The three-dimensional structure of C. freundii l-methionine γ-lyase has been determined at 1.9 Å resolution.
Keywords: l-methionine γ-lyase, pyridoxal 5′-phosphate, l-methionine, Citrobacter freundii
Abstract
l-Methionine γ-lyase (MGL) is a pyridoxal 5′-phosphate (PLP) dependent enzyme that catalyzes γ-elimination of l-methionine. The crystal structure of MGL from Citrobacter freundii has been determined at 1.9 Å resolution. The spatial fold of the protein is similar to those of MGLs from Pseudomonas putida and Trichomonas vaginalis. The comparison of these structures revealed that there are differences in PLP-binding residues and positioning of the surrounding flexible loops.
1. Introduction
l-Methionine γ-lyase (MGL; EC 4.4.1.11) catalyzes PLP-dependent γ-elimination and γ-replacement reactions of l-methionine and its derivatives as well as β-elimination and β-replacement reactions of l-cysteine and S-substituted l-cysteines (Tanaka et al., 1977 ▶, 1985 ▶). MGL has been isolated from a number of bacteria, including Pseudomonas putida, Aeromonas sp., Clostridium sporogenes, P. taetrolens and Brevibacterium linens, and from the primitive protozoa Entamoeba hystolytica and Trichomonas vaginalis. There has been increasing interest in this enzyme as methionine-dependency has been reported in cancer cell lines and primary tumours. The enzyme has been found to be an effective anti-tumour agent in vitro and in vivo and to be of potential value in the treatment of Parkinson’s disease, arteriosclerosis, aging and obesity (Hoffman, 1997 ▶). Sulfur amino-acid metabolism in bacteria is not yet fully understood and it is likely that many bacteria possess MGL. Thus, inhibitors of this enzyme could ultimately prove to be effective against pathogens. Moreover, since mammals apparently do not possess MGL, the enzyme is a promising target for anti-trichomonad and anti-entamoeba chemotherapy.
Despite the potential importance of MGL in medicine, the mechanism of the enzyme has not been well studied. Recently, crystal structures of MGL from P. putida (Motoshima et al., 2000 ▶; PDB codes http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1gc0, http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1ukj and http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1pg8) and from T. vaginalis (PDB codes http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1e5f and http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1e5e) have been determined. This opens a route for study of the structure–functional basis of the catalysis of the γ- and β-elimination reactions that are catalyzed by MGL.
It has been shown that Citrobacter intermedius cells produce MGL when grown on a medium containing lactate and l-methionine (Faleev et al., 1994 ▶). We have cloned and sequenced the gene of C. freundii MGL and overexpressed the protein in Escherichia coli BL21 (DE3) cells containing the plasmid pET-15b (Manukhov et al., 2005 ▶). A comparison of the kinetic parameters of MGLs from C. freundii, P. putida and T. vaginalis showed that the K m values of these enzymes were relatively similar, while significant variations in reaction rates were observed (Demidkina et al., unpublished work). To explain the observed differences in the kinetic behaviour of enzymes from different species, further mechanistic and structural studies must be undertaken. In this paper, we report details of the crystallization of C. freundii MGL and the three-dimensional structure of the holoenzyme at 1.9 Å resolution.
2. Materials and methods
2.1. Crystallization
Crystals of MGL were obtained using the hanging-drop vapour-diffusion technique at 303 K. All drops were generated by mixing 2.0 µl of the enzyme dialyzed into 50 mM Tris–HCl pH 8.5, 0.5 mM PLP, 0.2 mM DTT with 2.0 µl of a precipitant solution on siliconized cover slides and were equilibrated against 1.0 ml of the same precipitant solution. MGL formed crystals with two precipitant solutions: (i) 35–37% polyethylene glycol monomethyl ether (PEG MME) 2000, 200 mM ammonium sulfate, 50 mM Tris–HCl pH 8.5, 0.2 mM PLP, 25 mM DTT and (ii) the same solution without ammonium sulfate. Rhombic shaped crystals appeared after a week and attained dimensions of 0.3 mm within two weeks. Crystals obtained using solution (i) were used to collect a data set. These crystals belong to space group I222, with unit-cell parameters a = 56.35, b = 121.83, c = 127.16 Å, and contain one subunit in the asymmetric unit. Prior to freezing in liquid nitrogen, the crystals were transferred to 37% PEG MME 2000, 200 mM ammonium sulfate, 50 mM Tris–HCl pH 8.5, 0.2 mM PLP, 25 mM DTT. Data from a single crystal were collected on the EMBL PX beamline BW7A at the DORIS storage ring, DESY (Hamburg, Germany) using a MAR CCD detector and were processed using the XDS program (Kabsch, 1993 ▶). Detailed data-collection statistics are shown in Table 1 ▶.
Table 1. Data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| Space group | I222 |
| Unit-cell parameters (Å) | a = 56.35, b = 121.83, c = 127.16 |
| Wavelength (Å) | 0.843 |
| Resolution (Å) | 20–1.9 (2.0–1.9) |
| Completeness (%) | 95.2 (83.9) |
| I/σ(I) | 18.3 (4.1) |
| Redundancy | 4.0 (3.7) |
| Rmerge (%) | 5.7 (38.2) |
| No. non-H protein atoms | 3024 |
| No. solvent atoms | 104 |
| Resolution range (Å) | 20–1.9 |
| No. reflections | 33874 |
| R (%) | 20.6 |
| Rfree (%) | 20.9 |
| Mean temperature factor B (Å2) | 32.4 |
| R.m.s. deviation from ideal values | |
| Bond lengths (Å) | 0.022 |
| Bond angles (°) | 1.6 |
| Dihedral angles (°) | 24.1 |
| Improper angles (°) | 2.08 |
| Ramachandran plot | |
| Most favoured | 303 [87.8%] |
| Additionally allowed | 37 [11.0%] |
| Generously allowed | 4 [1.2%] |
2.2. Structure determination and refinement
The AMoRe program package (Navaza, 1994 ▶) was used to solve the structures by the molecular-replacement method. A monomer of MGL from P. putida (PDB code http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1gc0) with appropriate changes in the sequence was used as a model. For diffraction data between 15 and 3.0 Å, the model gave clear solutions with a correlation coefficient of 44.8 and an R factor of 59.8%. The structure was further subjected to several rounds of computational refinement and map calculation with CNS (Brünger et al., 1998 ▶) and manual model inspection and modification with O (Jones et al., 1991 ▶). A free R factor calculated from 5% of reflections set aside at the outset was used to monitor the progress of refinement. The initial anisotropic overall B factor was replaced successively with per-residue B factors, separate per-residue B factors for main- and side-chain atoms and, finally, restrained individual atomic B factors. The model bias present in the initial molecular-replacement solutions was tackled using composite omit cross-validated σA-weighted maps implemented in CNS. The electron-density map was of sufficient quality (Fig. 1 ▶) to trace whole polypeptide chains, including residues 2–398.
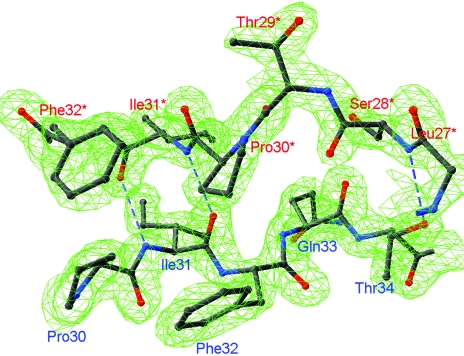
Figure 1.
A fragment of the final 2F o − F c map contoured at the 2.0σ level in the region of the short antiparallel β-sheet connecting two MGL monomers.
When the R-factor value reached 30%, water molecules were placed into 3σ peaks in F o − F c maps when they were within suitable hydrogen-bonding distance of the existing model. After refinement, water molecules whose positions were not supported by the electron density at 1σ contouring in a σA-weighted 2F o − F c map were deleted. The final model refined to 1.9 Å incorporates 3024 non-H atoms (Table 1 ▶) and was deposited in the PDB (code http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1y4i).
3. Results and discussion
MGL belongs to the evolutionary γ-family of PLP-dependent enzymes (Alexander et al., 1994 ▶) involved in the metabolism of sulfur-containing amino acids. The three-dimensional structures of PLP-dependent enzymes belong to five distinct folds: types I–V (John, 1995 ▶; Jansonius, 1998 ▶; Alexander et al., 1994 ▶; Grishin et al., 1995 ▶; Qu et al., 1998 ▶). Aspartate aminotransferase (AAT; EC 2.6.1.1) is the prototype of the α-family enzymes (Alexander et al., 1994 ▶), which are also named the ‘AAT family’ and have a type I fold. Type I fold enzymes were divided into subclasses based on the different structures of their N-terminal parts (Käck et al., 1999 ▶). The crystal structure of E. coli cystathionine β-lyase (EC 4.4.1.8), the first representative of the γ-family, revealed that it belongs to the type I fold (Clausen et al., 1996 ▶) and a structural subclass of enzymes belonging to the γ-family was named the cystathionine β-lyase subclass. The crystal structures of the MGLs from P. putida (PDB codes http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1gco, http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1ukj and http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1pg8) and T. vaginalis (PDB codes http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1e5f and http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1e5e) demonstrate features characteristic of enzymes of the cystathionine β-lyase subclass.
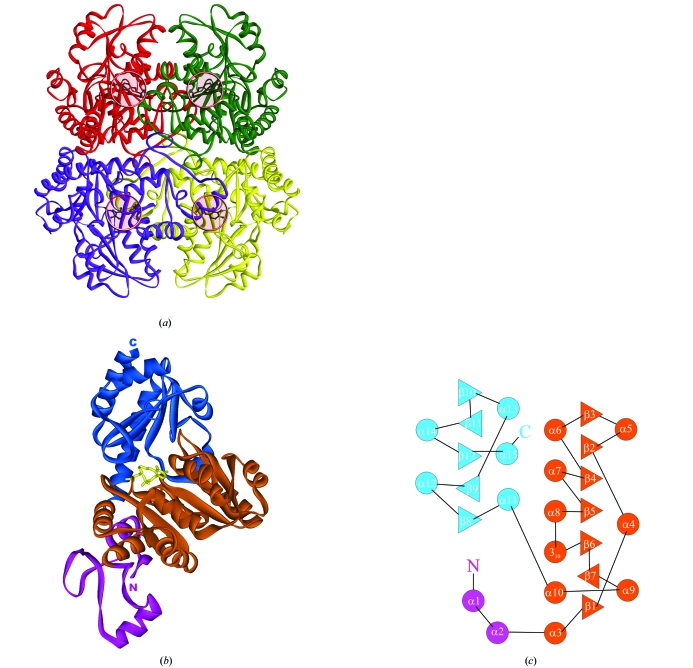
The MGLs from P. putida, T. vaginalis and C. freundii, like other enzymes from the cystathionine β-lyase subclass, exist as homotetramers with a molecular weight of about 200 kDa that posses 222 symmetry. As in the case of many PLP-dependent enzymes of the AAT family, the tetrameric molecule of MGL can be subdivided into two so-called catalytic dimers in which the active sites contain residues from the other subunit (Fig. 2 ▶ a, red/green and blue/yellow subunits). Two catalytic dimers make up the tetrameric molecule of the enzyme.
Figure 2.
(a) Schematic model of MGL tetramer. Subunits are shown in different colours. PLP-binding sites are shown by pink circles. (b) Schematic representation of a monomer. The N-terminal domain is shown in magenta, the PLP-binding domain is in orange and the C-terminal domain is in blue. PLP is shown in ball-and-stick representation. (c) Topology diagram of a monomer.
Each subunit consists of three different domains: the N-terminal domain, the PLP-binding domain and the C-terminal domain (Figs. 2 ▶ b and 2 ▶ c).
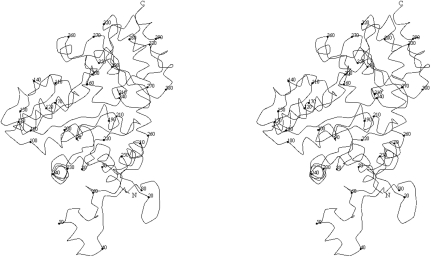
The extended N-terminal domain (residues 1–63; Fig. 3 ▶) is composed of helices α1 and α2 which connect a long loop structure containing 25 residues. C. freundii MGL has a short 310-helix at the N-terminus in contrast to the unstructured N-termini of the MGLs from P. putida and T. vaginalis. The N-terminal domain protrudes from the PLP-binding domain of the subunit and provides most of the contacts to neighbouring subunits. In the catalytic dimer, residues of the α1 helix from one subunit (Fig. 2 ▶ a, red) contact with the β12/α15 and β10/β11 loops of the C-terminal domain of the second subunit (Fig. 2 ▶ a, green). Residues 28–34 make a β-strand structure paired with the same region of the subunit from the other catalytic dimer (Fig. 2 ▶ a, blue), thus stabilizing the whole MGL tetramer by making a short antiparallel β-sheet (Fig. 1 ▶). Additional stabilization of the tetramer packing is provided by the contacts of the α2 helix and the preceding five residues with β11 and the adjacent short 310-helix of the C-terminal domain of another subunit of the neighbouring catalytic dimer (Fig. 2 ▶ a, yellow).
Figure 3.
Stereoview of the Cα trace of a monomer. Every tenth Cα atom is represented by a sphere.
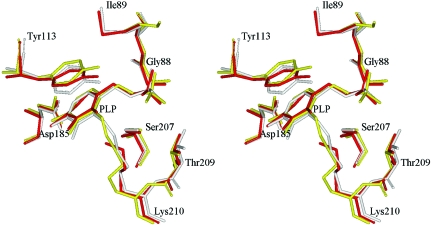
The large PLP-binding domain (residues 64–259; Fig. 3 ▶) includes a seven-stranded mainly parallel β-sheet (β1–β7). Characteristically for PLP-dependent enzymes, it has directions +−+++++, with eight α-helices (3–10) and one 310-helix arranged on both sides of the β-sheet. Helices α3, α6, α7, α8, α10 and a short 310-helix adjacent to α8 are located on one side of the β-sheet and shield it from solvent. Helices α4, α5 and α9 are located on the other side of the β-sheet and are included in the intermolecular interface. PLP is covalently attached to Lys210 and is located near the N-terminus of helix α4 and the C-termini of strands β5, β6 and β7. There are no contacts between PLP and the C-terminal domain. The arrangement of the PLP-binding residues is almost identical in all known MGL structures (Fig. 4 ▶) and can be superimposed with root-mean-square deviations of 0.27 Å (P. putida versus C. freundii) and 0.19 Å (T. vaginalis versus C. freundii). The main discrepancies are in the region of the contacts between PLP and the N-terminal domain of the other subunit of a catalytic dimer. In T. vaginalis MGL two highly conservative residues (Tyr56 and Arg58) make contacts with the phosphate group of PLP, but in C. freundii MGL only the corresponding Arg60 makes a contact (Table 2 ▶), whereas Tyr58 is directed in the opposite direction.
Figure 4.
The superposition of the PLP-binding sites of C. freundii (yellow), T. vaginalis (red) and P. putida (grey) MGLs.
Table 2. The distances between PLP and protein atoms in known MGL structures.
* represents residues from the other subunit of a catalytic dimer.
| C. freundii MGL (PDB code http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1y4i) | T. vaginalis MGL (PDB code http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1e5f) | P. putida MGL (PDB code http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1ukj) | ||||||
|---|---|---|---|---|---|---|---|---|
| Residue | PLP atom | Distance (Å) | Residue | PLP atom | Distance (Å) | Residue | PLP atom | Distance (Å) |
| Tyr58* OH | O1P | — | Tyr56* OH | O1P | 2.42 | Tyr59* OH | O2P | 2.45 |
| Arg60* NE | O3P | — | Arg58* NE | O3P | 2.59 | Arg61* NE | O3P | 3.08 |
| Arg60* NH2 | O2P | 2.97 | Arg58* NH2 | O1P | 2.93 | Arg61* NH2 | O2P | 3.03 |
| Gly88 N | O1P | 2.71 | Gly86 N | O2P | 2.81 | Gly89 N | O1P | 2.82 |
| Gly88 N | O3P | 3.16 | Gly86 N | O3P | 3.10 | Gly89 N | O3P | 3.04 |
| Ile89 N | O3P | 2.92 | Met87 N | O3P | 2.87 | Met90 N | O3P | 2.80 |
| Asp185 OD2 | N1 | 2.73 | Asp184 OD2 | N1 | 2.64 | Asp186 OD2 | N1 | 2.70 |
| Ser207 OG | O1P | 2.82 | Ser206 OG | O1P | 2.81 | Ser208 OG | O1P | 3.05 |
| Ser207 OG | O4P | 2.92 | Ser206 OG | O4P | 2.92 | Ser208 OG | O4P | 2.77 |
| Thr209 OG1 | O1P | 2.67 | Thr208 OG1 | O2P | 2.78 | Thr210 OG1 | O1P | 2.71 |
The C-terminal domain (residues 260–398; Fig. 3 ▶) consists of a five-stranded β-sheet with five α-helixes (α11, α12, α13, α14, α15) located on the both sides of the β-sheet. Helices α11, α12, α13 and α15 continue the solvent shield of the α-helices layer of the PLP-binding domain. Helix α14 is in the PLP-binding/C-terminal interdomain interface, but no atoms of α14 make contacts with PLP-domain atoms. The position of this helix is different from that in T. vaginalis MGL, where α14 is located closer to the PLP-binding domain; it is located very close to the position of the corresponding helix in P. putida MGL.
Supplementary Material
PDB reference: l-methionine γ-lyase, 1y4i, r1y4isf
Acknowledgments
We thank N. P. Fomenkova for excellent technical assistance. This work was supported by the Russian Academy of Sciences, the Russian Foundation for Basic Research (grant No. 05-04-48010), the Program of the RAS on Molecular and Cellular Biology and the Council of the RF President (grants for outstanding scientific schools Nos. 1969.2003.4 and 1800.2003.4). The research of AN was supported in part by the Russian Science Support Foundation. The research of MG was supported in part by an International Research Scholar’s award from the Howard Hughes Medical Institute. Partial support for TVD was provided by the International Fogarty Foundation (grant No. 1 R03 TW006045-01A2)
References
- Alexander, W., Sandmeir, E., Mehta, P. K. & Christen, P. (1994). Eur. J. Biochem.219, 953–960. [DOI] [PubMed] [Google Scholar]
- Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst. D54, 905–921. [DOI] [PubMed] [Google Scholar]
- Clausen, T., Huber, R., Laber, B., Pohlenz, H. D. & Messershmidt, A. (1996). J. Mol. Biol.262, 202–204. [DOI] [PubMed] [Google Scholar]
- Faleev, N. G., Troitskaya, M. V., Ivoilov, V. S., Karpova, V. V. & Belikov, V. M. (1994). Prikl. Biokhim. Microbiol.30, 458–463.
- Grishin, N., Phillips, M. A. & Goldsmith, E. J. (1995). Protein Sci.4, 1291–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, R. M. (1997). Hum. Cell, 10, 69–80. [PubMed] [Google Scholar]
- Jansonius, J. N. (1998). Curr. Opin. Struct. Biol.8, 759–769. [DOI] [PubMed] [Google Scholar]
- John, R. A. (1995). Biochim. Biophys. Acta, 1248, 81–96. [DOI] [PubMed] [Google Scholar]
- Jones, T. A., Zou, J. Y., Cavan, S. W. & Kjeldgaard, M. (1991). Acta Cryst. A47, 110–119. [DOI] [PubMed] [Google Scholar]
- Kabsch, W. (1993). J. Appl. Cryst.26, 795–800. [Google Scholar]
- Käck, H., Sandmark, J., Gibson, K., Schneider, H. & Lindqvist, Y. (1999). J. Mol. Biol.291, 857–876. [DOI] [PubMed] [Google Scholar]
- Manukhov, I. V., Mamaeva, D. V., Rastorguev, S. M., Faleev, N. G., Morozova, E. A., Demidkina, T. V. & Zavilgelsky, G. B. (2005). In the press. [DOI] [PubMed]
- Motoshima, H., Imagaki, K., Kumasaka, T., Furuichi, T., Inoe, H., Tamura, T., Esaki, N., Soda, K., Tanaka, N., Yamamoto, M. & Tanaka, H. (2000). J. Biochem.128, 349–354. [DOI] [PubMed] [Google Scholar]
- Navaza, J. (1994). Acta Cryst. A50, 157–163. [Google Scholar]
- Qu, K., Martin, D. L. & Lawrence, C. E. (1998). Protein Sci.7, 1092–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, H., Esaki, N. & Soda, K. (1977). Biochemistry, 16, 100–106. [DOI] [PubMed] [Google Scholar]
- Tanaka, H., Esaki, N. & Soda, K. (1985). Enzyme Microb. Technol.7, 530–537.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: l-methionine γ-lyase, 1y4i, r1y4isf