Abstract
Background
The heterogeneity of clinical findings in studies evaluating the efficacy of naltrexone in the treatment of alcohol dependence has led to growing efforts to explore novel approaches to data analysis. The objective of this study was to identify distinct trajectories of daily drinking over time in two negative clinical trials and to determine whether naltrexone affected the probability to follow a particular trajectory.
Methods
The VA Cooperative Study #425 and the Women’s Naltrexone Study of naltrexone failed to demonstrate efficacy on primary outcome variables. Separately for each study we analyzed daily indicators of any drinking and heavy drinking using the semi-parametric group-based approach of Nagin (1999).
Results
We estimated three distinct trajectories of daily drinking (both any and heavy drinking) which we describe as “abstainer”, “sporadic drinker” and “consistent drinker”. Naltrexone doubled the odds of following the “abstainer” trajectory instead of the “consistent drinker” trajectory but did not significantly change the odds of following the “abstainer” trajectory as contrasted with the “sporadic drinker” trajectory.
Conclusions
Naltrexone may have a clinically meaningful effect for alcohol dependent patients with a high chance of consistent drinking, even in studies where it failed to show efficacy in planned analyses.
Keywords: alcohol research, naltrexone, clinical trial, latent class model, trajectory-based analysis, population heterogeneity
Introduction
The heterogeneity of clinical findings in studies evaluating the efficacy of naltrexone in the treatment of alcohol dependence has led to growing efforts to explore novel approaches to study design and data analysis. Naltrexone is approved by the FDA for the treatment of alcoholism but evidence for its efficacy is not unequivocal. A number of small to medium size studies (Volpicelli et al 1992, 1997; O’Malley et al 1992, 1996; Kranzler et al 1998; Anton et al 1999; Heinala et al 2001; Morris et al 2001, Guardia et al 2002; Latt et al 2002; Balldin et al 2003; Kiefer et al 2003, 2004) and two large clinical trials (Garbutt 2005, Anton 2006) reported that naltrexone was effective in delaying relapse to heavy drinking, reducing the intensity of drinking or increasing percent days abstinence. Several systematic reviews indicated that naltrexone efficacy was associated with a small to moderate effect size (Garbutt et al 1999, Kranzler and Van Kirk 2001, Streeton and Whalen 2001, Bouza et al 2004, Berglund 2005, Srisurapanont and Jarusuraisin 2005). In contrast, a large randomized VA clinical trial (Krystal et al 2001) and other clinical trials (Kranzler et al 2000, Chick et al 2000, Gastpar et al 2002, Davidcon et al 2004, Killeen et al 2004) found no significant benefit associated with naltrexone treatment. Some meta-analyses also found no effect of naltrexone on abstinence (Garbutt et al 1999, Bouza et al 2004).
The sources of heterogeneity in these clinical trials are not clear. Since naltrexone is not an aversive treatment, it is not expected to stop people from drinking but may decrease frequency and volume of drinking. Hence, it may affect some measures of drinking but not others which may explain the findings in some studies of a protective effect of naltrexone on heavy drinking but not on any drinking. Naltrexone is thought to act by reducing craving, thereby promoting self-management of drinking behavior. The efficacy of naltrexone might also be directly related to changes in compliance. Furthermore, multi-site study effect sizes are generally smaller than single-site study effect sizes due to their larger population heterogeneity (Feinn and Kranzler 2005). The possibility that subgroups of alcohol dependent patients might differ predictably in their response to naltrexone also has been suggested on the basis of molecular genetic data (Oslin et al 2003).
The impact of heterogeneous patient responses to naltrexone on clinical trial results may be exacerbated by the reliance of all published studies on summary drinking measures. The use of analytic approaches that evaluate patterns of drinking rather than single events or summary measures may be better suited to evaluate naltrexone efficacy (O’Malley and Froehlich 2003, Wang et al 2002). We hypothesize that by using daily drinking data and by accounting for compliance, we will not only be able to elicit meaningful patterns of alcohol use over time but may also see an increase in power for detecting treatment effects.
Recent advances in longitudinal statistical modeling (Rose, 2000) provide methods that enable the use of daily drinking data. Traditional growth modeling (Longford 1993, Lindsey 1993, Diggle 1994, Raudenbush and Bryk 2002, Goldstein 2003) assumes that every individual follows the same type of trajectory over time, while latent-class based approaches (Nagin 1999, Muthen and Muthen 2000a, b, Dolan et al 2005) allow data-driven identification of distinct classes of developmental trajectories. Thus, it is possible to identify subgroups of subjects who show distinct patterns of clinical response within a clinical trial based on the structure of the data generated by that trial, i.e., subgroups that might not have been hypothesized a priori by the investigative team.
In the field of alcoholism research, trajectory-based analyses have been selectively applied to large-scale observational studies of developmental patterns of alcohol use (Hill et al 2000, Muthen 2000, Muthen and Muthen 2000a, b, Khoo and Muthen 2000, Chassin 2002, Del Boca 2004, Greenbaum 2005). To our knowledge however, trajectory-based approaches have not yet been applied to treatment research studies. The objective of this work was to determine whether trajectory-based methods would provide insights into two adequately powered trials that failed to demonstrate significant naltrexone efficacy.
Methods
VA Cooperative Studies Program (CSP) study #425 Naltrexone in the Treatment of Alcohol Dependence (Krystal et al 2001)
In this double-blind randomized trial, 627 veterans with chronic, severe alcohol dependence were assigned to 3 months of naltrexone, 12 months of naltrexone, or placebo. Of the 627 subjects, 567 (10 females) provided drinking data and were included in the analyses. Patients receiving naltrexone started with 25 mg once daily for 2 days, followed by 50 mg once daily for 3 or 12 months. Time to first day of heavy drinking (six or more drinks for men and four or more for women) was defined as the primary outcome measure in the first 13 weeks of the trial.
Women’s Naltrexone Trial
The second double-blind randomized clinical trial was conducted at the Substance Abuse Treatment Unit of the Connecticut Mental Health Center and enrolled 103 women with current alcohol dependence (O’Malley et al 2006). Eligible subjects were randomized to either naltrexone or matching placebo for 12 weeks. Of the 103 subjects, 98 had drinking data and were included in analyses. Patients receiving naltrexone started with 25 mg once daily for 2 days, followed by 50 mg once daily. The primary outcomes included time to first day of drinking and time to first day of heavy drinking defined as consuming four or more drinks on an occasion.
In both trials no significant naltrexone effects were observed on the primary outcome measures (Krystal et al 2001, O’Malley et al 2006).
In both studies, the “Timeline Follow Back” (TLFB) was used to retrospectively collect daily drinking data. TLFB is the most comprehensive self-report measure and has good reliability and internal consistency on summary drinking measures (Sobell & Sobell 1992, 1995). In the trajectory-based reanalysis we focused on the daily binary indicator of drinking (1 if any drinks were consumed by the subject on that day, 0 otherwise) or heavy drinking (1 if 6 or more drinks were consumed by males and 4 or more drinks were consumed by females on that day, 0 otherwise). Although there is large individual variability in accuracy of reporting daily drinking (Searle et al, 2000), the trajectory-based approach smoothes the day-to-day data, thus creating stable data-driven patterns with much smaller variability than daily measures. At the same time, this approach captures drinking over time more precisely than traditional summary measures.
We used the approach of Nagin (1999, 2001b) to identify distinct trajectories of drinking patterns during the first 3 months of the trials (considered separately) and to estimate how naltrexone affects the probability of following a particular trajectory. The models assumed fixed polynomial trends over time within each trajectory class and modeled the effect of treatment and covariates on trajectory membership via a generalized logistic regression model. Model selection (number of trajectory classes and degree of the polynomial trends over time) was based on the Schwartz Bayesian criterion. Baseline covariates (age; percent drinking days and drinks per drinking day in the 90 days prior to study entry; post-traumatic stress disorder and major depression diagnosis in last month prior to study entry) were entered as main effects one at a time and jointly, and their effects on trajectory membership and on the relationship between naltrexone and trajectory membership were assessed. Lifetime cocaine dependence was also tested as a covariate in the VA trial. Medication compliance was measured using microelectronic monitoring (MEMS) of the medication bottle to remove the single daily pill (APREX Corporation, Fremont, CA), was recorded as a binary variable (1 if bottle was opened on a particular day, 0 otherwise) and was treated as a time-dependent covariate in the analyses.
This modeling strategy allowed the data to guide the choice of the number of trajectories that best fit the data and to determine the shape of each trajectory over time. It also allowed estimation of the proportion of the population whose treatment response corresponds most closely to each trajectory group. For the analysis we used a customized SAS procedure (PROC TRAJ) developed by Jones et al (1998).
We performed parallel analyses of the two clinical trials and then calculated a weighted average of the naltrexone effect estimates to obtain a meta-analytic estimate of the overall naltrexone effect. For model selection, we considered two and three class models, and quadratic and cubic polynomials.
Results
Any drinking outcome
Figures 1 and 2 plot the estimated trajectories in the VA and women’s clinical trials, respectively. The three trajectory patterns over time were similar for the two studies and were interpreted as “abstainers”, “sporadic drinkers” and “consistent drinkers”. The “abstainers” were not necessarily complete abstainers but had close to 0% chance of drinking on any particular day. Estimated percentages of each treatment group that conform most closely to each trajectory and estimated odds ratios for the naltrexone effect adjusted for compliance are presented in Table 1. In both studies, subjects on naltrexone were more likely to be “abstainers” than were subjects on placebo, were about as likely to be “sporadic drinkers” and were less likely to be “consistent drinkers”. In the VA trial, the odds of being an “abstainer” vs. a “consistent drinker”, and of being a “sporadic drinker” vs. a “consistent drinker” were doubled for subjects on naltrexone as compared to subjects on placebo and these comparisons were statistically significant (odds ratio [OR]=2.02, 95% CI: 1.16,3.52 and OR= 1.85, 95% CI: 1.06, 3.22, respectively). In the trial in women, the corresponding odds were also doubled but these comparisons were not statistically significant. The combined estimates from the two studies confirmed that subjects on naltrexone had significantly higher odds to be “abstainers” or “sporadic drinkers” vs. “consistent drinkers” than subjects on placebo (OR=2.05, 95% CI: 1.24, 3.39 and OR=1.90, 95% CI: 1.08, 3.34, respectively). The odds ratio estimates for comparing “abstainers” and “consistent drinkers” in the VA trial and in the combined results were still significant after Bonferroni correction while the odds ratio estimates for comparing “sporadic drinkers” and “consistent drinkers” were no longer statistically significant.
Figure 1.
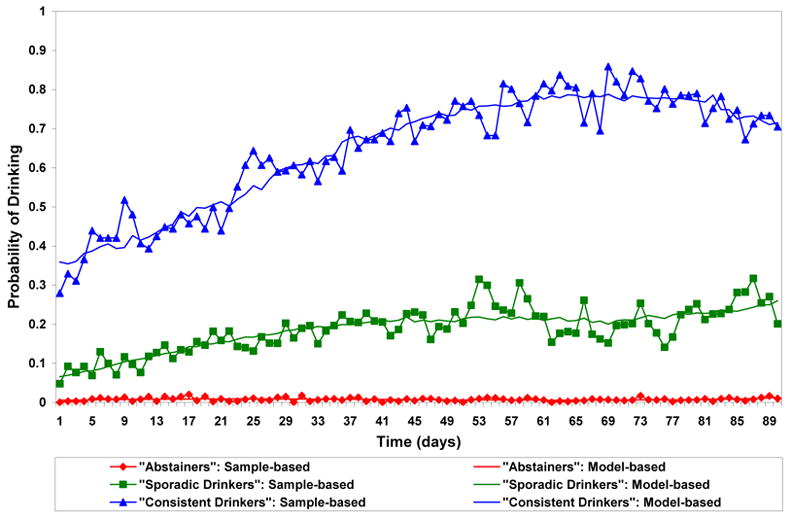
Figure 2.
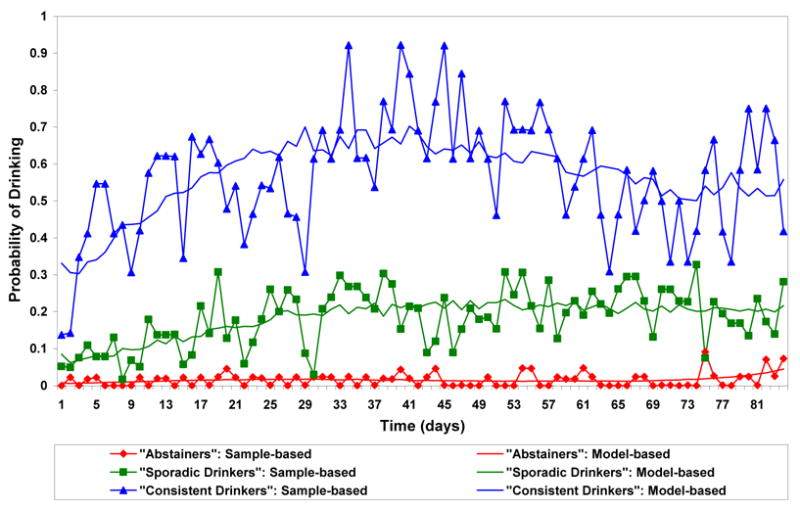
Trajectories of Any Drinking over Time in the Women's Trial a,b
Table 1.
Estimated probabilities of any drinking for a particular trajectory by treatment and adjusted odds ratios for naltrexone effect.
| Study group | Probability Traj. 1 (abstainers) | Probability Traj. 2 (sporadic drinkers) | Probability Traj. 3 (consistent drinkers) | Odds ratio (abstainers vs sporadic drinkers) | Odds ratio (abstainers vs consistent drinkers) | Odds ratio (sporadic vs consistent drinkers) |
|---|---|---|---|---|---|---|
| VA (98% men)
Naltrexone Placebo |
65.2%
67.4% 60.8% |
24.1%
24.2% 23.9% |
10.8%
8.4% 15.3% |
1.09 (0.71,1.68) |
2.02 (1.16,3.52) |
1.85 (1.06,3.22) |
| Women
Naltrexone Placebo |
45.7%
48.3% 41.7% |
35.7%
39.2% 34.3% |
18.6%
12.4% 24.0% |
1.01 (0.40,2.57) |
2.23 (0.68,7.37) |
2.20 (0.67,7.28) |
| Combined | 1.08 (0.73,1.60) | 2.05 (1.24,3.39) | 1.90 (1.08,3.34) |
Heavy drinking outcome
Figure 3 plots the estimated trajectories for heavy drinking in the VA clinical trial. In the women’s trial, due to the small sample size stable identification of trajectories of heavy drinking was not possible. In Figure 3 the three trajectory patterns over time were similar to the trajectories for any drinking and were depicted as “abstainers from heavy drinking”, “sporadic heavy drinkers” and “consistent heavy drinkers”. Estimated percentages of each treatment group that conform most closely to each trajectory and estimated odds ratios for the naltrexone effect adjusted for compliance are presented in Table 2. Subjects on naltrexone appeared slightly more likely to be “abstainers from heavy drinking” than subjects on placebo, were about as likely to be “sporadic heavy drinkers” and were less likely to be “consistent heavy drinkers”. The odds of being an “abstainer from heavy drinking” vs. being a “consistent heavy drinker” were doubled for subjects on naltrexone as compared to subjects on placebo (OR=2.07, 95% CI: 1.11, 3.84). This comparison was statistically significant before but not after Bonferroni correction.
Figure 3.
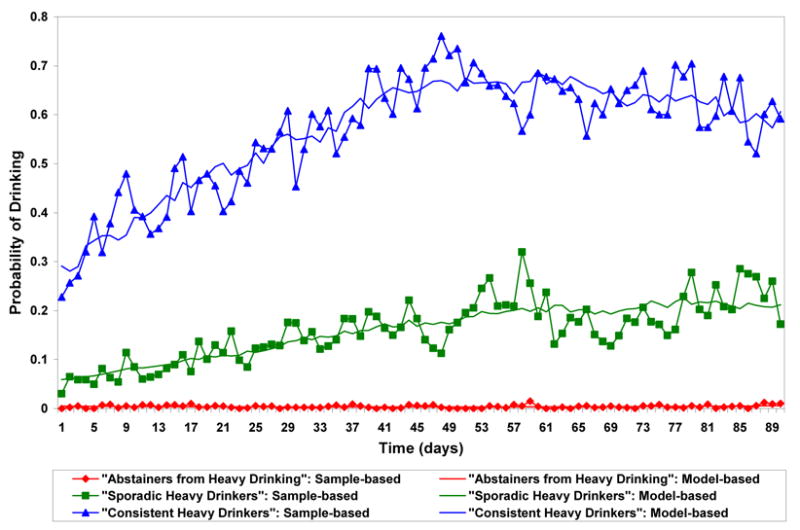
Trajectories of Heavy Drinking over Time in the VA Trial a,c
Table 2.
Estimated probabilities of heavy drinking for a particular trajectory by treatment and adjusted odds ratios for naltrexone effect.
| Study group | Probability Traj. 1 (abstainers from heavy drinking) | Probability Traj. 2 (sporadic heavy drinkers) | Probability Traj. 3 (consistent heavy drinkers) | Odds ratio (abstainers vs sporadic heavy drinkers) | Odds ratio (abstainers vs consistent heavy drinkers) | Odds ratio (sporadic vs consistent heavy drinkers) |
|---|---|---|---|---|---|---|
| VA
Naltrexone Placebo |
72.9%
75.2% 68.2% |
18.9%
18.4% 19.9% |
8.2%
6.4% 11.9% |
1.19 (0.75,1.89) |
2.07 (1.11,3.84) |
1.74 (0.85,3.57) |
Covariate Analyses
None of the baseline covariates significantly altered the effect of naltrexone on trajectory membership for both outcomes. Ignoring medication compliance did not change the significance of the findings regarding the efficacy of naltrexone on any drinking but decreased the significance of the efficacy of naltrexone on heavy drinking. Medication compliance had a significant effect on the trends over time by decreasing the odds of drinking in all trajectories (p<.01). For “consistent drinkers” in the VA trial, the odds of drinking on a particular day for compliers were only one-third of the odds of non-compliers (OR=0.35, 95%CI: 0.30, 0.40). For “sporadic drinkers” and “abstainers” in the VA trial the odds of drinking on a particular day for compliers were about one-half of the odds of non-compliers (OR=0.45, 95% CI: 0.40, 0.50 and OR=0.47, 95% CI: 0.34, 0.65). For all trajectory groups in the women’s trial, the odds of drinking on a particular day for compliers were slightly less than one-half of the odds of non-compliers (OR=0.46, 95% CI: 0.35, 0.60 for “consistent drinkers”; OR=0.43, 95% CI: 0.32, 0.57 for “sporadic drinkers”; OR=0.44, 95% CI: 0.23, 0.84 for “abstainers”). The effect of medication compliance on heavy drinking was most pronounced for the “consistent heavy drinkers” where the odds of heavy drinking on a particular day for compliers were only one-fifth of the odds of non-compliers (OR=0.20, 95%CI: 0.17, 0.23). For “sporadic heavy drinkers” the odds of heavy drinking on a particular day for compliers were only one-third of the odds of non-compliers (OR=0.35, 95% CI: 0.30, 0.40), while for “abstainers from heavy drinking” the odds were halved with compliance (OR=0.54, 95% CI: 0.37, 0.80).
Number Needed to Treat (NNT) Analyses
NNT analyses provide a clinical context of how many people need to receive naltrexone to have them move from one trajectory to another. The “adverse” outcome is membership in the “consistent drinkers” or “consistent heavy drinkers” trajectory. Using the probability estimates of trajectory membership from Table 1, we estimate that 15 and 9 subjects need to be treated with naltrexone to have one subject move out of the “consistent drinkers” trajectory in the VA and women’s trial, respectively. Using the probability estimates from Table 2 we estimate that 19 subjects need to be treated with naltrexone to have one subject move out of the “consistent heavy drinkers” trajectory in the VA trial.
Discussion
In the trajectory-based reanalysis of the VA clinical trial we found statistically significant effects of naltrexone on increasing the likelihood of abstinence from any drinking and decreasing the likelihood of heavy drinking. These findings stand in contrast to the findings of no effect of naltrexone on the originally specified summary measures of alcohol consumption in the same data set. We hypothesize that both original studies failed to find a significant naltrexone effect because of the high abstinence rates and because of the reliance on summary measures of alcohol consumption. Since all patients in both studies received a form of psychotherapy in addition to medication, both studies explored, in essence, the ability to show a medication effect in addition to an active treatment. This may account for the high abstinence rates. When the abstinence group is highly represented, the clinical effects appear small and most clinical trials using standard analytic techniques will fail to find group differences. The trajectory-based analytic approach may be one useful strategy in these situations.
We found a significant effect of naltrexone in the VA trial that recruited almost entirely men but no significant effect of naltrexone in the trial in women. While this could be interpreted to concur with the finding of Garbutt (2005) of differential effects by gender, the sample size of the VA trial was 6 times larger than that of the trial in women. Moreover, the similarities in both the shape of the estimated trajectories and in the magnitude of effects between the VA and the women’s studies suggest that the lack of statistical significance in the women’s study is more likely due to low power and that the naltrexone effect is actually consistent across the gender.
Not surprisingly, medication adherence was found to decrease the chance of drinking regardless of treatment (Cramer et al. 2003). Accounting for daily medication compliance strengthened the effect of naltrexone both for any drinking and for heavy drinking. This is consistent with previous findings that suggest the effect of naltrexone can be enhanced by improving medication compliance (Volpicelli 1997).
A limitation of our compliance analyses is that the model we used (Nagin, 1999) does not allow direct testing of interactions between baseline variables (including treatment and time-dependent covariates). Hence, we were not able to assess whether effects of compliance differed by treatment or baseline covariates. Compliance may also be affected by treatment and mediate treatment response but we could not establish such relationships in the model used.
Our analysis is predicated on the assumption that different classes of trajectories exist. When no categorically different trajectories exist, a substantial percent of subjects will not be reliably classified into any one trajectory. In our analysis, over 86% of subjects had a posterior probability greater than 95% to belong to one of the trajectory classes and over 99% of the subjects had a posterior probability greater than 60% to belong to one trajectory class. This gives reassurance that in the clinical trials we reanalyzed, categorically different trajectory classes indeed exist.
We considered models limited to either two or three trajectory classes because of limited sample sizes and computational feasibility. In the analysis of developmental behavioral data, a larger number of distinct developmental trajectory classes have typically been identified, although the additional classes have usually been formed by splitting already existing classes. Since ours is the first trajectory-based analysis of treatment data, it is not clear whether more trajectory classes would significantly improve the fit of our models to the data. Even if more trajectory classes are needed to adequately describe the data, stable identification of these trajectories will require even larger sample sizes to ensure precise estimation of probabilities of class membership for each trajectory class. It is thus possible that we may have missed a finer categorization of the trajectories over time.
The problem of negative or failed clinical trials for medications where there is substantial evidence supporting efficacy is a major problem in psychiatric research (Greist et al 2002, Katz et al 2002, Yang et al 2005, Khan and Schwartz 2005). The current findings suggest that trajectory-based statistical methods may play a role in the analysis of clinical trials by empirically estimating the heterogeneity in the study population and identifying subgroups of subjects with similar response patterns for whom treatment is effective.
Acknowledgments
In addition to the authors, the members of the Department of Veterans Affairs Cooperative Study 425 Group were as follows: K. Drexler, F. Mohammad, L. Siklosky, K. Walker, C. Arnold-Hunter, and R. Head, Atlanta; J. Hermos, H. Behr, B. Kinne, D. Savage, and J. Wickis, Boston; L. Rugle, O. Kausch, H. Zegarna, K. Conti, H. Adkins, G. Harris, and C. Cartier, Cleveland; B. Adinoff, L. Burney, J. Fields, B. Hudson, J. Corder, and A. Quintero, Dallas; J. Grabowski, R. Wancha, Y. Ruiz, S. Chermack, S. Fleming, K. Gamel, and B. Sullivan, Detroit; L. Madlock, R. Murray, J. Williams, R. Lewandowski, and T. Owens, Memphis, Tenn.; M. FeBornstein, J. Pena, B. Cotton-Brown, M. Cowie, A. Connelly, W. Hill, A. Holmes, and J. Fiery, New Orleans; P. Casadonte, S. Kushner, S. Johnson, J. Siegris, N. Lynch, E. Richardson, and A. Butcher, New York; S. Nixon, C. Shaw, R. Joswick, D. Bertoch, and H. Engebretson, Oklahoma City; L. Haynes-Tucker, L. Moffet, J. Weintraub, R. Lutz, S. Clinton, F. Pohlman, R. Royal, and S. Harris, Menlo Park, Calif.; I. Maany, J. DeStefano, M. Andem, C. Hackett, J. McNeely, S. Dyanick, D. Torpey, S. Poole, E. Moeller, and A. Scheamania, Philadelphia; G. Kaplan, H. MacAskill, P. Charnley, and C. Williams, Providence, R.I.; C. Stock, P. Stevenson, S. Plumb, M. Dean, and J. Hunter, Salt Lake City; P. Banys, I. Rhew, S. Staccone, J. Kelly, and S. Shives, San Francisco; A. Saxon, M. Willey-Allen, J. Williams, K. Lunna, V. Ruscigno, S. Brown, and K. Shaffer, Seattle; J. Collins, S. Kilby, T. Burke, L. Linzy, C. Dalzell, M. Rhoads, J. Kelly, N. Banks, J. Arflin, and D. Briones, Perry Point, Md.; and M. Miller and C. Messick, Albuquerque, N.M.
Supported by Department of Veterans Affairs Cooperative Study Program and Alcohol Research Center, the Center for Translational Neuroscience of Alcoholism (P50 AA012870-05), and grants RO1-AA10225 and K05-AA014715
Footnotes
Medication compliance is considered as a time-dependent covariate.
Solid lines without symbols represent model-based probabilities of any drinking over time for each trajectory group corresponding to average daily compliance rates. Solid lines with symbols represent sample-based probabilities of any drinking based on all subjects weighted by the posterior probability of trajectory membership.
Solid lines without symbols represent model-based probabilities of heavy drinking over time for each trajectory group corresponding to average daily compliance rates. Solid lines with symbols represent sample-based probabilities of any drinking based on all subjects weighted by the posterior probability of trajectory membership.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156:1758–64. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- 2.Anton RF, O’Malley SS, Giraulo D, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The Combine study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 3.Balldin J, Berglund M, Borg S, et al. A 6-month controlled naltrexone study: Combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcohol Clin Exp Res. 2003;27:1142–1149. doi: 10.1097/01.ALC.0000075548.83053.A9. [DOI] [PubMed] [Google Scholar]
- 4.Berglund M. A better widget? Three lessons for improving addiction treatment from a meta-analytical study. Addiction. 2005;100 (6):742–750. doi: 10.1111/j.1360-0443.2005.01106.x. [DOI] [PubMed] [Google Scholar]
- 5.Bouza C, Angeles M, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 6.Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high risk sample: predictors and substance abuse outcomes. J Consult Clin Psychol. 2002;70(1):67–78. [PubMed] [Google Scholar]
- 7.Chick J, Anton R, Checinski K, et al. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000;35:587–93. doi: 10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- 8.Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed? A novel assessment technique. JAMA. 1989;261:3273–3277. [PubMed] [Google Scholar]
- 9.Cramer J, Rosenheck R, Kirk G, et al. Medication compliance feedback and monitoring in a clinical trial: predictors and outcomes. Value in Health. 2003;6(5):566–573. doi: 10.1046/j.1524-4733.2003.65269.x. [DOI] [PubMed] [Google Scholar]
- 10.Davidson D, Saha C, Scifres S, Fyffe J, O’Connor S, Selzer C. Naltrexone and brief counseling to reduce heavy drinking in hazardous drinkers. Addictive behaviors. 2004;29:1253–1258. doi: 10.1016/j.addbeh.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Del Boca FK, Darkes J, Greenbaum PE, Goldman MS. Up close and personal: temporal variability in the drinking of individual college students during their first year. J Consult Clin Psychol. 2003;72(2):155–164. doi: 10.1037/0022-006X.72.2.155. [DOI] [PubMed] [Google Scholar]
- 12.Diggle PJ. Analysis of Longitudinal Data. Oxford: Clarendon Press; 1994. 1993. [Google Scholar]
- 13.Dolan CV, Schmittman VR, Lubke GH, Neale MC. Regime switching in the latent growth curve mixture model. Structural Equation Modeling. 2005;12(1):94–120. [Google Scholar]
- 14.Feinn R, Kranzler HR. Does effect size in naltrexone trials for alcohol dependence differ for single-site vs. multi-center studies? Alcohol Clin Exp Res. 2005;29(6):983–988. doi: 10.1097/01.alc.0000171061.03686.bc. [DOI] [PubMed] [Google Scholar]
- 15.Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 16.Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT. Pharmacological treatment of alcohol dependence: a review of the evidence. JAMA. 1999;281:1318–25. doi: 10.1001/jama.281.14.1318. [DOI] [PubMed] [Google Scholar]
- 17.Gastpar M, Bonnet U, Boning J, et al. Lack of efficacy of naltrexone in the prevention of alcohol relapse: Results from a German multicenter study. J Clin Psychology. 2002;22:592–598. doi: 10.1097/00004714-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein H. Multilevel Statistical Models. 3. London: Edward Arnold; 2003. [Google Scholar]
- 19.Greenbaum PE, Del Boca FK, Darkes J, Wang CP, Goldman MS. Variation in the drinking trajectories of freshmen college students. J Consult Clin Psychol. 2004;73(2):229–238. doi: 10.1037/0022-006X.73.2.229. [DOI] [PubMed] [Google Scholar]
- 20.Greist JH, Mundt JC, Kobak K. Factors contributing to failed trials of new agents: can technology prevent some problems? J Clin Psychiatry. 2002;63(Suppl 2):8–13. [PubMed] [Google Scholar]
- 21.Guardia J, Caso C, Arias F, et al. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: Results from a multicenter clinical trial. Alcohol Clin Exp Res. 2002;26:1381–1387. doi: 10.1097/01.ALC.0000030561.15921.A9. [DOI] [PubMed] [Google Scholar]
- 22.Heinala P, Alho H, Kiianmaa K, Lonnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: A factorial double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2001;21:287–292. doi: 10.1097/00004714-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Hill KG, White HR, Chung IJ, Hawkins JD, Catalano RF. Early adult outcomes of adolescent binge drinking: person and variable-centered analyses of binge drinking trajectories. Alcohol Clin Exp Res. 2000;24(6):892–901. [PMC free article] [PubMed] [Google Scholar]
- 24.Jones B, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29(3):374–393. [Google Scholar]
- 25.Katz MM, Halbreich UM, Bowden CL, et al. Enhancing the technology of clinical trials and the trials model to evaluate newly developed, targeted antidepressants. Neuropsychopharmacology. 2002;27(3):319–28. doi: 10.1016/S0893-133X(02)00329-9. [DOI] [PubMed] [Google Scholar]
- 26.Khan A, Schwartz K. Study designs and outcomes in antidepressant clinical trials. Essent Psychopharmacol. 2005;6(4):221–6. [PubMed] [Google Scholar]
- 27.Khoo SK, Muthen BO. Longitudinal Data on Families: Growth Modeling Alternatives. In: Rose JS, Chassin L, editors. Multivariate Applications in Substance Use Research: New Methods for New Questions. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2000. pp. 43–78. [Google Scholar]
- 28.Kiefer F, Jahn H, Tarnaske T, et al. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism - A double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60:92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- 29.Kiefer F, Andersohn F, Otte C, Wolf K, Jahn H, Wiedemann K. Long-term effects of pharmacotherapy on relapse prevention in alcohol dependence. Acta Neuropsychiatrica. 2004;16:233–238. doi: 10.1111/j.0924-2708.2004.00093.x. [DOI] [PubMed] [Google Scholar]
- 30.Killeen TK, Brady KT, Gold PB, Simpson KN, Faldowski RA, Tyson C, Anton RF. Effectiveness of naltrexone in a community treatment program. Alcohol Clin Exp Res. 2004;28:1710–1717. doi: 10.1097/01.alc.0000145688.30448.2c. [DOI] [PubMed] [Google Scholar]
- 31.Kranzler HR, Modesto-Lowe V, Newayser ES. A sustained-release naltrexone preparation for treatment of alcohol dependence. Alcohol Clin Exp Res. 1998;22:1074–1079. [PubMed] [Google Scholar]
- 32.Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: A meta-analysis. Alcohol Clin Exp Res. 2001;25 (9):1335–1341. [PubMed] [Google Scholar]
- 33.Kranzler HR, Modesto-Lowe V, Van Kirk J. Naltrexone vs. nefazodone for treatment of alcohol dependence: a placebo-controlled trial. Neuropsychopharmacology. 2000;22:493–503. doi: 10.1016/S0893-133X(99)00135-9. [DOI] [PubMed] [Google Scholar]
- 34.Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the Treatment of Alcohol Dependence. NEJM. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- 35.Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Medical Journal of Australia. 2002;176:530–534. doi: 10.5694/j.1326-5377.2002.tb04550.x. [DOI] [PubMed] [Google Scholar]
- 36.Lindsey JK. Models for Repeated Measurements. Oxford: Clarendon Press; 1993. [Google Scholar]
- 37.Longford NT. Random Coefficient Models. Oxford: Claredon Press; 1993. [Google Scholar]
- 38.Morris PLP, Hopwood M, Whelan G, Gardiner J, Drummond E. Naltrexone for alcohol dependence: a randomized controlled trial. Addiction. 2001;96:1565–1573. doi: 10.1046/j.1360-0443.2001.961115654.x. [DOI] [PubMed] [Google Scholar]
- 39.Muthen BO, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 40.Muthen BO. Methodological issues in random coefficient growth modeling using a latent variable framework: Applications to the development of heavy drinking in ages. In: Rose JS, Chassin L, editors. Multivariate Applications in Substance Use Research: New Methods for New Questions. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2000. pp. 18–37.pp. 113–140. [Google Scholar]
- 41.Muthen B, Muthen L. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000a;24(6):882–891. [PubMed] [Google Scholar]
- 42.Muthen BO, Muthen LK. The development of heavy drinking and alcohol-related problems from ages 18 to 37 in a U.S. national sample. J Stud Alcohol. 2000b:290–300. doi: 10.15288/jsa.2000.61.290. [DOI] [PubMed] [Google Scholar]
- 43.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods. 1999;4(2):139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 44.Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol Methods. 2001;6(1):18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 45.O’Malley SS, Jaffe A, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Arch Gen Psychiatry. 1992;49:881–7. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 46.O’Malley SS, Jaffe A, Chang G, Rode S, Schottenfeld RS, Meyer RE, Rounsaville B. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Arch Gen Psychiatry. 1996;53:217–224. doi: 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- 47.O’Malley SS, Froehlich JC. Advances in the use of naltrexone: an integration of preclinical and clinical findings. Recent Dev Alcohol. 2003;16:217–45. [PubMed] [Google Scholar]
- 48.O’Malley S, Sinha R, Grilo CM, et al. Naltrexone and cognitive behavioral coping skills therapy for the treatment of alcohol drinking and eating disorder features in alcohol dependent women: a randomized, double-blind, placebo controlled trial. Under review. Journal of Clinical Psychiatry. 2006 doi: 10.1111/j.1530-0277.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 49.Oslin DW, Berrettinni W, Kranzler HR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–52. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- 50.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 51.Rose JS, Chassin L, editors. Multivariate Applications in Substance Use Research: New Methods for New Questions. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- 52.Searles JS, Helzer JE, Walter DE. Comparison of drinking pattern measures by daily reports and timeline follow back. Psych of Addict Beh. 2000;14(3):277–286. doi: 10.1037//0893-164x.14.3.277. [DOI] [PubMed] [Google Scholar]
- 53.Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: Psycosocial and biological methods. New Jersey: Human Press; 1992. [Google Scholar]
- 54.Sobell LC, Sobell MB. Alcohol consumption measures. In: Allen JP, Columbus M, editors. Assessing alcohol problems: A guide for clinician and researchers. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. pp. 55–73. [Google Scholar]
- 55.Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8(2):267–280. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- 56.Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: A meta-analysis of randomized controlled trials. Alcohol Alcohol. 2001;36(6):544–552. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- 57.Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 58.Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence: role of subject compliance. Arch Gen Psychiatry. 1997;54:737–42. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- 59.Wang SJ, Winchell CJ, McCormick CG, Nevius SE, O’Neill RT. Short of complete abstinence: An analysis exploration of multiple drinking episodes in alcoholism treatment trials. Alcohol Clin Exp Res. 2002;26:1803–1809. doi: 10.1097/01.ALC.0000042009.07691.12. [DOI] [PubMed] [Google Scholar]
- 60.Yang H, Cusin C, Fava M. Is there a placebo problem in antidepressant trials? Curr Top Med Chem. 2005;5(11):1077–86. doi: 10.2174/156802605774297092. [DOI] [PubMed] [Google Scholar]


