Crystallization of human enhancer of rudimentary homologue protein.
Keywords: transcription, pyrimidine biosynthesis, HNF1, PCD, DCoH
Abstract
The human gene coding for the enhancer of rudimentary homologue (ERH) protein was overexpressed in Escherichia coli. The ERH protein was purified by anion-exchange, hydrophobic interaction and gel-filtration chromatography. Well diffracting single crystals were obtained by the vapour-diffusion method in hanging drops. The crystals belong to the trigonal space group P3121 or its enantiomorph P3221, with unit-cell parameters a = b = 53.74, c = 67.45 Å, α = β = 90, γ = 120°. They diffract to at least 1.75 Å. A selenomethionine derivative of the protein was prepared and crystallized for multiwavelength anomalous diffraction (MAD) phasing.
1. Introduction
Regulation of gene expression at the transcriptional level involves a well controlled balance between transcription factors and their positive and negative cofactors (reviewed in Kadonaga, 2004 ▶; Martinez, 2002 ▶). The enhancer of rudimentary homologue (ERH) gene encodes the ERH protein, which is evolutionarily highly conserved (Gelsthorpe et al., 1997 ▶; Wojcik et al., 1994 ▶). ERH was first found as a genetic interactor of the Drosophila rudimentary gene, which encodes a protein with enzymatic activities in the pyrimidine-biosynthesis pathway (Jarry & Falk, 1974 ▶; Norby, 1973 ▶; Rawls & Fristrom, 1975 ▶). Subsequently, RNAi experiments in Caenorhabditis elegans showed that disruption of ERH resulted in an embryonic lethal phenotype (Gonczy et al., 2000 ▶; http://worm-srv1.mpi-cbg.de/dbScreen/). Thus, ERH was believed to have evolutionarily conserved functions associated with a basic cellular process. Recently, in a two-hybrid study aiming at identifying protein interactors with the dimerization cofactor of HNF1/pterin-4-carbinolamine dehydratase (DCoH/PCD), Pogge von Strandmann et al. (2001 ▶) found that the Xenopus ERH may interfere with HNF-1-dependent gene regulation through its interaction with DCoH/PCD and proposed ERH to be a cell-type-specific transcriptional repressor. Here, we describe the details of the overexpression, purification and crystallization of human ERH, as well as the preliminary crystallographic data.
2. Methods, results and discussion
2.1. Cloning, expression and purification
The coding region of an ERH cDNA was PCR-amplified from a HeLa cDNA library. The PCR was performed with CCCCGAATTCCATATGTCTCACACCATTTTGCTGGTA and CCCCTCGAGGATCCTTATTTCCCAGCCTGTTGGGCC as 5′ and 3′ primers, respectively. The PCR product was cloned into the pBluescript II SK vector via an EcoRI and an XhoI site introduced by the 5′ and 3′ primer, respectively, to create pBlueERH. The ERH coding region was then released from pBlueERH with NdeI and XhoI and cloned into the vector pET29b to create pET29ERH. The insert of pET29ERH was confirmed by sequencing.
Escherichia coli BL21(DE3) cells (Novagen, Madison, WI, USA) transformed with pET29ERH were grown in 1 l of LB medium containing 50 mg l−1 kanamycin at 310 K. At an OD600 of 1.2, expression of ERH was induced by adding IPTG to the medium to a final concentration of 1 mM. The cells were collected by centrifugation after 4 h induction. An SDS–PAGE analysis of the total cell lysate showed an intense band with the expected molecular weight for ERH.
For purification, the cell pellet was resuspended in 50 ml buffer A (10 mM phosphate pH 7.9, 5 mM DTT) plus a protease-inhibitor cocktail. The cocktail contained aprotinin, antipain, leupeptin and pepstatin. Their final concentrations were 100 nM, 50 µM, 50 µM and 0.5 µg ml−1, respectively. Cells were sonicated for 10 min in a beaker surrounded by a mixture of ice and water. The cell extract was subjected to centrifugation at 25 000g for 30 min at 277 K. The supernatant was filtered with 0.20 µm syringe filters and the sample was loaded onto a Source 15Q (Pharmacia, now GE Healthcare, Piscataway, NJ, USA) ion-exchange column with a bed volume of 10 ml, pre-equilibrated with buffer A. The column was washed with three bed volumes of buffer A and then run with buffer A and a linear 0–0.5 M NaCl gradient (five bed volumes) with a flow rate of 1 ml min−1. The ERH protein eluted at 0.12 M NaCl. ERH-containing fractions were collected and loaded onto two coupled 5 ml Hitrap phenyl HP columns (Pharmacia) after adding an equal volume of 2 M (NH4)2SO4. After washing with five bed volumes of buffer A plus 1 M (NH4)2SO4, ERH was eluted with a linear 1–0 M (NH4)2SO4 gradient (20 bed volumes) with a flow rate of 1 ml min−1. ERH eluted at 0.26 M (NH4)2SO4 and the ERH-containing fractions were concentrated to ∼5 ml using an Ultrafree-5K centrifugal filter device (Millipore, MA, USA). The concentrated sample was loaded onto a Superdex75 gel-filtration column (diameter 26 mm, height 65 cm). The column was pre-equilibrated and eluted with buffer A at a flow rate of 1 ml min−1. ERH eluted from the gel column with an apparent molecular weight close to that of an ERH tetramer, as assessed by comparison with gel-filtration runs of standard proteins. Gel filtration of ERH was also performed at lower pH using the above Superdex75 column. At pH 5.6, at which ERH was crystallized (see below), ERH eluted from the gel column with an apparent molecular weight close to that of an ERH monomer. This procedure yielded approximately 20 mg of homogeneous ERH from a 1 l culture.
All chromatographic steps were carried out at 277 K using an FPLC system (Pharmacia). The molecular weight of the pure protein was determined to be 12 127 Da by electrospray mass spectrometry, consistent with the ERH protein lacking the N-terminal methionine.
2.2. Crystallization
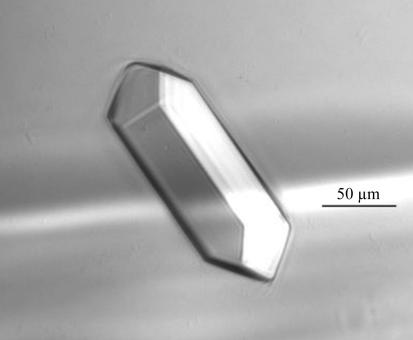
The pure protein was desalted and concentrated to 90 mg ml−1 in Milli-Q H2O using an Ultrafree-5K centrifugal filter device. The sample was filtered with a 0.2 µm filter and crystallization experiments were performed at 277 K in Linbro plates using the hanging-drop vapour-diffusion technique. An initial crystallization screen was performed using Hampton Research Crystal Screen and Crystal Screen Lite kits. 1 µl protein sample was mixed with 1 µl reservoir solution and sealed against 0.5 ml reservoir solution. All reservoir solutions contained 5 mM DTT and 0.02%(w/v) sodium azide. Several crystals appeared in the well containing solution No. 40 of the Crystal Screen Lite kit [0.1 M trisodium citrate dihydrate pH 5.6, 10%(v/v) isopropanol, 10%(w/v) polyethylene glycol 4000] in 3 d. Single crystals of dimensions ∼0.2 × 0.05 × 0.05 mm were obtained within a month using this condition (Fig. 1 ▶). No crystals were obtained at 295 K and crystals grown at 277 K redissolved in 10 min after being moved to 295 K. Currently, we are still screening for conditions to grow ERH crystals at higher pH values where ERH would assume a tetramer in solution.
Figure 1.
Crystal of ERH obtained by vapour diffusion in hanging drops.
2.3. X-ray diffraction experiments and crystal characterization
To collect data at low temperature, single crystals were transferred with nylon loops to a cryoprotectant, which was a mixture of an equal volume of the reservoir solution and 50%(v/v) ethylene glycol solution. The crystals were then picked up with nylon loops and flash-frozen in liquid nitrogen. Data were collected at IMCA-CAT beamline 17-ID (Advanced Photon Source, US Argonne National Laboratory) equipped with an ADSC Quantum 210 detector. A complete data set of 1° frames with 4 s exposures was collected at 12 398 eV. The ERH crystals diffracted to 1.75 Å and the data were processed with the HKL2000 suite of programs (Otwinowski & Minor, 1997 ▶) and X-GEN (Howard, 2000 ▶), revealing a trigonal crystal system with unit-cell parameters a = b = 53.74, c = 67.45 Å, α = β = 90, γ = 120°. From systematic absences in specific reflections in the diffraction, the space group was determined to be P3121 or its enantiomorph P3221 (Table 1 ▶). Assuming one molecule in the asymmetric unit, the Matthews coefficient V M was 2.32 Å3 Da−1, corresponding to a solvent content of 47%.
Table 1. X-ray data-collection statistics.
Values in parentheses are for the outer shell.
| Resolution (Å) | 50–1.75 (1.81–1.75) |
| Wavelength (Å) | 1.0 |
| Space group | P3121 or P3221 |
| Unit-cell parameters | |
| a (Å) | 53.74 |
| b (Å) | 53.74 |
| c (Å) | 67.45 |
| α (°) | 90 |
| β (°) | 90 |
| γ (°) | 120 |
| Data-collection temperature (K) | 110 |
| No. of observed reflections | 124880 |
| No. of unique reflections | 11671 |
| Completeness (%) | 99.9 (100) |
| 〈I/σ(I)〉 | 46.7 (9.7) |
| Rsym† (%) | 4.5 (25.1) |
R
sym = 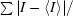
 .
.
2.4. Purification and crystallization of selenomethionine ERH
To facilitate multiwavelength anomalous diffraction (MAD) phasing, the met− E. coli strain B834(DE3) (Novagen, Madison, WI, USA) transformed with pET29ERH was grown in 1 l M9 medium supplemented with 0.15 mM thiamine and 50 mg l-methionine at 310 K in the presence of 50 mg l−1 kanamycin. When the OD600 of the culture reached 1.0, cells were spun down by centrifugation and resuspended in 1 l of the above medium devoid of methionine. After 30 min, 15 mg l-selenomethionine was added to the culture. After an additional 30 min, induction of selenomethionine ERH expression was initiated by adding IPTG to a final concentration of 1 mM. Induction was allowed to continue for 4 h. Purification of the selenomethionine ERH was identical to that of the native protein. The incorporation of selenomethionine was confirmed by electrospray mass spectrometry. Assuming equal efficiency of selenomethionine incorporation at all three sites, the success rate of selenomethionine labelling was determined to be 70%. Selenomethionine ERH crystals were grown under conditions identical to those for growing native ERH crystals. Selenomethionine ERH single crystals were obtained within a month that were morphologically identical and grew to the same size as the native ERH crystals. Selenomethionine ERH crystals diffracted equally well and had the same unit-cell parameters as crystals of natural ERH. For selenomethionine ERH, a complete data set has been collected at the IMCA-CAT beamline 17-ID and attempts to solve the structure of ERH by multiwavelength anomalous diffraction (MAD) methods are currently under way.
Acknowledgments
Use of the IMCA-CAT beamline is supported by the companies of the Industrial Macromolecular Crystallography Association through a contract with Illinois Institute of Technology. Use of the Advanced Photon Source was supported by US DOE, Office of Science, Office of Basic Energy Sciences under Contract No. W-31-109-Eng-38.
References
- Gelsthorpe, M., Pulumati, M., McCallum, C., Dang-Vu, K. & Tsubota, S. I. (1997). Gene, 186, 189–195. [DOI] [PubMed] [Google Scholar]
- Gonczy, P. et al. (2000). Nature (London), 408, 331–336. [Google Scholar]
- Howard, A. J. (2000). In Crystallographic Computing 7, edited by P. E. Bourne & K. D. Watenpaugh. Oxford University Press.
- Jarry, B. & Falk, D. (1974). Mol. Gen. Genet.135, 113–122. [DOI] [PubMed] [Google Scholar]
- Kadonaga, J. T. (2004). Cell, 116, 247–257. [DOI] [PubMed] [Google Scholar]
- Martinez, E. (2002). Plant Mol. Biol.50, 925–947. [DOI] [PubMed] [Google Scholar]
- Norby, S. (1973). Hereditas, 73, 11–16. [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Pogge von Strandmann, E., Senkel, S. & Ryffel, G. U. (2001). Biol. Chem.382, 1379–1385. [DOI] [PubMed] [Google Scholar]
- Rawls, J. M. & Fristrom, J. W. (1975). Nature (London), 255, 738–740. [DOI] [PubMed] [Google Scholar]
- Wojcik, E., Murphy, A. M., Fares, H., Dang-Vu, K. & Tsubota, S. I. (1994). Genetics, 138, 1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]