Abstract
The basic helix-loop-helix (bHLH) transcription factor Hand2 has been shown to play a role in the development of the mammalian sympathetic nervous system (SNS); however, its precise role could not be uncovered because Hand2 is required for early embryonic survival. We therefore generated a conditional Hand2 knockout mouse line by excising Hand2 in Wnt1-Cre-expressing neural crest-derived cells. These mice die at 12.5 dpc with embryos showing severe cardiovascular and facial defects. Crest-derived cells, however, populate sites of SNS development and proliferate normally. Sympathetic precursors differentiate into neurons and express the pan-neuronal markers, β3-tubulin (Tuj1) and Hu showing that Hand2 is not essential for SNS neuronal differentiation. To determine whether Hand2 regulates noradrenergic differentiation, the levels of the norepinephrine biosynthetic enzymes, tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DBH) was examined. Both enzymes were dramatically reduced in mutant embryos suggesting that the primary role of Hand2 in the SNS is determination of neuronal phenotype. Loss of Hand2 did not affect the expression of other members of the transcriptional circuit regulating SNS development, including Phox2a/b, Mash1 and Gata2/3; however, Hand2 was required for Hand1 expression. Our data suggest that the major role of Hand2 during SNS development is to permit sympathetic neurons to acquire a catecholaminergic phenotype.
Keywords: Hand2, bHLH, sympathetic nervous system, conditional knockout, neuronal differentiation, catecholaminergic, noradrenergic
INTRODUCTION
The sympathetic nervous system (SNS) regulates homeostasis in vertebrates, in part, by coordinating the organs response to internal and external stimuli. SNS neurons and supporting cells are both derived exclusively from the neural crest (NC). Cells in the NC lineage develop from ectoderm at the most dorsal aspect of the embryo and migrate to the dorsal aorta at the time the neural tube closes. At the dorsal aorta, crest-derived cells are induced by bone morphogenetic proteins (BMPs) to differentiate into sympathetic neurons, which form a primary sympathetic chain. BMP stimulation activates a SNS transcription cascade that includes Gata2/3, Hand2, Mash1, and Phox2a/b (Reviewed in (Goridis and Rohrer, 2002; Howard, 2005).
The paired-homeodomain factor, Phox2b, is expressed early during SNS development and is essential for survival of SNS precursors. In Phox2b mutant embryos, SNS precursors arise and express Mash1 but not other members of the transcriptional cascade, and die prior to SNS differentiation (Pattyn et al., 1999). Phox2a is expressed after Phox2b but its function remains unclear. Loss of Phox2a does not appear to effect SNS development or transcription of Phox2b (Morin et al., 1997). The Phox2 genes do not play a redundant role because Phox2a is unable to rescue animals from the loss of Phox2b (Coppola et al., 2005). The Phox2 genes are candidate factors for specification of a noradrenergic phenotype. Ectopic expression of Phox2a protein activates the noradrenergic differentiation program in cultured NC stem cells (Lo et al., 1999) and ectopic expression of either Phox2a or Phox2b activates this program in cells that colonize peripheral nerves (Stanke et al., 1999). Both Phox2 proteins directly bind to the promoters of the noradrenergic biosynthesis genes Th and Dbh and regulate their expression (Kim et al., 1998; Yang et al., 1998), suggesting that at least Phox2b, plays multiple roles in SNS development.
The bHLH factor, Mash1, is expressed in the CNS and in the autonomic nervous system (Blaugrund et al., 1996; Guillemot and Joyner, 1993; Guillemot et al., 1993; Johnson et al., 1992). In the autonomic nervous system, Mash1 expression is transient; expression begins in neural precursor cells but is extinguished before they differentiate into neurons. In embryo lacking Mash1, the SNS ganglia form but are greatly reduced in size due to a reduction in the number of neurons (Guillemot et al., 1993; Sommer et al., 1995). Mash1 is also expressed in parasympathetic neurons, which are predominantly cholinergic, and in the enteric nervous system where it is required for a subset of crest-derived cells that is transiently catecholaminergic but terminally differentiates into non-catecholaminergic neurons, including all of those that are serotonergic neurons (Blaugrund et al., 1996). The role of Mash1 in SNS development remains unclear, but its expression in non-sympathetic neurons, which are not catecholaminergic, indicates that Mash1 is not the determinant of the noradrenergic phenotype.
Noradrenergic differentiation of the SNS requires Gata transcription factors which are expressed during differentiation of sympathetic neurons. Mouse embryos lacking Gata3 in the sympathoadrenal system die at mid-gestation due to the deficiency of norepinephrine brought about by a greatly reduced level of TH expression and subsequent loss of the sympathoadrenal lineage (Lim et al., 2000; Moriguchi et al., 2006). Gata3 can directly bind the regulatory region of the Th gene suggesting the loss of TH in these mutant embryos is a direct effect and not mediated through other genes (Hong et al., 2006). In chickens, ectopic expression of Gata2 in brachial nerve is able to induce generic neurogenesis but not the noradrenergic phenotype (Tsarovina et al., 2004). This study suggests that Gata factors may have proneural activity but require co-factors to regulate noradrenergic gene expression.
Hand2 (previously called dHand) is a member of the twist family of bHLH factors. It is expressed in a number of tissues during development including the heart, limbs and crest-derived tissues where it regulates their development (McFadden et al., 2002; Srivastava et al., 1995; Srivastava et al., 1997; Yanagisawa et al., 2003). In the peripheral nervous system, Hand2 is expressed in the three lineages of the autonomic nervous system (sympathetic, parasympathetic, and enteric) but not in dorsal root or cranial nerve sensory ganglia. Hand2 is expressed in the enteric nervous system from early in development through adulthood (D’Autréaux et al., 2007). An extensive developmental analysis of Hand2 expression in the parasympathetic nervous system has not been undertaken, but Hand2 is expressed during development in at least a subset of ganglia (Dai et al., 2004). In the SNS, Hand2 is first expressed in differentiating precursor at 10 dpc with expression continuing throughout development in both sympathetic neurons and in the chromaffin cells of the adrenal gland (Morikawa et al., 2005).
Ectopic expression studies in vitro (Howard et al., 1999; Xu et al., 2003) and in vivo (Howard et al., 2000) suggest that Hand2 promotes differentiation of crest-derived precursors into catecholaminergic, presumably sympathetic neurons. The activation of the catecholaminergic differentiation program is accompanied by the activation of Phox2a and b (Howard et al., 2000) and Mash1 (Morikawa et al., 2005). After initiating the catecholaminergic differentiation program, Hand2 may also directly regulate genes synthesizing catecholamines. Dbh promoter analysis in vitro suggests that Hand2 interacts with Phox2a to regulate its expression (Rychlik et al., 2003; Xu et al., 2003). Recent studies using chromatin immunoprecipitation (ChIP) support these findings (Rychlik et al., 2005). The ability of Hand2 to activate the neuronal and catecholaminergic differentiation programs suggests that it plays multiple roles during SNS development. Hand2, however, is expressed after crest-derived cells have been specified to become sympathetic neurons and have started to express Phox2b (Howard et al., 2000). The late expression of Hand2 during SNS development suggests that it is not essential for precursor cells to be specified as neurons, although Hand2 might still function in later steps of differentiation, such as the choice of a neurotransmitter.
Although ectopic expression studies suggest that Hand2 plays a role in mammalian SNS development, its role has not been fully examined because when Hand2 is deleted, embryos die prior to SNS formation (Srivastava et al., 1997). To circumvent early embryonic lethality, we generated a Hand2 conditional knockout mouse line. When Hand2 is deleted in NC cells, embryos survive until 12.5 dpc, at which time they succumb to severe cardiovascular defects. In our analysis of SNS development, we show that loss of Hand2 does not prevent formation of the SNS and differentiation of neurons. However, loss of Hand2 severely compromises the ability of sympathetic neurons to acquire catecholamine biosynthetic enzymes. Recently, an analysis of SNS development in the zebrafish carrying the Hand2 mutation, hands off, showed that Hand2 plays a similar role in SNS development in fish (Lucas et al., 2006).
METHODS
Targeting the Hand2 gene
Construction of targeting vector
PGKneoF2L2DTA (a gift from Dr. P. Soriano, Fred Hutchinson Cancer Res. Center, Seattle) was used as the backbone for constructing the Hand2 targeting vector (Fig. 1B). Three regions of the Hand2 gene were cloned into PGKneoF2L2DTA. The 5′ upstream region of Hand2 flanking the loxP site was obtained from plasmid dH5.5lacZ (a gift from Dr. A. Firulli, Herman B. Wells Center for Pediatric Research, Indiana University) and cloned as a 3.0 kb SmaI fragment into the SacII site of PGKneoF2L2DTA after blunting with VENT polymerase (NEB). The internal fragment that was placed between the first loxP site and the neomycin gene was obtained in two steps; a 6.7 kb SmaI-EcoRI fragment containing the 5′ untranslated region to 2 kb past the gene was purified from a P1 clone and cloned into SmaI-EcoRI the site of pBKSII (Stratagene) to generate dH6.7pBKSII. A 2.0 kb internal fragment was obtained from dH6.7pBKSII by partial digestion with SmaI followed by complete digestion with BsrDI. The BsrDI end was polished with T4 DNA polymerase (TaKaRa) and cloned into the EcoRV site of PGKneoF2L2DTA. The 3′ fragment of Hand2 used to flank the 3′ loxP site was obtained from dH6.7pBKSII as a 2.7 kb SmaI fragment and was cloned into SmaI site of PGKneoF2L2DTA. Prior to ES cell electroporation, the targeting vector was linearized with XhoI.
Fig. 1. Targeting strategy for generating the conditional allele of Hand2.
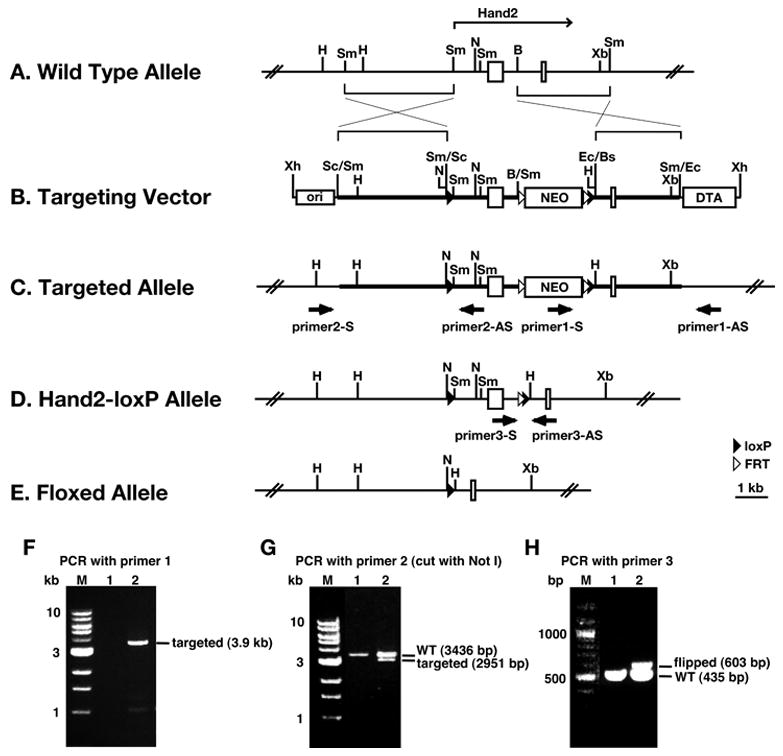
A–C: Schematic representation of the strategy used to insert loxP sites and the neomycin cassette flanked by FRT sites into the Hand2 locus. D: Schematic representation of targeted allele after neomycin cassette has been recombined out with FLT recombinase. E: Schematic representation of targeted allele after recombination with Cre. F, G: PCR analysis of the 3′ and 5′ ends of the targeted Hand2 gene. H: PCR analysis of the conditional Hand2 allele showing that the neomycin cassette was removed by FLT recombinase. A details description of the targeting strategy is presented in Materials and Methods
Gene targeting and generation of the conditional Hand2 mouse line
The targeting vector was electroporated into CJ7 ES cells (gift from Dr. T. Gridley, Jackson Laboratory, Bar Harbor) (Swiatek and Gridley, 1993). A total of 920 neomycin resistant ES cell colonies was picked and screened for homologous recombination by PCR. The primer set consisted of one primer 1-S (ATGCGGTGGCTCTATGGCTTCT), within the neomycin gene, and another primer 1-AS (GGTTGGGGGGTGGGGTGTATTC), outside the region that was used to construct the KO vector (Fig. 1C). PCR with these primers produced a 3.9 kb amplification product that identified the recombinant allele. To determine whether correct recombination occurred at the 5′ end of the targeted gene, that region was PCR amplified using primer 2-S (CCTAAACCTGGGCTGCCTTCCTGTGATGAT), located outside the region used in constructing the KO vector, and primer 2-AS (ATTTGGTTGTTGTTTTGGTCCTTGAGTGTGATTTG), located within the KO vector. To distinguish between the wild-type and targeted alleles, the PCR product was digested with NotI to produce a 3.4 kb wild-type and 2.9 kb targeted fragments.
The screen identified 3 correctly targeted ES cell lines. One ES cell line was injected into blastocysts (Tulane Transgenic Mouse Facility). One chimeric mouse with germ line transmission was used to establish the conditional mouse line. To recombine out the neomycin gene located within the intron of Hand2, the conditional mouse line was crossed with the mouse line FLP1/FLP1 (129S4/SvJaeSor-Gt(ROSA)26Sortm1(FLP1)Dym/J, the Jackson Laboratory). Recombination between the FLP sites was identified by PCR, using primer 3-S (TGACCGCCAACCCAACTTT) and primer 3-AS (TGCCCTATCCAGAACTCCATTACT) (Fig. 1D).
Generation of embryos in which Hand2 is ablated in crest-derived cells
Wnt1-Cre mice (Danielian et al., 1998) were crossed with Hand2+/− animals (Srivastava et al., 1997) to obtain Hand2+/−; Wnt1-Cre/+ males. These mice were crossed with Hand2loxP/loxP or Hand2loxP/loxP; R26R (Gt(ROSA)26Sortm1Sho) (Mao et al., 1999) females. All mouse lines were periodically crossed with Black Swiss mice (Taconic).
In situ hybridization
In situ hybridization of whole mount mouse embryos was carried out with digoxygenin labeled probes, which were synthesized with digoxigenin labeling mix (Roche Diagnostics) and T7 or T3 polymerase (Roche Diagnostics). Plasmids used to generate these probes were pBSK-Hand1 (Cserjesi et al., 1995), pBSK-Hand2 (Dai et al., 2004), Mash1 (Guillemot and Joyner, 1993), and cRet9-pBKSII (provided by F. Costantini, Columbia University, New York). Gata2, Gata3, Phox2a, and peripherin probes were obtained from a mouse embryonic cDNA (Ambion) by PCR amplification and cloning of the PCR products into the transcription vector pCRII (Invitrogen).
Embryos were collected at 11.5 dpc, fixed overnight with 4% formaldehyde (from paraformaldehyde) and dehydrated in 100% ethanol for storage. Prior to hybridization, embryos were re-hydrated, treated with 10 μg/ml protenase K (Sigma) for 15 min, and pre-hybridized for 1 hr. Probes were added at a concentration of 0.5 μg/ml then embryos were hybridized at 70° C overnight. After extensive washing, digoxigenin was detected with antibodies to digoxygenin conjugated with alkaline phosphatase (Roche Diagnostics). Color development was with nitro blue tetrazolium (NBT) and 5-Bromo-4-Chloro-3′-Indolyphosphate p-Toluidine (BCIP) (Roche Diagnostics). After documentation, embryos were embedded in paraffin for sectioning.
Immunocytochemistry
Cryosections were washed with PBST and preincubated with 10% serum for 1 hr and incubated at 4°C overnight with primary antibodies. Antibodies used in this study were: a rabbit monoclonal (Covance) against β3-Tubulin (TuJ1; diluted 1:1000); a biotinylated mouse monoclonal against HuC/D (Invitrogen-Molecular Probes; diluted 1:10); a sheep polyclonal against TH (Chemicon; diluted 1:500); a rabbit polyclonal against TH (Chemico; diluted 1:500); a rabbit polyclonal against Phox2b (provided by J.F. Brunet, CNRS, École Normale Supérieure, Paris; diluted 1:1000). Secondary antibodies used for detection were labeled with Alexa 488 or Alexa 596. Biotinylated antibodies were detected with streptavidin-HRP and HRP activity was visualized with 3, 3′-diaminobenzindine (DAB; Vector labs). DNA was stained with bisbenzimide for cell density determination.
Quantification
Ten micrometer cryostat cross sections were collected on Superfrost plus slides (Fisher). For each genotype 3 embryos were analyzed. TH- or DBH-immunocytochemistry was co-localized with Tuj1-immunocytochemistry. Five sections for each co-immunolabeling were selected per embryo so that the entire rostro-caudal sympathetic chain was represented. A 10x field containing the sympathetic ganglia from each section was photograph using a cooled CCD camera (Retiga;Q Imaging Inc) installed on a Leica DMRXA2 microscope. The density of labeled cells was determined by computer-assisted morphometry (Openlab software; Improvision Inc., Lexington, MA). Density slicing tool was used to create a binary image of each channel with intensity above the threshold intensity. The threshold was set individually for each channel so that the binary image included all pixels with an intensity above the background of the surrounding tissue. Density areas were measured excluding all objects with a size inferior or equal to 1 pixel. For each field TH and DBH density areas were expressed as a percentage of Tuj1 density area.
RESULTS
Analysis of the Hand2 conditional knockout mouse line
When the mouse Hand2 gene is deleted, embryos die prior to formation of the SNS (Srivastava et al., 1997). To enable studies into the role Hand2 plays during SNS development, a conditional Hand2 knockout line was therefore generated to circumvent the early lethality. The targeting strategy is presented in Fig. 1. The targeting vector consists of two loxP sites with one located upstream of the gene and one within the intron and the neomycin cassette, which is flanked by Frt sites and placed within the intron of the gene (Fig. 1B). One mouse line carrying the targeted allele of Hand2 was crossed with the Flp recombinase expressing mouse line, FLP1, to excise the neomycin gene leaving a single Frt site flanked by a loxP site (Hand2loxP/+) (Fig. 1D). The targeted allele was analyzed by PCR for correct integration and excision of the neomycin gene (Fig. 1E).
To begin the characterization of the targeted allele of Hand2, Hand2loxP/+ mice were back-crossed and their offspring were examined for viability. Homozygous targeted mice (Hand2loxP/loxP) were born at the expected ratio of 1:3. The adult animals did not have discernable physical or behavioral abnormalities, and when bred, produced litters of normal size.
The targeted allele of Hand2 was further characterized by examining how it functions when it is the only active allele. Since Hand2−/+ mice have no discernable phenotype (Srivastava et al., 1997), Hand2loxP/− animals would be expected to be phenotypically normal if the conditional allele is expressed at wild-type levels. Hand2loxP/+ and Hand2 null heterozygote (Hand2−/+) mice were crossed to generate Hand2loxP/− animals. All Hand2loxP/− pups were unable to feed and died within a day because of a cleft palate (Fig. 2A, B). The primary palate formed in Hand2loxP/− mice but growth was incomplete and the primary palate failed to fuse with the secondary palate. The secondary palate contained a wide cleft in the anterior and mid-section revealing the bones of the nasal cavity.
Fig. 2. Development of a cleft palate in Hand2loxP/− mice.
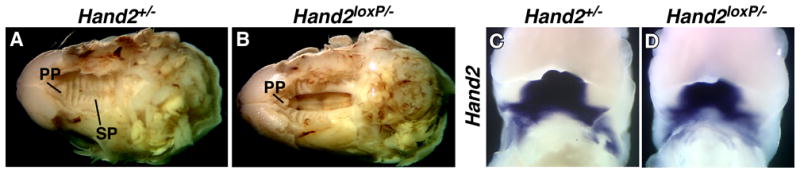
A–B: The skin and lower jaws from the heads of Hand2+/− (A) and Hand2loxP/− (B) newborn pups were removed to reveal the palate. A single copy of the wild-type Hand2 gene does not produce a cleft palate; however, a single copy of the conditional targeted allele produces a severe cleft palate defect. PP; primary palate, SP; secondary palate. C–D: Whole mount in situ hybridization to detect transcripts encoding Hand2 in Hand2+/− (C) and Hand2loxP/− (D) 12.5 dpc embryos.
Hand2 is expressed in crest-derived cells within branchial arches during development (Srivastava et al., 1995) and mice lacking the Hand2 branchial arch enhancer have craniofacial defects including a cleft palate (Yanagisawa et al., 2003). The presence of a cleft palate suggests that expression of Hand2 from the conditional allele is affected. To determine whether there are changes in the pattern of Hand2 expression from the conditional allele during development, we examined the expression of Hand2 in Hand2loxP/− embryos by whole mount in situ hybridization. In the jaw, the expression level and the region of expression are reduced from the floxed Hand2 allele (Fig. 2C, D). The distribution of transcripts encoding Hand2 in other regions of the Hand2loxP/− embryo, including the heart and limbs, appeared to be normal and development of these structures was unaffected (data not shown).
In situ hybridization was employed to investigate Hand2 expression in the developing SNS of Hand2loxP/− mice. No abnormalities were discerned in the expression of Hand2 driven by the conditional allele in sympathetic ganglia. The expression of SNS neuronal markers tyrosine hydroxylase (TH) and peripherin were also indistinguishable from wild-type in Hand2loxP/− embryos (data not shown). Because neither Hand2loxP/loxP nor Hand2−/+ mice exhibit an abnormal phenotype, and the expression pattern of Hand2 in Hand2loxP/− embryos is similar to wild-type, the appearance of a cleft palate in Hand2loxP/− mice suggests that the level of Hand2 expression from the conditional allele is slightly reduced. These results are also consistent with the idea that the development of the palate is more sensitive than other Hand2-expressing tissues to the level of Hand2 protein.
Expression of Hand2 by crest-derived cells is required for embryonic viability
To address the role of Hand2 in SNS formation, the Hand2 gene was deleted in NC by crossing Hand2loxP/loxP with the Wnt1-Cre mouse line (Danielian et al., 1998). The efficiency of Hand2 recombination was determined by examining the level of Hand2 expression using whole mount in situ hybridization (Fig. 3A, B). Hand2 expression was undetectable in crest-derived cells in Hand2loxP/−;Wnt1-Cre/+ embryos including the outflow tract of the heart, autonomic ganglia, and branchial arches. In contrast, in Hand2 expressing non-crest derived tissues such as the limbs, right ventricle, and epicardium, Hand2 continues to be expressed. The reduction of Hand2 expression in the hind limb of Hand2loxP/−;Wnt1-Cre/+ embryos may be due to developmental delay resulting from loss of Hand2 in NC.
Fig. 3. Neural crest-specific deletion of Hand2 results in vascular defects.
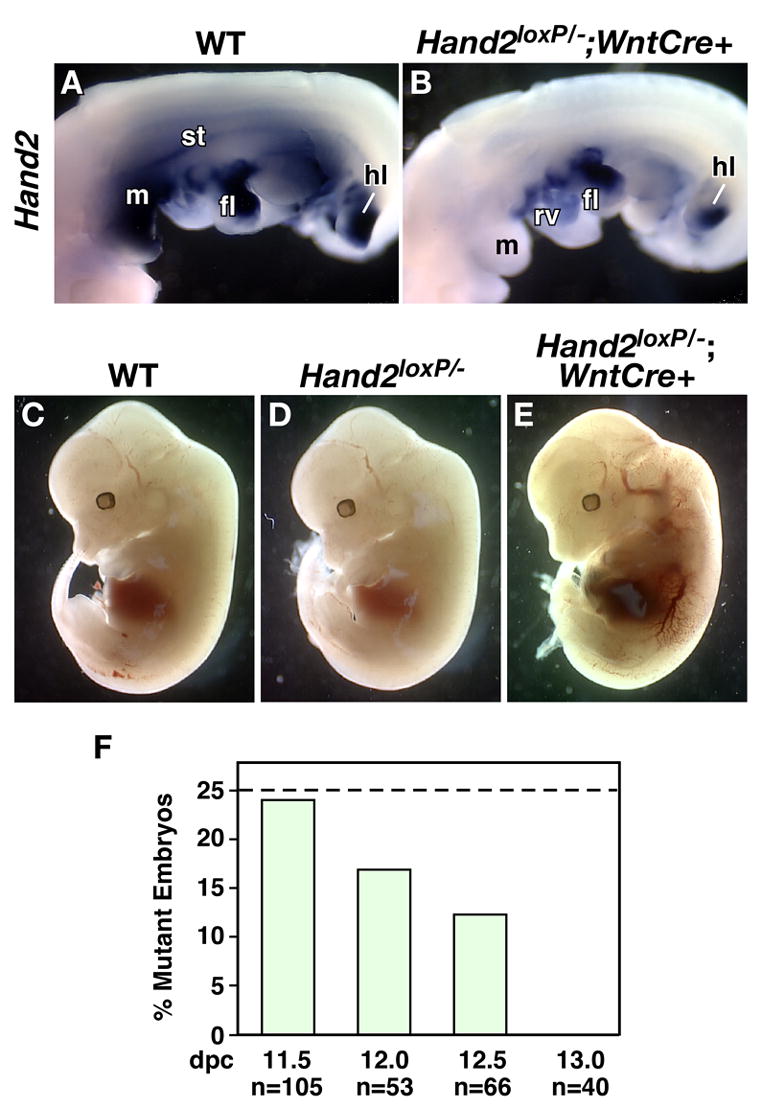
Wnt1-Cre was used to delete Hand2 in crest-derived cells. A–B: The extent of Hand2 recombination was assessed by whole mount in situ hybridization. To visualize Hand2 expression in the heart, the epicardium was removed. fl: forelimb, h: hindlimb, m: mandible, rv: right ventricle, st: sympathetic trunk. In wild-type embryos (A), Hand2 is expressed in a number of crest- and non-crest-derived cells while Hand2 was not detected in crest-derived cells of Hand2loxP/−; Wnt1-Cre/+ embryos (B) but is expressed in tissues without NC contribution. C–E: Deletion of Hand2 mediated by Wnt1-Cre in the NC lineage results in lethality at 12.5 dpc. When compared with Hand2+/+ (C) and Hand2loxP/− (D), Hand2loxP/−; Wnt1-Cre/+ embryos (E) show extensive hemorrhaging of the vasculature throughout the embryo. F: Survival rate of Hand2loxP/−; Wnt1-Cre/+ embryos during development.
An examination of Hand2loxP/−; Wnt1-Cre/+ embryos showed that circulatory defects developed by 12.0 dpc (Fig. 3C–E). Blood pooled in the major vessels, heart, liver and celom (Fig. 3E). By comparison, Hand2loxP/− embryos did not exhibit cardiovascular defects during development (Fig. 3D) or as newborns (data not shown). Constitutive loss of Hand2 expression results in embryonic lethality at approximately 10 dpc due to cardiovascular defects with severe degeneration of the right ventricle (Srivastava et al., 1997). Degeneration of the right ventricle does not occur in Hand2loxP/−; Wnt1-Cre/+ embryos which may account for their survival to 12.0 dpc. In addition to the requirement for Hand2 in maintenance of the right ventricle, Hand2 is expressed in the cardiac crest (Srivastava et al., 1995). The cardiac crest is essential for the patterning and development of the outflow tract and valves. The vascular defects resulting from Hand2 loss in NC suggests it plays an important role cardiac crest development.
To determine when these embryos begin to die, a survival analysis was undertaken (Fig. 3F). Live Hand2loxP/−; Wnt1-Cre/+ embryos were recovered at the expected ratios until 11.5 dpc. By 12 dpc, the ratio of live Hand2loxP/−; Wnt1-Cre/+ embryos began to decrease and no live embryos were recovered by 13 dpc.
Hand2 is not required for NC colonization of the SNS
Hand2 is not expressed in the migrating crest-derived precursors of the SNS but is expressed soon after they arrive at sites of ganglion formation. To determine whether Hand2 is required for the formation and maintenance of the SNS, we used in situ hybridization to examine how the NC-specific loss of Hand2 affects the expression of markers of migratory and early post-migratory crest-derived cells. Expression of Hand2 was not detected in the SNS of Hand2loxP/−; Wnt1-Cre/+ embryos, indicating that recombination of the targeted allele had occurred with high efficiency (Fig. 4A, B). Sympathetic neuronal precursors express the tyrosine kinase receptor gene c-ret and the neurotrophin receptor p75NTR at the time crest-derived cells begin to migrate away from the neural tube. In situ analysis of c-ret expression (Fig. 4C, D) and immunocytochemical analysis of p75NTR (data not shown) was undertaken in wild-type and Hand2loxP/−; Wnt1-Cre/+ embryos. Levels of c-ret and p75NTR expression comparable to those of wild-type embryos were observed at the dorsolateral aspect of the dorsal aorta, which is the site of SNS ganglion formation. The loss of Hand2 does not appear to affect the initial formation, migration, or early survival of SNS precursors.
Fig. 4. Hand2 is not required for colonization of sites of sympathetic ganglion formation.
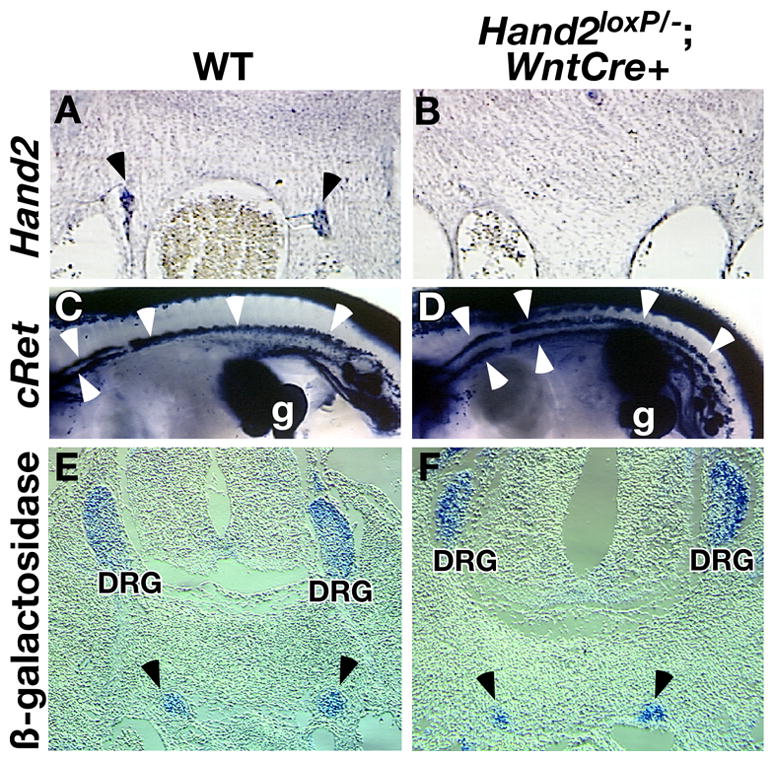
The level of Hand2 transcript was examined in wild-type (A) and Hand2loxP/−; Wnt1-Cre/+ (B) embryos by in situ hybridization. Hand2 expression (arrowheads) is undetectable in mutant embryos. C–D: Precursors of the SNS were identified by in situ hybridization with c-ret No difference in c-ret expression was detected between wild-type (C) and Hand2loxP/−; Wnt1-Cre/+ (D) embryos. E-F: The size of sympathetic paravertebral ganglia was analyzed by marking all sympathetic neurons genetically by Wnt1-Cre and R26R genes. R26R line was crossed into Wnt1-Cre/+ (E) and Hand2loxP/−; Wnt1-Cre/+ (F) lines, resulting in β-galactosidase activation in all NC cells and their descendents. The number of sympathetic neurons was not affected by the loss of Hand2. Arrowheads; sympathetic trunk. DRG: dorsal root ganglia.
Sympathetic neuronal precursors continue to proliferate after their migration (Rothman et al., 1978) with the ultimate size of the forming SNS is regulated by proliferation of precursors. To determine whether Hand2 plays a role in the proliferation of sympathetic neuronal progenitors, the size of the developing SNS was examined by genetically marking all progenitor NC cells with β-galactosidase. Mice carrying the R26R allele (Mao et al., 1999) were crossed with the Hand2loxP/loxP line and offspring crossed with Hand2+/−; Wnt1-Cre/+ to generate embryos in which Hand2 is deleted and β-galactosidase is activated in crest-derived cells. Cross sectional analysis shows that Hand2 is not required for sympathetic trunk development but a discrete boundary between the ganglia and surrounding mesenchyme does not form (Fig. 4E, F). To determine if loss of Hand2 effects the size of the SNS, the area occupied by β-galactosidase expressing cells in the cervical region of mutant and wild-type embryos was quantified using ImageJ. The mean area of the sympathetic ganglia of Hand2+/+ embryos was 182.6 (+/−33.7) μm2 and Hand2loxP/−; Wnt1-Cre/+ embryos were 165.2 (+/− 32.6) μm2. The loss of Hand2 does not affect the size of the developing SNS.
Loss of Hand2 does not hinder SNS neuronal differentiation
The ability of Hand2 to activate the neuronal differentiation program in NC cultures (Howard et al., 1999; Howard and Cserjesi, 1996) and in P19 embryonal carcinoma cells (Morikawa et al., 2005) suggests that it can behave as a proneural gene; nevertheless, the specification of sympathetic neurons prior to their expression of Hand2 argues against such a role in SNS development. To address the question of whether Hand2 functions in the determination and/or differentiation of sympathetic neurons, we investigated the ability of the crest-derived precursors to differentiate into neurons in the sympathetic ganglion promordia of Hand2loxP/−; Wnt1-Cre/+ embryos (Fig. 5). We analyzed the expression of the early pan-neuronal marker TuJ1 in Hand2loxP/−; Wnt1-Cre/+ animals to determine whether neurons can develop at sites of SNS ganglion formation. The number of cells expressing TuJ1 was comparable in wild-type and mutant embryos (Fig. 5A, B). Because neuronal differentiation can occur in stages, the expression of the late expressing pan-neuronal marker, Hu was also examined. Like those of cells expressing TuJ1, the numbers of cells expressing Hu throughout the SNS of Hand2loxP/−; Wnt1-Cre/+ embryos was found to be comparable to those observed in their wild-type littermates (Fig. 5C, D). When Hand2 is expressed in P19 embryonal carcinoma cells, many of the cells that form neurons also express the neuronal intermediate filament protein, peripherin, which is found predominantly in peripheral neurons, including those of the SNS (Morikawa et al., 2005). Expression of the peripherin gene was examined by in situ hybridization at 11.5 dpc embryos (Fig. 5E, F). The level of peripherin transcripts was found to be the same in wild-type (Fig. 5E) and mutant (Fig. 5F) animals. The observed expression of pan-neuronal genes suggests that Hand2 is not essential for generic neuronal differentiation.
Fig. 5. Hand2 does not regulate sympathetic neuron development.
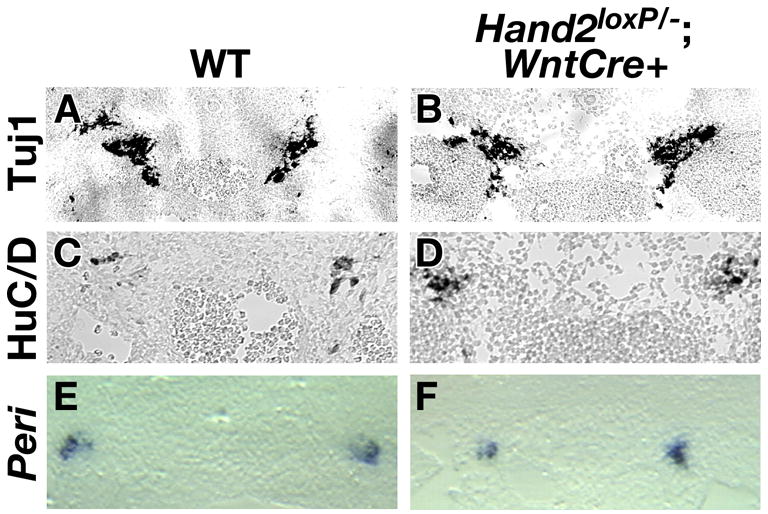
The development of neurons in the SNS was determined by immunocytochemical detection of the pan-neuronal proteins β3-tubulin (TuJ1) (A, B) and Hu (C, D) and by in situ hybridization analysis of the peripheral neuronal gene, peripherin (E, F). The level of neuronal marker expression was the same in wild-type (A, C, E) as in Hand2loxP/−; Wnt1-Cre (B, D, F) embryos. peri; peripherin.
Hand2 regulates catecholaminergic differentiation
Ectopic expression of Hand2 activates the noradrenergic biosynthetic program in vitro (Howard et al., 1999; Xu et al., 2003) and in vivo (Howard et al., 2000; Lucas et al., 2006), which is consistent with the hypothesis that Hand2 determines whether crest-derived precursors acquire a noradrenergic phenotype. The biosynthetic pathway for the synthesis of norepinephrine includes the enzymes tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DBH). Sympathetic neuroblasts start to express TH and DBH at approximately 10.5 dpc with expression continuing throughout development and in the majority of adult sympathetic neurons (Groves et al., 1995; Howard et al., 2000; Tsarovina et al., 2004). Analysis of the regulatory region of the Dbh gene has shown that Hand2 binds to (Rychlik et al., 2005) and regulates the transcriptional activity of Dbh (Rychlik et al., 2003; Xu et al., 2003), suggesting that Hand2 directly regulates norepinephrine biosynthesis.
To determine whether Hand2 is essential for development of the noradrenergic phenotype, the expression of TH and DBH in the SNS was examined (Fig. 6). In wild-type mice, TH was expressed in most neurons of the SNS by 12.0 dpc (Fig. 6A) while in Hand2loxP/−; Wnt1-Cre/+ embryos, the number of cells expressing TH was reduced dramatically (Fig. 6B). Immunocytochemical analysis of DBH expression showed that it is also greatly reduced in the absence of Hand2 (Fig. 6C, D). The differences were quantified by determining the densities of TH- and DBH-immunoreactive relative to the density of the pan-neuronal marker, Tuj1 (Fig. 6E). In mutant embryos, TH expression was less than 10% of that of wild-type embryos (p<0.0001) and DBH is expressed less than 15% found in the SNS of wild-type embryos (p<0.0071). These results demonstrate that catecholaminergic differentiation of the SNS is dependent on Hand2.
Fig. 6. Hand2 regulates TH and DBH in the SNS.
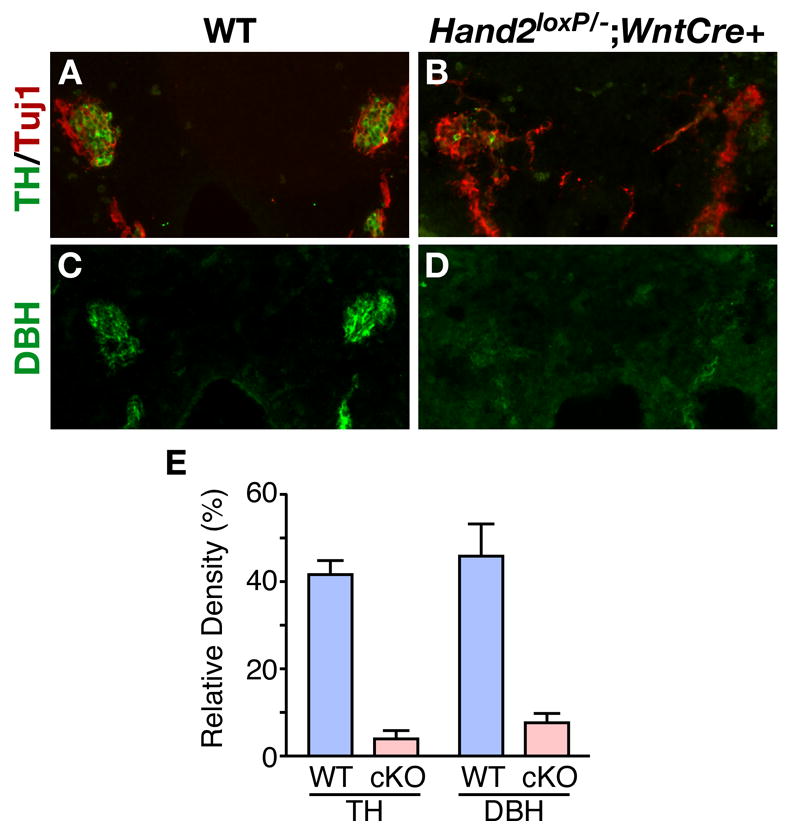
Noradrenergic differentiation was investigated by immunocytochemical analysis of the expression of the catecholamine biosynthetic enzymes, TH and DBH. The immunoreactivities of TH (A, B) and DBH (C, D) were analyzed in sections of the abdominal region of 12.5 dpc embryos in wild-type (A, C) and Hand2loxP/−; Wnt1-Cre (B, D) embryos. E: The level TH and DBH was quantified relative the level of expression of the pan-neuronal marker TuJ1 by density determination of immunoreactivity. The level of TH expression was reduced 10 fold and DBH by 8 fold in mutant sympathetic neurons.
Hand2 regulation of TH and DBH expression is independent of Gata2/3, Phox2a/b and Mash1
Members of the Gata family of zinc finger-containing transcription factors regulate the expression of TH (Lim et al., 2000; Moriguchi et al., 2006; Tsarovina et al., 2004). The loss of Gata3 in mice leads to a dramatic decrease in catecholamine production, which in turn leads to embryonic lethality. In vitro, Gata3 has been shown to directly bind to the Th promoter and regulate its transcription, suggesting that Gata3 plays a direct role in catecholamine biosynthesis (Hong et al., 2006). The role of Gata2 in mammalian sympathetic neuronal development remains unknown because of the early death of Gata2 null embryos (Tsai et al., 1994). To determine whether the loss of TH and DBH expression in the Hand2 null SNS is mediated by the regulation of the genes encoding Gata factors, expression of Gata factors was investigated by in situ hybridization (Fig. 7A–D). Loss of Hand2 in sympathetic neurons was found not to affect the expression of Gata2 (Fig. 7A, B) or Gata3 (Fig. 7C–D).
Fig. 7. Expression of Gata, Phox2, and bHLH transcription factors in the SNS of Hand2 mutant embryos.
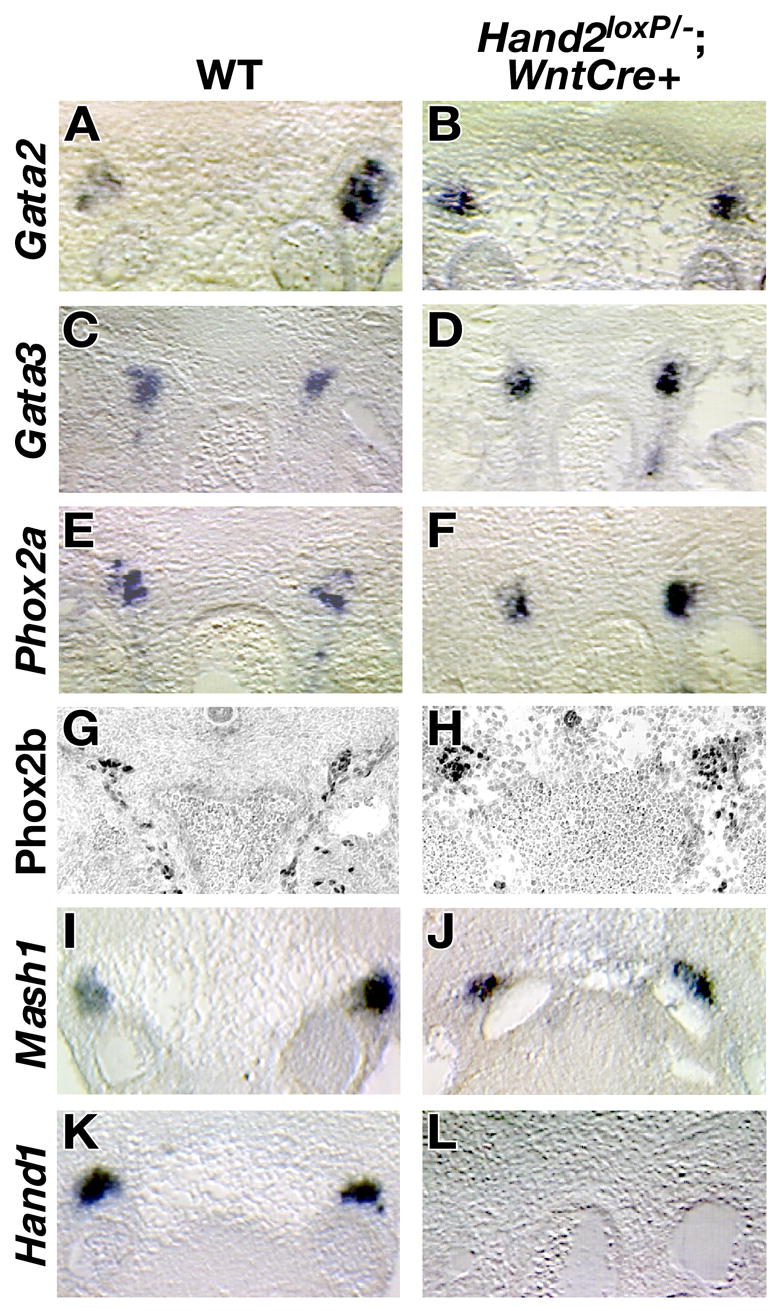
The expression of Gata2 (A, B), Gata3 (C, D), Phox2a (E, F), Mash1 (I, J), and Hand1 (K, L) was examined in wild-type (A, C, E, G, I, K) and Hand2loxP/−; Wnt1-Cre (B, D, F, H, J, L) 11.5 dpc animals by in situ hybridization while Phox2b (G, H) expression was monitored by immunocytochemical analysis. All transcription factors, with the exception of Hand1, were expressed in the mutant SNS. Hand1 expression could not be detected in the SNS of Hand2loxP/−; Wnt1-Cre mice.
The homeodomain transcription factor Phox2b is essential for survival of sympathetic neural precursors, catecholaminergic differentiation, and expression of Hand2 (Goridis and Rohrer, 2002; Pattyn et al., 1999). In contrast, Phox2a is not required for sympathetic neuronal development but can regulate expression of TH and DBH (Lo et al., 1999; Stanke et al., 1999). Ectopic expression of Hand2 can activate the Phox2 genes (Howard et al., 1999) suggesting that Hand2 might contribute to the maintenance of Phox2 gene expression. In situ analysis showed that expression of Phox2a (Fig. 7E, F) or Phox2b (Fig. 7G, H) was unaffected by the loss of Hand2. Our data suggest that Hand2 does not regulate the expression of noradrenergic biosynthetic enzymes through the activation of either the Gata or the Phox2 transcription factors.
The bHLH transcription factor Mash1 is expressed in sympathetic neuronal precursors and regulates their development (Guillemot et al., 1993; Sommer et al., 1995). We have previously shown that Mash1 is not required for Hand2 expression but ectopic expression of Hand2 can activate Mash1 expression (Morikawa et al., 2005). These observations are consistent with the possibility that Mash1 is a downstream target of Hand2. To directly address the role of Hand2 in Mash1 gene regulation, the expression of Mash1 in sympathetic neurons of Hand2 null embryos was examined by in situ hybridization (Fig. 7I, J). The expression of Mash1 in wild-type (Fig. 7I) and mutant embryos (Fig. 7J) was found to be comparable. Hand2, therefore, does not regulate Mash1. Taken together, our results suggest that Hand2 is an essential gene for catecholaminergic determination of sympathetic neurons.
Hand2 regulates sympathetic neuronal expression of Hand1
The Hand2-related bHLH factor, Hand1, is unique in that its neuronal expression is restricted to the SNS, even though it is also expressed in a number of non-neuronal tissues. Initial Hand1 expression follows that of Hand2 and the Phox2 factors and Hand1 is down-regulated in late SNS development (Cserjesi et al., 1995; Morikawa and Cserjesi, 2004). The function of Hand1 in development of sympathetic neurons has not been determined because embryos that lack Hand1 die before sympathetic neurons arise (Firulli et al., 2003; Morikawa and Cserjesi, 2004). To determine whether Hand2 regulates expression of Hand1 in the SNS, in situ hybridization was used to compare the level of Hand1 transcripts in wild-type embryos (Fig. 7K) and in Hand2 mutant embryos (Fig. 7L). In the absence of Hand2, no Hand1 expression could be detected in the SNS. Although the function of Hand1 in SNS development is still undetermined, Hand1 is the only transcription factor that known to be a target of Hand2 in the mammalian SNS.
DISCUSSION
Studies of the expression pattern of Hand2 and its ectopic expression have suggested that Hand2 is an important member of the transcriptional cascade that regulates sympathetic neuronal development. Following its ectopic expression, Hand2 can induce competent cells to develop as neurons (Howard et al., 1999; Howard and Cserjesi, 1996; Morikawa et al., 2005) and it activates and maintains a noradrenergic phenotype in sympathetic neurons of avians and fish (Howard et al., 2000; Lucas et al., 2006). Until now, assignment of a definitive role for Hand2 function during mammalian SNS development has not been possible because knockout animals that lack Hand2 die before the onset of SNS formation. We have circumvented the early lethality associated with the complete knockout of Hand2 by generating a conditional knockout mouse line in which the Hand2 gene was specifically excised in cells derived from the NC. These mice survived long enough to reach the stage of sympathetic ganglion formation, which enabled us to study the function of Hand2 development of sympathetic neurons. Our observations reveal that Hand2 is not essential for the formation of neurons in presumptive sympathetic ganglia but is essential in for these neurons to express the noradrenergic phenotype that characterizes most of the postganglionic neurons of the SNS.
Conditional targeting of the Hand2 gene
Our targeting strategy placed loxP sites upstream of the Hand2 gene and within its intron. A cleft palate was found to be present in all Hand2loxP/− embryos, suggesting that the insertion of these loxP sites interferes with Hand2 gene expression; nevertheless, animals survived to birth, indicating that the severe developmental defects and embryonic lethality associated with the complete loss of Hand2 function did not occur. In situ analysis of Hand2 expression in Hand2loxP/− embryos shows that the introduction of loxP sites into the gene changed the level and distribution of transcripts in the jaw relative to the wild-type allele. Hand2 expression is reduced in the lateral region of the first branchial arch. This loss of expression is similar to, but less severe than found in embryos lacking the branchial arch enhancer located 7 kb upstream (Yanagisawa et al., 2003). Loss of the enhancer leads to a cleft palate similar to what we observed but in addition, embryos exhibit jaw defect. The change in Hand2 expression due to the insertion of loxP site did not produce an overt morphological defect in the jaws of Hand2loxP/− embryos or pups. Limbs, which are severely affected by the deletion of Hand2 (McFadden et al., 2002), developed normally. An immunocytochemical analysis of the early Hand2loxP/− SNS showed that it differentiates normally, suggesting that Hand2 expression in the SNS of Hand2loxP/− mice is sufficient to support normal development. Although a thorough examination of the cardiovascular system of Hand2loxP/− animals was not undertaken, the ability of Hand2loxP/− mice to survive to birth suggests that severe cardiovascular developmental defects do not arise. These observations suggest that targeting the Hand2 gene resulted in the generation of a hypomorphic allele and a moderate decrease in Hand2 protein expression. A high level of Hand2 expression occurs in the developing face, which may reflect a higher than normal need for the protein in this region and an exquisite sensitivity of palate development to even a small decrease in Hand2 expression.
The conditional knockout of Hand2 in crest-derived cells leads to developmental abnormalities in the cardiovascular system resulting in embryonic death at mid-gestation. The most prominent defect is in the vasculature. At 12.5 dpc, embryos hemorrhage and blood pools in a number of regions including the heart, liver and peripheral vasculature. The cardiac crest contributes smooth muscle cells to the great vessels of the heart including the ascending and transverse aorta and the pulmonary trunk, but not to more distal peripheral vessels (Jiang et al., 2000). Since the atria and ventricles of Hand2loxP/−; Wnt1-Cre/+ embryos appear grossly normal, the lethality observed in mutant embryos is probably due to defects in the great vessels that lead, in turn, to poor blood flow and secondary defects in the peripheral vascular system.
Role of Hand2 in noradrenergic expression
The development of the SNS starts soon after crest-derived cells reach the dorsal aorta. Pleuripotent crest-derived cells receive an inductive signal from the dorsal aorta that specifies them to become sympathetic precursors. In situ analysis of Hand2 expression shows that Hand2 is not expressed until after crest-derived cells have begun to express the neuronal lineage marker, Phox2b and have started to differentiate (Morikawa et al., 2005). The timing of Hand2 expression thus suggests that Hand2 does not specify crest-derived cells to a neuronal fate, although it might play a role in determining what type of neurons ultimately differentiates. When Hand2 is ectopically expressed, however, it does have the ability to activate a neuronal program of differentiation in competent cells. In NC cultures (Howard et al., 1999; Howard and Cserjesi, 1996) and in P19 embryonal carcinoma cells, (Morikawa et al., 2005), Hand2 is able to promote neuronal differentiation. Although the Hand2 protein can exhibit proneural activity, the current observations show that its expression is not essential for neurons to develop in the SNS. Neurons do appear, despite the conditional knockout of Hand2.
The present study, which is consistent with prior reports (Howard et al., 1999; Howard and Cserjesi, 1996; Lucas et al., 2006; Morikawa et al., 2005), shows that Hand2 expression is required for development of noradrenergic neurons. Noradrenergic determination requires the activation of genetic pathway that leads to the expression of the norepinephrine biosynthetic pathway, which includes the genes encoding the enzymes, TH and DBH. Transcription factors that are required for neurons to be noradrenergic have been shown to interact to activate TH and DBH genes. Hand2, for example, can bind to the Dbh promoter in vitro and synergistically regulate its expression with Phox2a (Rychlik et al., 2003; Xu et al., 2003). ChIP analysis has shown that Hand2 and Phox2a also bind to the promoter region of Dbh in vivo (Rychlik et al., 2005). These studies are consistent with the idea that Hand2 is a part of transcriptional complex that directly activates the expression of Dbh.
Previous studies of limb development (McFadden et al., 2002), and Dbh expression (Rychlik et al., 2003) show that Hand2 can function independently of direct DNA binding. The ability of Hand2 to function independent of DNA binding suggests that at least some of its actions require the participation of transcriptional co-factors. Hand2 interacts with Phox2a at the Dbh promoter and this interaction enhances the transcriptional activity of Phox2a (Rychlik et al., 2003; Xu et al., 2003) suggesting that Phox2a may be a co-factor of Hand2. It is also possible that Hand2 interacts with Gata factors and this interaction with Hand2 may be essential for Gata regulation of Th and Dbh expression in noradrenergic neurons. In support of this idea, Hand2 and Gata factors are known to interact physically to regulate expression of heart-specific genes (Dai et al., 2002). The complex of Hand2 with Gata4 also recruits the histone acetyltransferase p300/CBP. That Hand2 interacts with p300/CBP has also been demonstrated by the ability of Hand2 to synergize with p300/CBP to regulate Mash1 expression, although it is not known whether this interaction includes the participation Gata factors (Morikawa et al., 2005). However suggestive they may be, observations made to date do not rule out the possibility that an essential role for Hand2 in Th and Dbh gene regulation is to activate an unidentified transcription factor that is essential for their expression.
The recent analysis of SNS development in zebrafish suggests that in fish, as in mice, development of noradrenergic sympathetic neurons is severely compromised when the Hand2 orthologue, hands-off, is mutated and non-functional (Lucas et al., 2006). Phox2a, phox2b and zasha/ascl1 expression are not affected by the loss of hands-off, however, there is a marked reduction in gata2 expression in zebrafish. The observed difference between Hand2-deficient mice and zebrafish in the level of Gata2 expression may be explained by the different SNS developmental stages that were examined in the two species. The cervical sympathetic trunk was investigated in zebrafish, while pre- and paravertebral ganglia were investigated in mice. Unlike mice, where the SNS develops rapidly along the length of the embryos after the initial arrival of crest-derived precursors at the cervical-thoracic junction, the zebrafish cervical sympathetic trunk develops several days prior to formation of the remainder of the SNS (An et al., 2002). It is possible that Hand2 regulates the expression of Gata2 differently in the early developing cervical sympathetic neurons in zebrafish than in the later-developing sympathetic ganglia analyzed in the present study. Another possibility is that there are differences between fish and mammals in the underlying genetic mechanisms regulating noradrenergic determination. This latter possibility is supported by a divergence in the role of mouse Ap2α and of the zebrafish homologue tfapa. In mice, deletion of Ap2α does not affect development of the SNS (Brewer et al., 2004), while in the zebrafish tfapa mutant, mont blanc, noradrenergic development in the SNS is disrupted (Holzschuh et al., 2003).
The regulatory cascade in SNS development
During the past few years, a number of different transcriptional regulatory circuits have been proposed to explain sympathetic neural development. The role of Hand2 in the developing mammalian SNS has remained enigmatic because of the lack of a mutant mouse line to employ in loss-of-function studies. Here we have shown that Hand2 regulates noradrenergic differentiation of sympathetic neurons and we propose a model to explain its position in the transcriptional regulatory cascade that controls sympathetic neuronal development (Fig. 8). The earliest detectable sympathetic precursors express Phox2b. If Phox2b is absent, sympathetic neuronal precursors develop and express Mash1, but then are rapidly lost through apoptosis in (Pattyn et al., 1999). The lack of dependence of Mash1 expression on Phox2b is consistent with the possibility that the signals, such as BMPs, which induce crest-derived precursors to become sympathetic neurons, directly active Mash1 expression. In mouse embryos that lack Mash1, most sympathetic precursors are lost during development (Guillemot et al., 1993; Sommer et al., 1995). The small numbers of sympathetic precursors that remain differentiate into neurons but their development is delayed (Morikawa et al., 2005; Pattyn et al., 2006), suggesting that Mash1 plays a dual role in the SNS; it supports the survival of neuronal precursor cells and accelerates their differentiation into neurons. All other members of the regulatory cascade, Hand2 (Goridis and Rohrer, 2002), Gata2 and Gata3 (Tsarovina et al., 2004) are dependent on expression of Phox2b.
Fig. 8. Model for the transcriptional cascade that regulates mammalian SNS development.
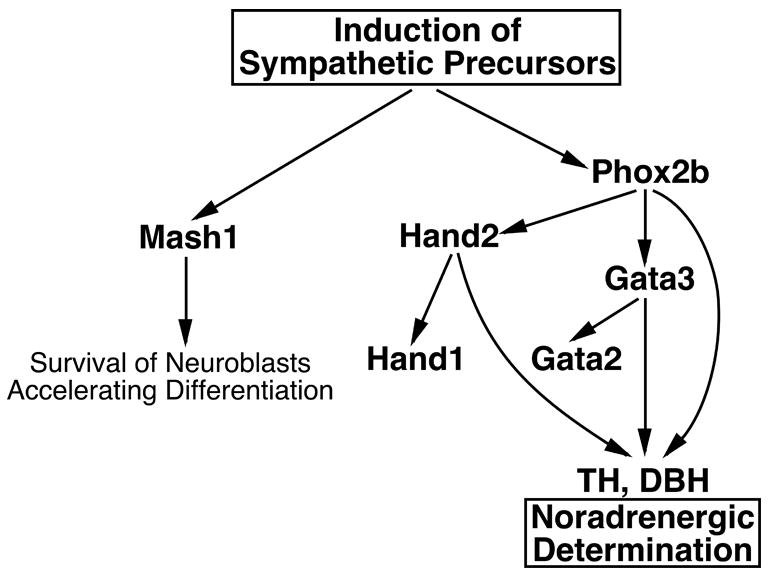
Crest-derived precursors are induced to give rise to the progenitors of the SNS. This induction results in the independent activation of Phox2b and Mash1. The function of Mash1 remains unclear but it appears to promote the survival of neuronal progenitors and possibly also, their differentiation. Phox2b expression is required for Gata2, Gata3, Hand2, and the genes encoding TH and DBH to be expressed. The regulation of Th and Dbh requires the interaction of Phox2b, Hand2 and Gata3 and the loss of these factors leads to the absence of, or a substantial reduction in the number of neurons expressing a noradrenergic phenotype. Hand2 is essential for Hand1 expression with the role of Hand1 remaining unknown.
Loss of Phox2b leads to the loss of the expression of Gata2 (Tsarovina et al., 2004) indicating that Gata2 is directly or indirectly regulated by Phox2b. Our model proposes that Phox2b activates Gata2 expression through Gata3 because mice that lack Gata3 do not express Gata2 (Tsarovina et al., 2004). If Gata2 expression is Phox2b-dependent, it requires Gata3 as a co-factor. The function of Gata2 in mammalian sympathetic neuronal development remains unknown because of the early embryonic lethality associated with the loss of its function (Tsai et al., 1994). The regulation of catecholamine biosynthesis is mediated by Gata3 (Lim et al., 2000; Moriguchi et al., 2006), but not through activation of Gata2, because neither factor is sufficient to active the expression of TH and DBH. The expression of TH and DBH requires Hand2 and the loss of Hand2 does not diminish expression of Gata2, Gata3 or Phox2b. Taken together, these observations suggest that the noradrenergic phenotype is acquired through the synergistic activities of Gata3, Phox2b and Hand2.
Hand2 expression is dependent on that of Phox2b (Goridis and Rohrer, 2002) but Hand2 does not regulate the expression of Phox2b, Phox2a, Mash1, Gata2, or Gata3 in vivo. Hand2 is, however, essential for Hand1 expression. Hand2 has been shown to bind the promoter region of the Hand1 gene, suggesting that Hand1 is a direct target of Hand2 (Rychlik et al., 2005). The SNS-restricted expression of Hand1 (Cserjesi et al., 1995; Morikawa and Cserjesi, 2004) is certainly consistent with a role for Hand1 in SNS development; nevertheless, the function of Hand1 in developing and/or mature sympathetic neurons has not been determined because of the early embryonic lethality of Hand1 null mice (Firulli et al., 1998; Morikawa and Cserjesi, 2004). During the development of the SNS, Hand1 is not expressed until after TH has already begun (Howard et al., 2000), suggesting that Hand1 may play a late or restricted role in development of sympathetic neurons, but that it is not a determinant of their transmitter choice.
Acknowledgments
We wish to thank Dr. E.N. Olson (University of Texas, Southwestern, Dallas) for providing the Hand2 null mouse line and Dr. H.M. Sucov (University of Southern California, Los Angeles) for providing the Wnt1-Cre mouse line. We thank Taneasha Washington and Emily Maska for technical assistance. This work was supported by grants from NSF (0529746 and 0609086) and AHA (Grant in Aid) to P.C. and an NIH grant (NS 15547) to M.D.G. and P.C. Y.M. is a Postdoctoral Fellow of the AHA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An M, Luo R, Henion PD. Differentiation and maturation of zebrafish dorsal root and sympathetic ganglion neurons. J Comp Neurol. 2002;446:267–75. doi: 10.1002/cne.10214. [DOI] [PubMed] [Google Scholar]
- Blaugrund E, Pham TD, Tennyson VM, Lo L, Sommer L, Anderson DJ, Gershon MD. Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers and Mash-1-dependence. Development. 1996;122:309–20. doi: 10.1242/dev.122.1.309. [DOI] [PubMed] [Google Scholar]
- Brewer S, Feng W, Huang J, Sullivan S, Williams T. Wnt1-Cre-mediated deletion of AP-2alpha causes multiple neural crest-related defects. Dev Biol. 2004;267:135–52. doi: 10.1016/j.ydbio.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Coppola E, Pattyn A, Guthrie SC, Goridis C, Studer M. Reciprocal gene replacements reveal unique functions for Phox2 genes during neural differentiation. Embo J. 2005;24:4392–403. doi: 10.1038/sj.emboj.7600897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserjesi P, Brown D, Lyons GE, Olson EN. Expression of the novel basic helix-loop-helix gene eHAND in neural crest derivatives and extraembryonic membranes during mouse development. Dev Biol. 1995;170:664–78. doi: 10.1006/dbio.1995.1245. [DOI] [PubMed] [Google Scholar]
- D’Autréaux F, Morikawa Y, Cserjesi P, Gershon MD. Hand2 is necessary for terminal differentiation of enteric neurons from crest-derived precursors but not for their migration into the gut or formation of glia. Development. 2007 doi: 10.1242/dev.003814. in press. [DOI] [PubMed] [Google Scholar]
- Dai YS, Cserjesi P, Markham BE, Molkentin JD. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J Biol Chem. 2002;277:24390–8. doi: 10.1074/jbc.M202490200. [DOI] [PubMed] [Google Scholar]
- Dai YS, Hao J, Bonin C, Morikawa Y, Cserjesi P. JAB1 enhances HAND2 transcriptional activity by regulating HAND2 DNA binding. J Neurosci Res. 2004;76:613–22. doi: 10.1002/jnr.20105. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–6. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat Genet. 1998;18:266–70. doi: 10.1038/ng0398-266. [DOI] [PubMed] [Google Scholar]
- Firulli BA, Howard MJ, McDaid JR, McIlreavey L, Dionne KM, Centonze VE, Cserjesi P, Virshup DM, Firulli AB. PKA, PKC, and the protein phosphatase 2A influence HAND factor function: a mechanism for tissue-specific transcriptional regulation. Mol Cell. 2003;12:1225–37. doi: 10.1016/s1097-2765(03)00425-8. [DOI] [PubMed] [Google Scholar]
- Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci. 2002;3:531–41. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- Groves AK, George KM, Tissier-Seta JP, Engel JD, Brunet JF, Anderson DJ. Differential regulation of transcription factor gene expression and phenotypic markers in developing sympathetic neurons. Development. 1995;121:887–901. doi: 10.1242/dev.121.3.887. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Joyner AL. Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech Dev. 1993;42:171–85. doi: 10.1016/0925-4773(93)90006-j. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–76. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Barrallo-Gimeno A, Ettl AK, Durr K, Knapik EW, Driever W. Noradrenergic neurons in the zebrafish hindbrain are induced by retinoic acid and require tfap2a for expression of the neurotransmitter phenotype. Development. 2003;130:5741–54. doi: 10.1242/dev.00816. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Huh Y, Chae H, Hong S, Lardaro T, Kim KS. GATA-3 regulates the transcriptional activity of tyrosine hydroxylase by interacting with CREB. J Neurochem. 2006;98:773–81. doi: 10.1111/j.1471-4159.2006.03924.x. [DOI] [PubMed] [Google Scholar]
- Howard M, Foster DN, Cserjesi P. Expression of HAND gene products may be sufficient for the differentiation of avian neural crest-derived cells into catecholaminergic neurons in culture. Dev Biol. 1999;215:62–77. doi: 10.1006/dbio.1999.9450. [DOI] [PubMed] [Google Scholar]
- Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol. 2005;277:271–86. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Howard MJ, Cserjesi P. Society for Neuroscience. Vol. 26. Washington: 1996. Chicken eHAND and dHAND influence neural crest cell differentiation; p. 7. [Google Scholar]
- Howard MJ, Stanke M, Schneider C, Wu X, Rohrer H. The transcription factor dHAND is a downstream effector of BMPs in sympathetic neuron specification. Development. 2000;127:4073–81. doi: 10.1242/dev.127.18.4073. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–16. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Zimmerman K, Saito T, Anderson DJ. Induction and repression of mammalian achaete-scute homologue (MASH) gene expression during neuronal differentiation of P19 embryonal carcinoma cells. Development. 1992;114:75–87. doi: 10.1242/dev.114.1.75. [DOI] [PubMed] [Google Scholar]
- Kim HS, Seo H, Yang C, Brunet JF, Kim KS. Noradrenergic-specific transcription of the dopamine beta-hydroxylase gene requires synergy of multiple cis-acting elements including at least two Phox2a-binding sites. J Neurosci. 1998;18:8247–60. doi: 10.1523/JNEUROSCI.18-20-08247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet. 2000;25:209–12. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- Lo L, Morin X, Brunet JF, Anderson DJ. Specification of neurotransmitter identity by Phox2 proteins in neural crest stem cells. Neuron. 1999;22:693–705. doi: 10.1016/s0896-6273(00)80729-1. [DOI] [PubMed] [Google Scholar]
- Lucas ME, Muller F, Rudiger R, Henion PD, Rohrer H. The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development. 2006;133:4015–24. doi: 10.1242/dev.02574. [DOI] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci U S A. 1999;96:5037–42. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DG, McAnally J, Richardson JA, Charite J, Olson EN. Misexpression of dHAND induces ectopic digits in the developing limb bud in the absence of direct DNA binding. Development. 2002;129:3077–88. doi: 10.1242/dev.129.13.3077. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Takako N, Hamada M, Maeda A, Fujioka Y, Kuroha T, Huber RE, Hasegawa SL, Rao A, Yamamoto M, Takahashi S, Lim KC, Engel JD. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development. 2006;133:3871–81. doi: 10.1242/dev.02553. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Cserjesi P. Extra-embryonic vasculature development is regulated by the transcription factor HAND1. Development. 2004;131:2195–204. doi: 10.1242/dev.01091. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Dai YS, Hao J, Bonin C, Hwang S, Cserjesi P. The basic helix-loop-helix factor Hand 2 regulates autonomic nervous system development. Dev Dyn. 2005;234:613–21. doi: 10.1002/dvdy.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X, Cremer H, Hirsch MR, Kapur RP, Goridis C, Brunet JF. Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron. 1997;18:411–23. doi: 10.1016/s0896-6273(00)81242-8. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Guillemot F, Brunet JF. Delays in neuronal differentiation in Mash1/Ascl1 mutants. Dev Biol. 2006;295:67–75. doi: 10.1016/j.ydbio.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–70. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- Rothman TP, Gershon MD, Holtzer H. The relationship of cell division to the acquisition of adrenergic characteristics by developing sympathetic ganglion cell precursors. Dev Biol. 1978;65:322–41. doi: 10.1016/0012-1606(78)90030-1. [DOI] [PubMed] [Google Scholar]
- Rychlik JL, Gerbasi V, Lewis EJ. The interaction between dHAND and Arix at the dopamine beta-hydroxylase promoter region is independent of direct dHAND binding to DNA. J Biol Chem. 2003;278:49652–60. doi: 10.1074/jbc.M308577200. [DOI] [PubMed] [Google Scholar]
- Rychlik JL, Hsieh M, Eiden LE, Lewis EJ. Phox2 and dHAND transcription factors select shared and unique target genes in the noradrenergic cell type. J Mol Neurosci. 2005;27:281–92. doi: 10.1385/JMN:27:3:281. [DOI] [PubMed] [Google Scholar]
- Sommer L, Shah N, Rao M, Anderson DJ. The cellular function of MASH1 in autonomic neurogenesis. Neuron. 1995;15:1245–58. doi: 10.1016/0896-6273(95)90005-5. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Cserjesi P, Olson EN. A subclass of bHLH proteins required for cardiac morphogenesis. Science. 1995;270:1995–9. doi: 10.1126/science.270.5244.1995. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16:154–60. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- Stanke M, Junghans D, Geissen M, Goridis C, Ernsberger U, Rohrer H. The Phox2 homeodomain proteins are sufficient to promote the development of sympathetic neurons. Development. 1999;126:4087–94. doi: 10.1242/dev.126.18.4087. [DOI] [PubMed] [Google Scholar]
- Swiatek PJ, Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 1993;7:2071–84. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–6. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Tsarovina K, Pattyn A, Stubbusch J, Muller F, Van Der Wees J, Schneider C, Brunet JF, Rohrer H. Essential role of Gata transcription factors in sympathetic neuron development. Development. 2004;131:4775–86. doi: 10.1242/dev.01370. [DOI] [PubMed] [Google Scholar]
- Xu H, Firulli AB, Zhang X, Howard MJ. HAND2 synergistically enhances transcription of dopamine-beta-hydroxylase in the presence of Phox2a. Dev Biol. 2003;262:183–93. doi: 10.1016/s0012-1606(03)00361-0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Clouthier DE, Richardson JA, Charite J, Olson EN. Targeted deletion of a branchial arch-specific enhancer reveals a role of dHAND in craniofacial development. Development. 2003;130:1069–78. doi: 10.1242/dev.00337. [DOI] [PubMed] [Google Scholar]
- Yang C, Kim HS, Seo H, Kim CH, Brunet JF, Kim KS. Paired-like homeodomain proteins, Phox2a and Phox2b, are responsible for noradrenergic cell-specific transcription of the dopamine beta-hydroxylase gene. J Neurochem. 1998;71:1813–26. doi: 10.1046/j.1471-4159.1998.71051813.x. [DOI] [PubMed] [Google Scholar]


