Mycobacterial PimA is an essential enzyme that catalyses the first mannosylation step in phosphatidyl-myo-inositol mannoside (PIM) biosynthesis. Crystals of the enzyme from M. smegmatis, obtained in the presence of GDP and myo-inositol, are orthorhombic (P212121) and diffract X-rays to 2.4 Å resolution.
Keywords: GT4 glycosyltransferase, tuberculosis, phosphatidylinositol mannoside, PIM, mycobacteria
Abstract
Phosphatidylinositol mannosyltransferase (PimA) is an essential enzyme for mycobacterial growth that catalyses the first mannosylation step in phosphatidyl-myo-inositol mannoside (PIM) biosynthesis. The enzyme belongs to the large GT4 family of glycosyltransferases, for which no structure is currently available. Recombinant purified PimA from Mycobacterium smegmatis has been crystallized in the presence of GDP and myo-inositol. The crystals belong to space group P212121, with unit-cell parameters a = 37.2, b = 72.4, c = 138.2 Å, and diffract to 2.4 Å resolution.
1. Introduction
Phosphatidyl-myo-inositol mannoside (PIM), lipomannan (LM) and lipoarabinomannan (LAM) are glycolipids/lipoglycans interspersed in the cell wall of mycobacteria (Brennan & Nikaido, 1995 ▶). Linked to the hydrophobic cell envelope through their phosphatidyl-myo-inositol anchor, these molecules play important roles both in mycobacterial physiology and in the modulation of the host immune response in the course of tuberculosis and leprosy (Briken et al., 2004 ▶).
PimA (MW 43.3 kDa) is an α-retaining glycosyltransferase that catalyses the first mannosylation step in PIM biosynthesis. The enzyme transfers a Manp residue from GDP-Man to the 2-position of the myo-inositol ring of phosphatidyl-myo-inositol (PI) to form phosphatidyl-myo-inositol monomannoside (PIM1; Kordulakova et al., 2002 ▶). The pimA gene is the fourth of a cluster of five genes conserved in all known mycobacterial genomes (Rv2613c–Rv2609c in Mycobacterium tuberculosis) that are involved in the early steps of PIM biosynthesis (Cole et al., 1998a ▶,b ▶; Jackson et al., 2000 ▶). The first gene of the cluster encodes a protein of unknown function, the second and third, respectively, encode the phosphatidyl-myo-inositol synthase PgsA (Jackson et al., 2000 ▶) and an acyltransferase responsible for the acylation of the Manp residue linked to the 2-position of the inositol ring in PIM1 and PIM2 molecules (Kordulakova et al., 2003 ▶), and the last encodes a putative GDP-Man hydrolase (Frick et al., 1995 ▶). Both PgsA and PimA have been shown to be essential for the growth of M. smegmatis; the acyltransferase Rv2611c mutants of M. smegmatis exhibit reduced rates of acylation of PIM molecules and show severe growth defects (Kordulakova et al., 2003 ▶).
Subsequent mannosylation steps in PIM biosynthesis are catalysed by PimB, which transfers a second Manp residue to the 6-position of the myo-inositol ring of PIM1 to form PIM2 (Schaeffer et al., 1999 ▶), and PimC, which transfers the third Manp residue to the growing molecule (Kremer et al., 2002 ▶). In contrast to PimA, however, PimB and PimC appear to be non-essential genes, suggesting the existence of alternative pathways for the synthesis of PIM2 and higher PIMs (Schaeffer et al., 1999 ▶; Kremer et al., 2002 ▶). The three mannosyltransferases PimA, PimB and PimC belong to the GT4 family of glycosyltransferases (see http://afmb.cnrs-mrs.fr/CAZY/index.html; Coutinho et al., 2003 ▶; Abdian et al., 2000 ▶), for which there is no structure available, and their amino-acid sequences contains the GPGTF (glycogen phosphorylase/glycosyl transferase) motif (Wrabl & Grishin, 2001 ▶), a signature present in enzymes of the GT-B fold superfamily (Coutinho et al., 2003 ▶).
The involvement of PimA in an essential pathway restricted to mycobacteria and a few other actinomycetes makes this enzyme an attractive drug target for anti-tuberculosis chemotherapy. The crystallographic characterization of PimA will shed light on the catalytic mechanism of the GT4 family of glycosyltransferases and will provide a structural framework for the development of specific inhibitors.
2. Results and discussion
2.1. Expression and purification
Escherichia coli BL21(DE3)pLysS cells transformed with pET-pimA (Kordulakova et al., 2002 ▶) were grown in 1000 ml of 2×YT medium supplemented with 100 µg ml−1 ampicillin and 34 µg ml−1 chloramphenicol at 310 K. When the culture reached an A 600 value of 0.6, PimA expression was induced by adding 0.5 mM isopropyl β-thiogalactopyranoside (IPTG). After 12 h at 293 K, cells were harvested and resuspended in 40 ml 25 mM Tris–HCl pH 7.5 (solution A) containing protease inhibitors (Complete EDTA-free, Roche). Cells were then disrupted by three compression–decompression cycles in a French press and the suspension was centrifuged for 20 min at 15 000g. The supernatant was subjected to Ni2+-affinity chromatography using a HiTrap Chelating column (5 ml, Amersham Biosciences) equilibrated with 25 mM Tris–HCl pH 7.5, 500 mM NaCl (solution B). The column was washed with solution B until no absorbance at 280 nm was detected. Elution was performed with a linear gradient of 0–250 mM imidazole in 50 ml solution B at 2 ml min−1. Fractions showing enzymatic activity were pooled and dialyzed overnight against solution A containing 5 mM dithiothreitol (DTT; solution C). The dialyzed solution was filtered through a Millipore 0.22 µm filter and applied onto a Mono Q HR 5/5 (Amersham Biosciences) column equilibrated in solution C. The enzyme was eluted at 200 mM NaCl with a linear gradient of 0–500 mM NaCl in 30 ml solution C at 1 ml min−1. The resulting preparation displayed a single protein band when run on 10% SDS–PAGE stained with Coomassie brilliant blue. The purified enzyme was stored at 193 K for further use in crystallization trials.
2.2. Crystallization
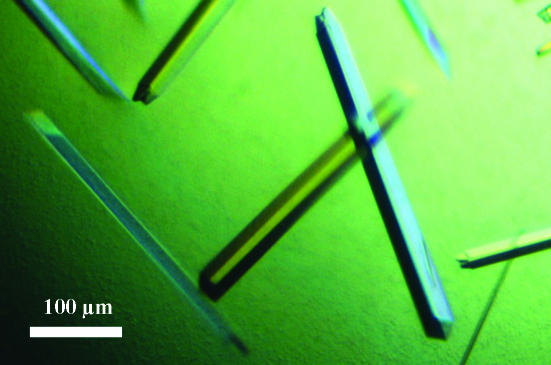
The protein was concentrated to 10 mg ml−1 using a Centricon YM-10 (Millipore) in 10 mM Tris–HCl pH 7.5 and 1 mM DTT. A broad screening of crystallization conditions using Crystal Screens I and II (Hampton Research), Structure Screens I and II (Molecular Dimensions Ltd) and JBScreens 1–10 (Jena Biosciences) was performed using a Cartesian Technologies workstation by the sitting-drop vapour-diffusion method. Crystallization conditions resulting in small crystals were manually reproduced and further optimized in terms of pH, precipitant concentration and drop volume using the hanging-drop vapour-diffusion method. All experiments were carried out at 291 K. The best crystals were obtained by mixing 8 µl protein (10 mg ml−1) preincubated with 10 mM guanosine 5′-diphosphate (GDP, Sigma) and 10 mM myo-inositol (Sigma) with 2 µl well solution consisting of 18%(w/v) PEG 8000, 200 mM calcium acetate and 50 mM HEPES pH 7.5. Crystals appeared after 2–3 d and grew as rods, reaching 0.4 × 0.06 × 0.06 mm (Fig. 1 ▶). Prior to data collection, crystals were transferred to a cryoprotectant solution [25%(v/v) glycerol in the well solution] for 1 min and flash-frozen in liquid nitrogen.
Figure 1.
Crystals of PimA from M. smegmatis in the presence of GDP and myo-inositol grown in 16–18%(w/v) PEG 8000, 200 mM calcium acetate and 50 mM HEPES pH 7.5.
2.3. Preliminary crystallographic characterization
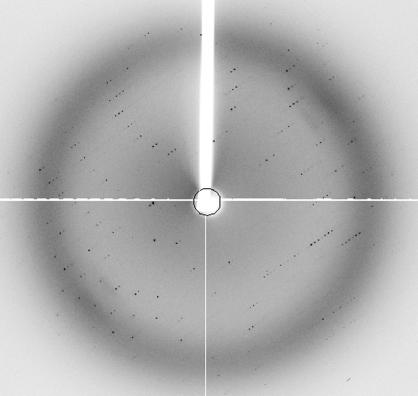
Crystals of PimA belong to the orthorhombic space group P212121 and have one molecule per asymmetric unit, corresponding to a Matthews coefficient of 2.2 Å3 Da−1 and a solvent content of 43%. X-ray diffraction data from a single crystal (Fig. 2 ▶) were collected at 2.4 Å on the ID29 beamline (λ = 0.9330 Å) at the ESRF (Grenoble, France) equipped with an ADSC Q210 two-dimensional detector and were processed with the programs MOSFLM v.6.2.3, SCALA v.3.2.0-3 and TRUNCATE from the CCP4 program suite (Collaborative Computational Project, Number 4, 1994 ▶). Data-collection statistics are shown in Table 1 ▶. Preliminary attempts to determine the crystal structure using molecular-replacement methods with other known GT-B glycosyltransferases as probe models were unsuccessful. The selenomethionine-labelled protein is thus being produced for structure determination using single- or multiple-wavelength anomalous diffraction (SAD/MAD) methods.
Figure 2.
X-ray diffraction image of a M. smegmatis PimA crystal.
Table 1. Data-collection statistics.
Values in parentheses are for the last resolution shell.
| Space group | P212121 |
| Crystal system | Orthorhombic |
| Unit-cell parameters (Å) | a = 37.2, b = 72.4, c = 138.2 |
| Matthews coefficient (Å3 Da−1) | 2.2 |
| Molecules per AU | 1 |
| Resolution range (Å) | 50–2.4 (2.53–2.4) |
| Mosaicity (°) | 0.66 |
| Total observations | 51389 |
| Unique reflections | 17229 |
| Completeness (%) | 99.7 (100) |
| Rsym† (%) | 5.8 (31.3) |
| Multiplicity | 3.4 |
| 〈I/σ(I)〉 (top shell) | 18.5 (2.4) |
R
sym = 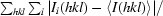
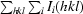 .
.
Acknowledgments
We thank A. Haouz (PF6, Institut Pasteur) and W. Shepard (ID29, ESRF, Grenoble, France) for help with data collection. This work was partially supported by the Institut Pasteur (GPH-5), the Ministere de la Recherche, France (contract 01-B-0095) and the European Commission, contracts QLG2-CT-2002-00988 (SPINE) and QLK2-CT-2001-02018 (X-TB).
References
- Abdian, P. L., Lellouch, A. C., Gautier, C., Ielpi, L. & Geremia, R. A. (2000). J. Biol. Chem.275, 40568–40575. [DOI] [PubMed] [Google Scholar]
- Brennan, P. J. & Nikaido, H. (1995). Annu. Rev. Biochem.64, 29–63. [DOI] [PubMed] [Google Scholar]
- Briken, V., Porcelli, S. A., Besra, G. S. & Kremer, L. (2004). Mol. Microbiol.53, 391–403. [DOI] [PubMed] [Google Scholar]
- Cole, S. T. et al. (1998a). Nature (London), 393, 537–544. [Google Scholar]
- Cole, S. T. et al. (1998b). Nature (London), 396, 190.
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Coutinho, P. M., Deleury, E., Davies, G. J. & Henrissat, B. (2003). J. Mol. Biol.328, 307–317. [DOI] [PubMed] [Google Scholar]
- Frick, D. N., Townsend, B. D. & Bessman, M. J. (1995). J. Biol. Chem.270, 24086–24091. [DOI] [PubMed] [Google Scholar]
- Jackson, M., Crick, D. C. & Brennan, P. J. (2000). J. Biol. Chem.275, 30092–30099. [DOI] [PubMed] [Google Scholar]
- Kordulakova, J., Gilleron, M., Mikusova, K., Puzo, G., Brennan, P. J., Gicquel, B. & Jackson, M. (2002). J. Biol. Chem.277, 31335–31344. [DOI] [PubMed] [Google Scholar]
- Kordulakova, J., Gilleron, M., Puzo, G., Brennan, P. J., Gicquel, B., Mikusova, K. & Jackson, M. (2003). J. Biol. Chem.278, 36285–36295. [DOI] [PubMed] [Google Scholar]
- Kremer, L., Gurcha, S. S., Bifani, P., Hitchen, P. G., Baulard, A., Morris, H. R., Dell, A., Brennan, P. J. & Besra, G. S. (2002). Biochem. J.363, 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer, M. L., Khoo, K. H., Besra, G. S., Chatterjee, D., Brennan, P. J., Belisle, J. T. & Inamine, J. M. (1999). J. Biol. Chem.274, 31625–31631. [DOI] [PubMed] [Google Scholar]
- Wrabl, J. O. & Grishin, N. V. (2001). J. Mol. Biol.314, 365–374. [DOI] [PubMed] [Google Scholar]