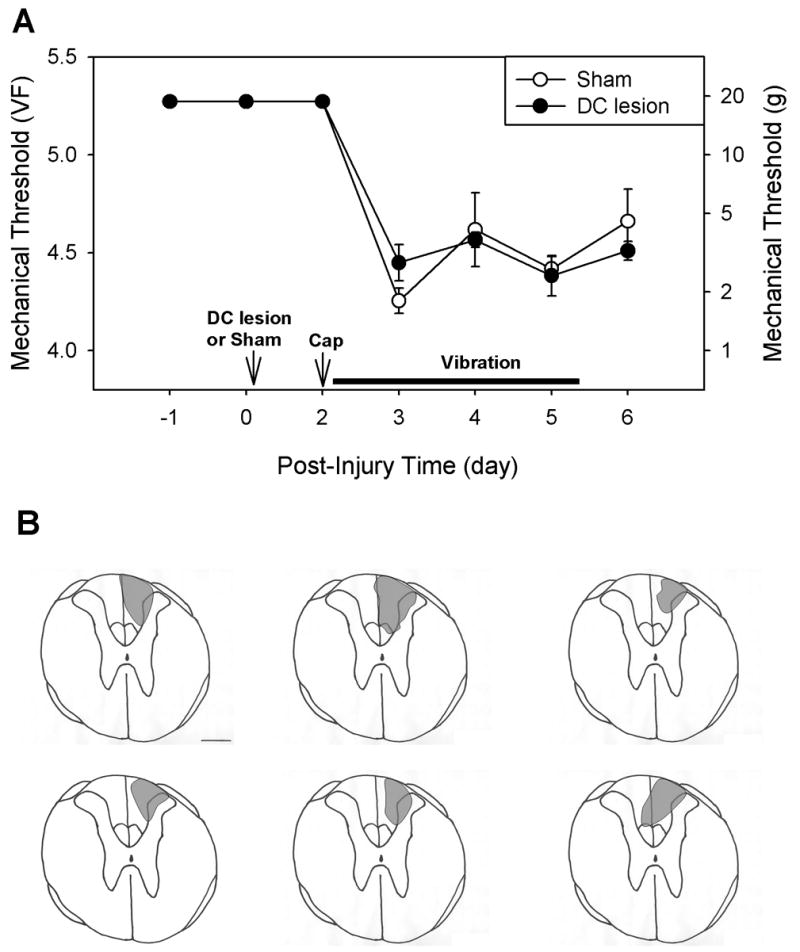
The effects of dorsal column lesion on the vibration-induced prolongation of hyperalgesia. (A) In a group of six rats, the right dorsal column (DC) was transected at the T11 spinal cord level, and in another five rats a sham operation was performed. Two days later, capsaicin was injected into the right hind paw, daily vibration stimulation was applied daily for 4 days. Rats with dorsal column lesions showed no significantly different level of vibration-induced prolongation of hyperalgesia as compared to sham-operated rats according to two-way ANOVA with one repeated factor (time). (B) Drawings of the histologically confirmed lesion site of each of six rats. Calibration bar = 0.5 mm.
RESULTS
Experiment 1: The effects of daily applications of innocuous mechanical stimuli on capsaicin-induced hyperalgesia
The effects of daily applications of three different conditioning mechanical stimuli on capsaicin-induced hyperalgesia were examined. As shown in Fig. 2, mechanical thresholds of all rats before capsaicin injection were 5.27 (18.6 g), which was the value set as the maximum cut-off point. Following capsaicin treatment but without daily conditioning mechanical stimuli (control group), mechanical thresholds declined to 4.41 (2.6 g) ± 0.06 at day 1 and then recovered by day 3 to 5.04 ± 0.15, a value that was not significantly different from the pre-capsaicin levels (Fig. 2). The rats that received vibration stimulation after capsaicin injection had significantly lower mechanical thresholds compared to the control group on post-capsaicin day 1 (4.18 ± 0.05). Furthermore, subsequent daily vibration stimulations produced prolongation of lower thresholds throughout the 2-week stimulation period (4.45 ± 0.04 at day 3 and 4.66 ± 0.06 at day 14). After termination of vibration stimulation, mechanical thresholds gradually returned to normal levels (Fig. 2).
In contrast, the group with daily pressure stimulation showed no detectable difference from the control group in mechanical sensitivity of the paw. The group that received daily soft brush stimulation showed slightly lower thresholds through the testing period, but none of the data points reached statistical significance.
All rats in this experiment were anesthetized with halothane (2% for induction, 1% for maintenance) for 30 minutes every day for 2 weeks. The average body weight of all rats in four groups was 228 ± 4 g (mean ± SEM, n = 31 rats) at day 0, increasing to 290 ± 6 g at day 14 and 349 ± 7 g at day 26, similar to normal weight gain according to the data provided by Harlan Sprague-Dawley Co. There was no change in the rats’ skin color. All rats were assessed for posture and righting reflexes based on the five-point scales described in the Methods section, and all scored 0 at all time points. These data suggest that repeated anesthesia produced no sedative effect and no change in the overall health condition of the rats.
The data suggest that non-noxious stimulation repeated daily, especially vibration, prolongs capsaicin-induced hyperalgesia for several weeks as long as the stimulation is continued.
Experiment 2: The effects of systemic injection of ionotrophic glutamate receptor antagonists and a ROS scavenger on prolonged capsaicin-induced hyperalgesia maintained by daily vibration stimulation
Since daily applications of vibration stimulation prolonged capsaicin-induced hyperalgesia, we attempted to study the underlying mechanism by pharmacological means. Three different types of chemical compounds were tested: ifenprodil (an NMDA-receptor blocker), CNQX (an AMPA/kainate receptor blocker), and PBN (a free radical scavenger).
The effect of ifenprodil on the prolonged capsaicin-induced hyperalgesia maintained by daily vibration stimulation is shown in Fig. 3A. Ifenprodil (30 mg/kg, i.p.) injected on either the 5th or 7th post-capsaicin day significantly increased mechanical thresholds from 4.25 ± 0.09 to 4.94 ± 0.24 at 1 hour after injection. A systemic injection of a lower dose (10 mg/kg) of ifenprodil at another time point produced only a mild increase in the mechanical thresholds (VF = 4.11 ± 0.22 prior to injection to 4.47 ± 0.12 at 1 hour after injection, n = 3). These data suggest that ifenprodil significantly reduces the prolonged capsaicin-induced hyperalgesia in a dose-dependent manner.
In contrast, systemic injection of CNQX (15 mg/kg, i.p.) or PBN (100 mg/kg, i.p.) produced no changes in the prolonged capsaicin-induced hyperalgesia established by daily vibration (Fig. 3B). These data suggest that the prolongation of hyperalgesia is maintained by NMDA receptors, but not by AMPA receptors. Furthermore, free radicals do not seem to be involved in this process.
Since ifenprodil is known to produce sedation, care was taken to determine whether the reduction of hyperalgesia was due to a sedative effect. However, a single injection of 30 mg/kg of ifenprodil (the dose used in the experiment) did not produce sedative behavior at any time point (score = 0 for posture and righting reflexes).
Experiment 3: The effect of dorsal column lesion on prolonged capsaicin-induced hyperalgesia maintained by daily vibration stimulation
To examine the possible contribution of the ascending dorsal column pathway for the prolongation of capsaicin-induced hyperalgesia by daily vibration stimulation, one group of rats was tested after dorsal column lesion. In six rats, the dorsal column was transected unilaterally on the right side at the T11 level of the spinal cord, while sham surgery was performed on an additional five rats. Two days later, capsaicin was injected intradermally on the right paw (ipsilateral to the dorsal column lesion). Daily vibration stimulations were applied for 4 consecutive days following capsaicin injection, and mechanical thresholds were measured daily. After termination of the experiment, the extent of the dorsal column lesion was determined histologically, and the lesion was reconstructed in all six lesioned rats. The rats with dorsal column lesions and sham-operated rats showed the same prolongation of capsaicin-induced hyperalgesia (Fig. 4), which suggests that the ascending dorsal column pathway is not involved in this phenomenon, although the dorsal column is a major ascending pathway conveying vibratory stimulation.
DISCUSSION
The present study investigated the effect of repeated innocuous mechanical stimulation on capsaicin-induced secondary hyperalgesia. Repeated daily applications of vibration stimulation prolonged the hyperalgesia for the entire 2-week stimulation period. However, neither soft brushing nor pressure stimulation produced significant changes in the level or duration of hyperalgesia. Systemic injection of ifenprodil, an NMDA-receptor antagonist, significantly reduced vibration-induced prolongation of the hyperalgesia, suggesting that the prolongation is an NMDA-receptor-mediated process. Finally, the ascending dorsal column pathway does not seem to be involved in this process. The data suggest that repeated brief daily vibration stimulation maintains capsaicin-induced secondary hyperalgesia for weeks and that the process is mediated by NMDA receptors.
In this study, capsaicin was injected intradermally into the middle of the plantar surface of the hind paw, and secondary hyperalgesia was assessed by measuring mechanical threshold at a site about 1.2 cm away from the injection site (Fig. 1). It is well known that secondary mechanical hyperalgesia after intradermal capsaicin injection is caused by changes within the spinal cord (Treede et al., 1992). A number of studies have demonstrated that nociceptive input can sensitize spinal dorsal horn neurons, with a reduction in nociceptive threshold, an increase in responsiveness to peripheral input, and an expansion of receptive fields (Woolf 1983; Baumann et al., 1991). This phenomenon, termed central sensitization, is generally produced by C-fiber input to the spinal cord, which, in turn, produces slow synaptic potentials via actions on glutamate (AMPA, NMDA, and metabotropic) and NK1 receptors (Dickenson, 1990; Dougherty et al., 1992, 1994; Haley et al., 1990; Neugebauer et al., 1999). The present study suggests that input from non-nociceptive afferent A fibers, particularly those from rapidly adapting mechanoreceptors, can modify central sensitization in such a way that brief but repeated input can maintain the sensitization for weeks. In earlier studies, innocuous mechanical stimulations applied to an area of secondary hyperalgesia were able to prolong the duration of mechanical hyperalgesia for hours (Ma and Woolf, 1996) or days (Kim et al., 2001). The present study shows that central sensitization can be maintained by repeated mechanoreceptor stimulation for the entire stimulation period of 2 weeks, thus confirming and expanding previous findings. The results of the present study further raise the possibility that spinal NMDA receptors are involved in the prolongation of the hyperalgesia and that a mechanism similar to that found in the present study may underlie certain chronic pain conditions. For example, in some patients with complex regional pain syndrome type 1 (CRPS-1), pain is maintained by repeated, seemingly innocuous, input.
CRPS-1 has been defined as a syndrome that develops after trauma with minor or no obvious nerve injury to an extremity (Harden et al., 2001; Jänig and Baron, 2004). It is characterized by spontaneous pain, allodynia, and hyperalgesia; by active and passive movement disorders; and by abnormal regulation of blood flow and sweating, edema, and trophic changes (Jänig and Baron, 2004). The underlying mechanisms of pain in CRPS-1 patients are not fully understood. In the present study, vibration stimulation sustained capsaicin-induced hyperalgesia for a much longer period of time than could be explained by the tissue injury caused by capsaicin injection. This model resembles the painful conditions seen in CRPS-1 patients. Currently three animal models of CRPS-1 are available: (1) the electrical stimulation of the sciatic nerve model (Vatine et al., 1998), (2) the tibial fracture model (Guo et al., 2004), and (3) the chronic post-ischemia pain (CPIP) model (Coderre et al., 2004). In the first model, brief, intense electrical stimulation is applied to the sciatic nerve without producing further injury. Rats subsequently develop hyperalgesia lasting 3–5 weeks (Vatine et al., 1998). In the rat tibial fracture model, the tibial bone is fractured and the hind limb is casted after the fractured bones are repositioned. These rats show signs of mechanical allodynia, which include plasma extravasation with edema, vasodilation, an increase in skin temperature, and a decrease in bone mineralization density. The NK1-receptor antagonist LY303870 attenuates the edema, vasodilation, and allodynia (Guo et al., 2004). The CPIP model is produced by placing a tourniquet on one hindlimb of an anesthetized rat just proximal to the ankle joint for 3 hours. These rats exhibit hyperemia, edema, plasma extravasation, hyperalgesia, and allodynia to innocuous mechanical stimulation and to cold (acetone) (Coderre et al., 2004). The phenomenon shown in the present study may also play a role in maintaining pain behaviors in one or more of these animal models of CRPS-1.
An NMDA receptor does not routinely participate in synaptic transmission because of its voltage-dependent block by extracellular magnesium. Noxious afferent input (such as following capsaicin injection) depolarizes membranes and remove the magnesium block, which then permits the involvement of NMDA receptors in synaptic transmission. Nociceptive input to the dorsal horn is further increased via positive feedback through presynaptic NMDA receptors (Petrenko et al., 2003). If the Mg2+ block is removed from the receptor by membrane depolarization, the release of glutamate is followed by an increased Ca2+ influx. The increase in intracellular Ca2+ activates several intracellular signaling pathways (Sluka and Willis, 1997), resulting in the phosphorylation of several proteins including the NMDA receptor (Zou et al., 2000). These processes may explain why hyperalgesia is maintained although the nociceptive discharges have disappeared. Ifenprodil, a novel NMDA-receptor antagonist that selectively inhibits the receptor containing the NR2B subunit (Williams, 2001), has a much lower side-effect profile than other NMDA-receptor antagonists (Chizh et al., 2001). The fact that prolongation of mechanical hyperalgesia was significantly reduced by ifenprodil suggests that continuous NMDA-receptor activation is involved in the prolongation of hyperalgesia by daily vibration stimulation. On the other hand, neither CNQX nor PBN, which can block certain forms of persistent pain (Nozaki-Taguchi and Yaksh, 2002; Kim et al., 2004a), failed to influence the prolonged hyperalgesia. Therefore, prolongation of capsaicin-induced hyperalgesia produced by repeated vibration stimulation seems to be mediated by NMDA receptors.
In this study, vibration was the most effective stimulation, followed by soft brushing. However, constant pressure stimulation did not prolong the capsaicin-induced hyperalgesia. Vibration stimulation (100 Hz) to the glabrous skin of the rat hind paw should mainly have activated both Meissner and Pacinian corpuscles, which are fast-adapting receptors type I and II (FA I and FA II), respectively. On the other hand, brush and pressure stimulations should have activated mostly FA I and slowly adapting type I receptors, respectively. The fact that vibration stimulation is most effective in prolonging the hyperalgesia suggests that activation of FA II receptors may be an important process. Furthermore, FA II receptors have large receptive fields and extremely low mechanical thresholds of less than 1 mN (Horch et al., 1977), making them easy receptors to activate. However, the exact mechanism by which activation of FA II prolongs central sensitization is not known, except that NMDA receptors seem to be involved. Since mechanoreceptors send branches of axons both to dorsal horn neurons of the spinal cord and to the dorsal column nuclei of the brainstem, their activation can influence spinal neurons both directly and indirectly through the brainstem loop. Ascending dorsal column projections can activate descending nociceptive modulatory pathways, which may maintain neuropathic pain behaviors (Burgess et al., 2002). However, the dorsal column lesion did not produce changes in the prolonged hyperalgesia by vibration stimulation in this study. Therefore, the vibration-induced prolongation of hyperalgesia seems to be maintained mainly by activation of FA II receptors or by their influence in spinal neurons, and the ascending dorsal column pathway does not seem to be involved.
In conclusion, brief, repeated application of vibration stimulation prolongs capsaicin-induced hyperalgesia for a long time. This phenomenon seems to be mediated by NMDA receptors. A similar prolongation of hyperalgesia by innocuous mechanical input may underlie persistent pain in some CRPS-1 patients.
Acknowledgments
This work was supported by NIH Grants R01 NS31680, AT01474, and P01 NS11255. JS was a visiting scholar supported by the Novartis Foundation, and also partially by (1) the Deutsche Forschungsgemeinschaft (DFG Ba 1921/1-3), (2) the German Research Network on “Neuropathic Pain” of the German Ministry of Research and Education (BMBF, 01EM0/1/04), and (3) an unrestricted educational grant from Pfizer, Germany. We would like to express our gratitude to Ms. Denise Broker for her excellent assistance in editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baik EJ, Chung JM, Chung K. Peripheral norepinephrine exacerbates neuritis induced hyperalgesia. J Pain. 2003;4:212–221. doi: 10.1016/s1526-5900(03)00617-5. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, et al. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Headley PM, Tzschentke TM. NMDA receptor antagonists as analgesics: focus on the NR2B subtype. Trends Pharmacol Sci. 2001;22:636–642. doi: 10.1016/s0165-6147(00)01863-0. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004;112:94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Devor M, Zalkind V. Reversible analgesia, atonia, and loss of consciousness on bilateral intracerebral microinjection of pentobarbital. Pain. 2001;94:101–112. doi: 10.1016/S0304-3959(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Dickenson AH. A cure for wind up: NMDA receptor antagonists as potential analgesics. Trends in Phar Sci. 1990;11:307–309. doi: 10.1016/0165-6147(90)90228-z. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Paleckova V, Sorkin LS, Willis WD. The role of NMDA and non-NMDA excitatory amino acid receptors in the excitation of primate spinothalamic tract neurons by mechanical, chemical, thermal, and electrical stimuli. J Neurosci. 1992;12:3025–3041. doi: 10.1523/JNEUROSCI.12-08-03025.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Paleckova V, Willis WD. Neurokinin 1 and 2 antagonists attenuate the responses and NK1 antagonists prevent the sensitization of primate spinothalamic tract neurons after intradermal capsaicin. J Neurophysiol. 1994;72:1464–1475. doi: 10.1152/jn.1994.72.4.1464. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108:95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Haley JE, Sullivan AF, Dickenson AH. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990;518:218–226. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- Harden RN, Baron R, Jänig W. Complex regional pain syndrome. Seattle, WA: IASP Press; 2001. [Google Scholar]
- Horch KW, Tuckett RP, Burgess PR. A key to the classification of cutaneous mechanoreceptors. J Invest Dermatol. 1977;69:75–82. doi: 10.1111/1523-1747.ep12497887. [DOI] [PubMed] [Google Scholar]
- Jänig W, Baron R. Experimental approach to CRPS. Pain. 2004;108:3–7. doi: 10.1016/j.pain.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kim HK, Park SK, Zhou J-L, Taglialatela G, Chung K, Coggeshall RE, et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004a;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Kim HK, Schattschneider J, Chung K, Chung JM. Persistence of capsaicin-induced hyperalgesia by brief daily innocuous cutaneous stimulations. Neurosci Abstr. 2004b:407–13. [Google Scholar]
- Kim HT, Park SK, Lee SE, Chung JM, Lee DH. Non-noxious A fiber afferent input enhances capsaicin-induced mechanical hyperalgesia in the rat. Pain. 2001;94:169–175. doi: 10.1016/S0304-3959(01)00351-7. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;67:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Chen PS, Willis WD. Role of metabotropic glutamate receptor subtype mGluR1 in brief nociception and central sensitization of primate STT cells. J Neurophysiol. 1999;82:272–282. doi: 10.1152/jn.1999.82.1.272. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Yaksh TL. Pharmacology of spinal glutamatergic receptors in post-thermal injury-evoked tactile allodynia and thermal hyperalgesia. Anesthesiology. 2002;96:617–626. doi: 10.1097/00000542-200203000-00018. [DOI] [PubMed] [Google Scholar]
- Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- Raja SN, Meyer RA, Campbell JN. Peripheral mechanisms of somatic pain. Anesthesiology. 1988;68:571–590. doi: 10.1097/00000542-198804000-00016. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Willis WD. The effects of G-protein and protein kinase inhibitors on the behavioral responses of rats to intradermal injection of capsaicin. Pain. 1997;71:165–178. doi: 10.1016/s0304-3959(97)03371-x. [DOI] [PubMed] [Google Scholar]
- Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Vatine JJ, Argov R, Seltzer Z. Brief electrical stimulation of c-fibers in rats produces thermal hyperalgesia lasting weeks. Neurosci Lett. 1998;246:125–128. doi: 10.1016/s0304-3940(98)00217-1. [DOI] [PubMed] [Google Scholar]
- Weng HR, Dougherty PM. Response properties of dorsal root reflexes in cutaneous C fibers before and after intradermal capsaicin injection in rats. Neuroscience. 2005;132:823–831. doi: 10.1016/j.neuroscience.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Curr Drug Targets. 2001;2:285–298. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- Willis WD. Long-term potentiation in spinothalamic neurons. Brain Res Brain Res Rev. 2002;40:202–214. doi: 10.1016/s0165-0173(02)00202-3. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang L, Lin Q, Willis WD. Nitric oxide synthase in spinal cord central sensitization following intradermal injection of capsaicin. Pain. 2001;94:47–58. doi: 10.1016/S0304-3959(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J Neurosci. 2000;20:6989–6997. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


