The isolation, characterization and preliminary crystallographic studies of two new toxic PLA2s from the venom of V. nikolskii, which has only recently been established as a new species, are reported.
Keywords: Vipera nikolskii, vipers, snake venoms, phospholipase A2, toxicity
Abstract
Snake-venom phospholipases A2 (PLA2s) represent a good model for studies of structure–function relationships, mainly because of their small size and diverse pharmacological and toxicological activities. To obtain new members of the abundant PLA2 family, the venom of the viper Vipera nikolskii was fractionated for the first time and two new proteins, VN5-3 and VN4-3, were isolated. Both proteins show phospholipase A2 activity and may possess neurotoxic activity. Based on the determined partial amino-acid sequences, the new proteins can be classified as basic Asp49 phospholipases A2. They were crystallized using the hanging-drop vapour-diffusion method and crystals of both proteins belong to space group R32, with similar unit-cell parameters: a = b = 76.29, c = 303.35 Å for protein VN5-3 and a = b = 76.28, c = 304.39 Å for protein VN4-3. Diffraction data sets to 3.0 and 2.2 Å resolution were collected and processed for the VN5-3 and VN4-3 crystals, respectively. Preliminary analysis indicates that there are two molecules in the asymmetric unit for both crystals. Further crystallographic studies will help in understanding the structural basis for the multiple functions of snake-venom PLA2s.
1. Introduction
Phospholipases A2 (PLA2s; EC 3.1.1.4) are enzymes that hydrolyze the sn-2 acyl ester bond of various phospholipids to produce free fatty acids and lysophospholipids. They are widely distributed in nature, occurring as both intracellular and extracellular forms (Six & Dennis, 2000 ▶), and play important roles in various biological processes such as phospholipid metabolism and remodelling, homeostasis of cellular membranes, host defence and mediator production as well as signal transduction (Valentin & Lambeau, 2000 ▶). In contrast to the mammalian enzymes, snake-venom PLA2s are toxic and possess diverse pharmacological and toxicological functions, e.g. neurotoxicity, myotoxicity, cardiotoxicity, anticoagulant and haemolytic activities etc. (Kini, 2003 ▶). The small size of snake-venom phospholipases A2 makes them good models for studies of protein structure–function relationships.
About 290 snake-venom PLA2 enzymes have been reported (Kini, 2003 ▶) and a number of three-dimensional structures of these enzymes have been determined by X-ray crystallography and nuclear magnetic resonance (e.g. Brunie et al., 1985 ▶; Arni et al., 1999 ▶; Ward et al., 1998 ▶; Wang et al., 1996 ▶; Tang et al., 1998 ▶). Some progress has been made in searching for the molecular determinants of their pharmacological or toxic activities. Nevertheless, further structural studies are still required in order to understand in detail the structural basis for the diverse biological functions of PLA2.
Some PLA2s, found mainly in snake venoms, are neurotoxins that block neuromuscular communication by inhibiting the release of acetylcholine from motor neurons (Lee et al., 1984 ▶) and are also highly neurotoxic on administration to the brain (Gubensek et al., 1980 ▶; Dorandeu et al., 1998 ▶). These neurotoxins have been extensively investigated as promising tools for studying various aspects of nerve function and dysfunction (Montecucco & Rossetto, 2000 ▶; Krizaj & Gubensek, 2000 ▶). It was found that neurotoxic PLA2s exist in monomeric, homodimeric and heterodimeric forms. Crystal structures have been reported both for heterodimeric and homodimeric enzymes (e.g. Westerlund et al., 1992 ▶; Tang et al., 1998 ▶; Banumathi et al., 2001 ▶; Georgieva et al., 2004 ▶). However, the toxic site and the mechanism of their action at the molecular level are still largely unknown.
The venom of the viper Vipera nikolskii was chosen as a source of new PLA2s. This black viper, which inhabits the forest steppes of southern Russia and Ukraine and was previously regarded as a form of V. berus, has only recently been established as an independent species. The venom of V. nikolskii is more toxic than that of V. berus (Starkov et al., unpublished results) and possesses neurotoxic activity. Nothing is known about the composition of V. nikolskii venom.
In the present work, the venom of V. nikolskii was fractionated for the first time and two new toxic proteins, VN5-3 and VN4-3, were isolated. These two proteins belong to the basic Asp49 PLA2s and may possess neurotoxity. Here, we report the isolation, characterization and preliminary crystallographic studies of the two new PLA2s.
2. Materials and methods
2.1. Isolation of proteins VN5-3 and VN4-3
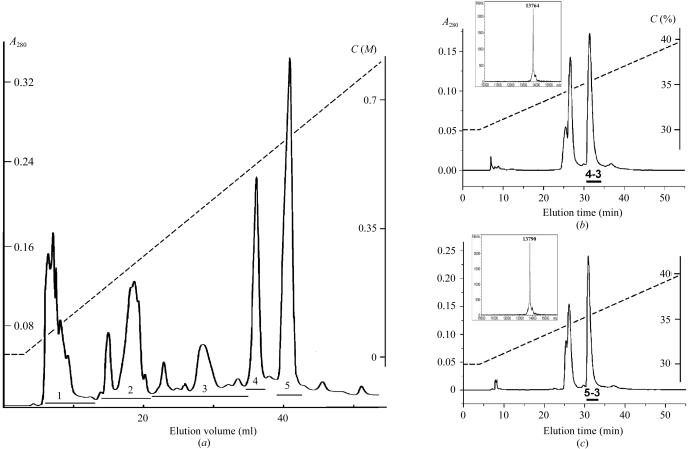
V. nikolskii venom was obtained from vipers kept in captivity at 298–299 K and fed mainly with mice. The snakes were milked by manual gland massage and the collected venom was dried over anhydrous calcium chloride. Dried crude venom was separated on a HEMA BIO 1000 CM column (8 × 250 mm, Tessek, Czech Republic) in an ammonium acetate gradient from 5 mM to 1 M (pH 7.5) at a flow rate of 1 ml min−1 (Fig. 1 ▶ a). Fractions 4 and 5 were further separated on a Vydac C18 column (10 × 250 mm) in a linear gradient of 30–40% acetonitrile in 0.1% trifluoroacetic acid at flow rate of 2 ml min−1 (Figs. 1 ▶ b and 1 ▶ c). The fractions VN4-3 and VN5-3 were obtained from this final separation step.
Figure 1.
Isolation of the proteins. (a) Separation of crude V. nikolskii venom by cation-exchange chromatography on HEMA BIO 1000 CM column (8 × 250 mm) in an ammonium acetate gradient from 5 mM to 1 M in 100 min. Flow rate 1 ml min−1. (b) and (c) Isolation of proteins VN4-3 and VN5-3 by reverse-phase HPLC on a Vydac C18 column (10 × 250 mm). The MALDI mass spectra for VN4-3 and VN5-3 are shown in insets. The dashed lines show the gradients of ammonium acetate (in a) or acetonitrile (in b and c); C are the concentrations of ammonium acetate (a) or acetonitrile (b and c).
2.2. Characterization of proteins
2.2.1. Molecular weight
The mass spectra of isolated proteins were recorded on a Vision 2000 (Thermo BioAnalysis, US) or Reflex III (Bruker Daltonics, Germany) spectrometer using 2,5-dihydroxybenzoic acid and sinapinic acid as matrices. The samples were prepared using the dried droplet technique. The spectra were recorded in positive-ion mode. Insulin from porcine pancreas (5777 Da) and lysozyme from chicken egg white (14 308 Da) were used as external calibrants.
2.2.2. Biological activity
Phospholipase A2 activity was determined by the method described by Radvanyi et al. (1989 ▶) using 1-palmitoyl-2-(10-pyrenyldecanoyl)-sn-glycero-3-phosphorylcholine (Molecular Probes, The Netherlands) as substrate.
Crickets (Gryllus assimilis) with body weight ranging from 0.3 to 0.5 g were used for testing toxicity. Protein solutions in 1–6 µl of water were injected intra-abdominally to the ventral site between the third and fourth segments. Doses of 0.5, 1, 1.5, 2, 4, 6, 8, 10 and 15 nmol per gram of body weight were applied. Five insects were used for each dose. LD50 was calculated using the ED50plus program (v.1.0), developed by M. H. Vargas (from the web page http://www.iner.gob.mx/docs/ed50.htm).
2.3. Crystal growth, X-ray data collection and processing
The proteins VN5-3 and VN4-3 were crystallized by the hanging-drop vapour-diffusion method using Linbro plates, in which 1 µl protein solution was typically mixed with 1 µl reservoir solution and suspended on a cover slip over 0.4 ml reservoir solution. The initial concentration of the protein dissolved in distilled water was about 20 mg ml−1. The two proteins were initially screened with Crystal Screen and Crystal Screen 2 (Hampton Research Co.), but those attempts did not lead to crystals. A simplified screen with higher efficiency (Gao et al., to be published elsewhere) was then used to screen crystallization conditions for the two proteins and crystal seeds were found within one week. By optimization, single crystals suitable for X-ray studies were obtained. Crystallization conditions for protein VN4-3 were obtained by screening and optimization in a manner similar to that used for protein VN5-3.
Data sets for the two proteins were collected at the Beijing Synchrotron Radiation Facility (BSRF), Institute of High Energy Physics, Chinese Academy of Sciences. All the diffraction data were indexed, integrated and scaled using the programs DENZO and SCALEPACK (Otwinowski & Minor, 1996 ▶). The parameters of X-ray diffraction and the data-collection statistics are listed in Table 1 ▶.
Table 1. X-ray diffraction and data-collection statistics.
The values in parentheses refer to the outermost shell.
| VN5-3 | VN4-3 | |
|---|---|---|
| Wavelength (Å) | 0.98 | 1.0 |
| Space group | R32 | R32 |
| Unit-cell parameters (Å, °) | a = b = 76.29, c = 303.35, α = β = 90, γ = 120 | a = b = 76.28, c = 304.39, α = β = 90, γ = 120 |
| No. of molecules in AU | 2 | 2 |
| No. of observed reflections | 83182 | 259886 |
| No. of unique reflections | 7107 | 20434 |
| Resolution (Å) | 30.0–3.0 (3.11–3.0) | 30.0–2.2 (2.25–2.2) |
| Completeness of data (%) | 100 (99.9) | 100 (100) |
| I/σ(I) | 17.7 (5.4) | 25.1 (4.1) |
| Rmerge† (%) | 17.0 (54.3) | 9.8 (49.7) |
R
merge = 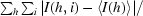
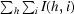 , where I(h, i) is the intensity of ith measurement of reflection h and 〈I(h)〉 is the mean value of I(h, i) for all i measurements.
, where I(h, i) is the intensity of ith measurement of reflection h and 〈I(h)〉 is the mean value of I(h, i) for all i measurements.
3. Results and discussion
Crude V. nikolskii venom was first separated by cation-exchange chromatography on a HEMA BIO 1000 CM column (Fig. 1 ▶ a) and five fractions were obtained. Fractions VN4 and VN5 possessing PLA2 activity were further fractionated by reverse-phase HPLC on a Vydac C18 column. Of the fractions obtained, only VN4-3 (Fig. 1 ▶ b) and VN5-3 (Fig. 1 ▶ c) showed enzymatic activity, the PLA2 activity of fraction VN4-3 being 1.5 times higher than that of fraction VN5-3. The MALDI mass spectrum of the VN4-3 fraction contained only one peak, corresponding to a molecular weight of 13 764 Da. Similarly, there was a single peak of 13 790 Da in the spectrum of fraction VN5-3. The observed difference in molecular weights clearly indicates that VN4-3 and VN5-3 are different proteins.
According to partial amino-acid sequences (Fig. 2 ▶) of the enzymes determined by combination of Edman degradation and MALDI mass spectrometry (the experimental details will be published elsewhere), the two proteins contain an aspartic acid residue at position 49 and thus belong to the group of Asp49 PLA2s. The amino-acid sequences indicate that the enzymes are highly homologous to the basic subunits of heterodimeric neurotoxin vipoxin (B chain) from V. ammodites ammodites (Mancheva et al., 1987 ▶), PLA2-I (subunit B) from V. aspis zinnikeri (Komori et al., 1996 ▶) and the neurotoxin (subunit Cb II) from Pseudocerastes fieldi (Francis et al., 1995 ▶). The differences in molecular weights (Fig. 2 ▶) between known PLA2s on the one hand and proteins isolated from V. nikolskii venom on the other suggest that the amino-acid sequences of VN4-3 and VN5-3 differ from those of all known enzymes. Based on their chromatographic behaviour (dissociation into two subunits under acidic conditions; Figs. 1 ▶ b and 1 ▶ c), the difference in enzymatic activity (to be published elsewhere) and the amino-acid sequence similarity to the basic subunit of the neurotoxins, we suggest that fractions 4 and 5 (Fig. 1 ▶ a) contain heterodimeric neurotoxic PLA2s. Isolation of VN4-3 and VN5-3 is the first indication of the presence of two isoforms of a heterodimeric neurotoxin in one species.
Figure 2.
Partial amino-acid sequences of proteins VN4-3 and VN5-3 in comparison with the sequences of several basic subunits of heterodimeric phospholipases A2 from viper venoms. gi:129419 is vipoxin non-toxic component from V. ammodytes meridionalis, gi:2914537 is chain B of the vipoxin complex from V. ammodytes meridionalis, gi:1709548 is PLA2-I complex B chain from V. aspis zinnikeri and gi:1345181 is Cb II heterodimeric neurotoxin basic subunit from P. fieldi.
The toxicity of VN5-3 and VN4-3 has been determined using insects, which have been shown to be a good substitution for mice in acute toxicity tests for PLA2s (Osipov et al., 2002 ▶). Both proteins are toxic to crickets. The LD50 is about 3 nmol g−1, being very close to the value determined previously for cobra PLA2 (Osipov et al., 2002 ▶).
The crystallization conditions of the protein VN5-3 were defined after optimization. The reservoir solution contained 15% PEG 4K, 0.2 M Li2SO4, 0.1 M sodium cacodylate pH 6.5, 0.1% sodium azide and the drop contained 1 µl 20 mg ml−1 protein VN5-3, 1 µl reservoir solution (15% PEG 4K, 0.2 M Li2SO4, 0.1 M sodium cacodylate pH 6.5, 0.1% sodium azide), 0.2 µl 0.9 mM Triton X-100 and 0.2 µl MPD. The plates were incubated at 291 K. Crystals of dimensions 0.1 × 0.1 × 0.05 mm were obtained after one week (Fig. 3 ▶ a). To grow crystals of VN4-3 suitable for X-ray studies, the reservoir solution contained 15% PEG 4K, 0.2 M Li2SO4, 0.1 M sodium cacodylate pH 6.5, 0.1% sodium azide and the hanging drop contained 1 µl 20 mg ml−1 VN4-3, 1 µl reservoir solution (15% PEG 4K, 0.2 M Li2SO4, 0.1 M sodium cacodylate pH 6.5, 0.1% sodium azide) and 0.4 µl 0.9 mM Triton X-100. The crystal dimensions reached 0.1 × 0.2 × 0.2 mm after more than one week (Fig. 3 ▶ b). The crystals could be obtained using a wide range of concentrations of Triton X-100 (0.15–0.3 mM).
Figure 3.
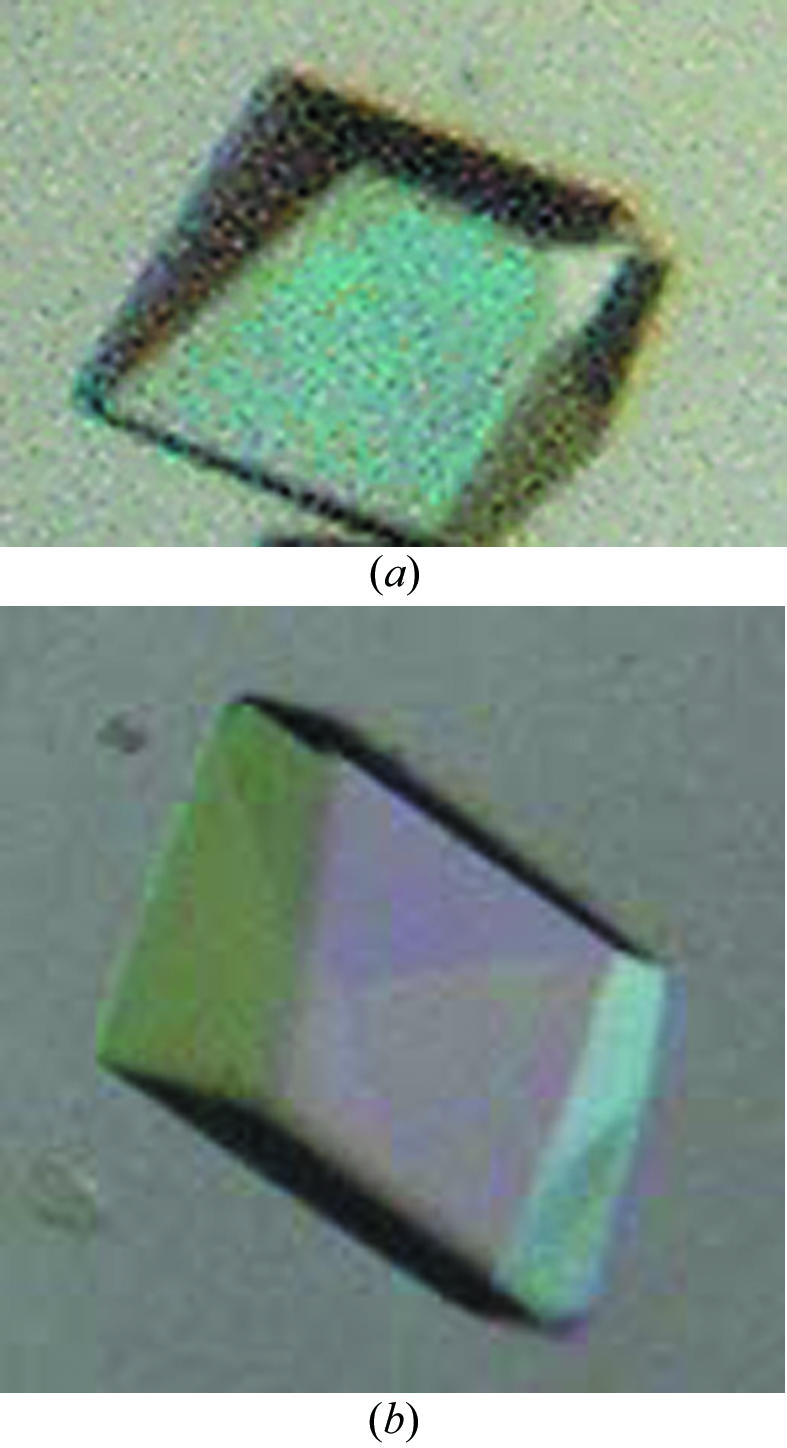
Crystal photos of proteins VN5-3 (a) and VN4-3 (b).
Statistics of data processing are given in Table 1 ▶. The R merge value [17.0 (54.3)] for VN5-3 crystals is quite high. This may be caused by the choice of a high resolution limit and by possible damage to the crystal from the synchrotron radiation. The R merge value, particularly for the highest resolution shell, could be improved by reducing the resolution limit, e.g. from 3.0 to 3.1 Å, or by the removal of some data that was collected late from the data processing. This indicates that the quality of the VN4-3 crystals is much better than that of the VN5-3 crystals.
The space group of the VN5-3 crystals is R32, with unit-cell parameters a = b = 76.29, c = 303.35 Å, a Matthews coefficient (Matthews, 1968 ▶) of 3.1 Å3 Da−1 and a solvent content of 59.8% assuming that the asymmetric unit contains two molecules. Moreover, a calculation of the self-rotation function using the program POLARRFN (Collaborative Computational Project, Number 4, 1994 ▶) indicated the presence of a non-crystallographic twofold axis. This suggests that the crystals have two molecules in the asymmetric unit that are related by the non-crystallographic twofold axis.
The result of data processing for VN4-3 crystals is similar to that for the VN5-3 crystal. The space group is R32, with unit-cell parameters a = b = 76.28, c = 304.39 Å (Table 1 ▶). The self-rotation function calculation for the VN4-3 crystal using the program POLARRFN also indicates the presence of a non-crystallographic twofold axis. The crystallization conditions, space group and unit cells are about the same for the two proteins, probably owing to similarity in the three-dimension structure and molecular packing. The preliminary studies given above suggest the presence of two molecules in the asymmetric units of both crystals. The two molecules form a homodimer or exist as two independent monomers. It is hoped that new information will be obtained from comparisons of the structures of these new venom PLA2s with known structures of other neurotoxic and non-neurotoxic venom PLA2s, in particular from comparisons with the structures of the vipoxin B subunit both in heterodimeric and monomeric forms (Banumathi et al., 2001 ▶; Georgieva et al., 2004 ▶). As mentioned above, the vipoxin B subunit is highly homologous to protein VN5-3 from V. nikolskii.
Acknowledgments
The authors are grateful to Professor A. Tu for help in establishing collaboration between the Chinese and Russian research groups, to Dr Yu-hui Dong for his help in diffraction data collection at BSRF, China, to Dr A. M. Surin for help in the determination of the enzymatic activity and to Mrs T. V. Andreeva for excellent technical assistance. This work was supported by the Russian Foundation for Basic Research (grant Nos. 02-04-39014 and 03-04-48496), the Natural Science Foundation of China (grant Nos. 30311120048 and 30411120080) and the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-SW-322).
References
- Arni, R. K., Fontes, M. R., Barberato, C., Gutierrez, J. M., Diaz, C. & Ward, R. J. (1999). Arch. Biochem. Biophys.366, 177–182. [DOI] [PubMed] [Google Scholar]
- Banumathi, S., Rajashankar, K. R., Notzel, C., Aleksiev, B., Singh, T. P., Genov, N. & Betzel, C. (2001). Acta Cryst. D57, 1552–1559. [DOI] [PubMed] [Google Scholar]
- Brunie, S., Bolin, J., Gewirth, D. & Sigler, P. B. (1985). J. Biol. Chem.260, 9742–9749. [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760– 763. [Google Scholar]
- Dorandeu, F., Pernot-Marino, I., Veyret, J., Perrichon, C. & Lallement, G. (1998). J. Neurosci. Res.54, 848–862. [DOI] [PubMed] [Google Scholar]
- Francis, B., Bdolah, A. & Kaiser, I. I. (1995). Toxicon, 33, 863–874. [DOI] [PubMed] [Google Scholar]
- Georgieva, D. N., Rypniewski, W., Gabdoulkhakov, A., Genov, N. & Betzela, C. (2004). Biochem. Biophys. Res. Commun.319, 1314–1321. [DOI] [PubMed] [Google Scholar]
- Gubensek, F., Ritonja, A., Zupan, J. & Turk, V. (1980). Period. Biol.82, 443–447.
- Komori, Y., Masuda, K., Nikai, T. & Sugihara, H. (1996). Arch. Biochem. Biophys.327, 303–307. [DOI] [PubMed] [Google Scholar]
- Kini, R. M. (2003). Toxicon, 42, 827–840. [DOI] [PubMed] [Google Scholar]
- Krizaj, I. & Gubensek, F. (2000). Biochemie, 82, 807–814. [DOI] [PubMed]
- Lee, C. Y., Tsai, M. C., Chen, Y. M., Ritonja, A. & Gubensek, F. (1984). Arch. Int. Pharmacodyn. Ther.268, 313–324. [PubMed] [Google Scholar]
- Mancheva, I., Kleinschmidt, T., Aleksiev, B. & Braunitzer, G. (1987). Biol. Chem. Hoppe-Seyler, 368, 343–352. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Montecucco, C. & Rossetto, O. (2000). Trends Biochem. Sci.25, 266–270. [DOI] [PubMed] [Google Scholar]
- Osipov, A. V., Starkov, V. G. & Utkin, Y. N. (2002). Toxicon, 40, 1507–1509. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1996). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Radvanyi, F., Jordan, L., Russo-Marie, F. & Bon, C. (1989). Anal. Biochem.177, 103–109. [DOI] [PubMed] [Google Scholar]
- Six, D. A. & Dennis, E. A. (2000). Biochim. Biophys. Acta, 1488, 1–19. [DOI] [PubMed] [Google Scholar]
- Tang, L., Zhou, Y. C. & Lin, Z. J. (1998). J. Mol. Biol.282, 1–11. [DOI] [PubMed] [Google Scholar]
- Valentin, E. & Lambeau, G. (2000). Biochim. Biophys. Acta, 1488, 59–70. [DOI] [PubMed] [Google Scholar]
- Wang, X. Q., Yang, J., Gui, L. L., Lin, Z. J., Chen, Y. C. & Zhou, Y. C. (1996). J. Mol. Biol.255, 669–676. [DOI] [PubMed] [Google Scholar]
- Ward, R. J., De Azevedo, W. F. & Arni, R. K. (1998). Toxicon, 36, 1623–33. [DOI] [PubMed] [Google Scholar]
- Westerlund, B., Nordlund, P., Uhlin, U., Eaker, D. & Eklund, H. (1992). FEBS Lett.301, 159–164. [DOI] [PubMed] [Google Scholar]