The periplasmic chaperone FaeE of E. coli F4 fimbriae crystallizes in three crystal forms.
Keywords: fimbriae, chaperone-usher pathway, periplasmic chaperones
Abstract
F4 (formerly K88) fimbriae from enterotoxigenic Escherichia coli are assembled via the FaeE/FaeD chaperone/usher pathway. The chaperone FaeE crystallizes in three crystal forms, all belonging to space group C2. Crystals of form 1 diffract to 2.3 Å and have unit-cell parameters a = 195.7, b = 78.5, c = 184.6 Å, β = 102.2°. X-ray data for crystal form 2 were collected to 2.7 Å using an SeMet variant of FaeE. The crystals have unit-cell parameters a = 136.4, b = 75.7, c = 69.4 Å, β = 92.8°. Crystals of form 3 were formed in a solution containing the FaeE–FaeG complex and diffract to 2.8 Å. Unit-cell parameters are a = 109.7, b = 78.6, c = 87.8 Å, β = 96.4°.
1. Introduction
Early steps in bacterial pathogenesis involve the attachment of the bacteria to the host cell, often mediated by recognition of carbohydrate moieties on cell-surface receptors by fimbrial adhesins (Hultgren et al., 1996 ▶). Assembly of fimbriae or pili on the surface of Gram-negative bacteria in many cases occurs via the chaperone/usher pathway (Thanassi et al., 1998 ▶). The crystal structures of the fimbrial chaperones PapD, FimC and SfaeE show that they all share the same overall boomerang-shaped structure containing two immunoglobulin-like domains (Holmgren et al., 1992 ▶; Pellecchia et al., 1998 ▶; Knight et al., 2002 ▶). The structures of the FimC–FimH and PapD–PapK complexes revealed that the fimbrial subunits also have an immunoglobulin-like fold, but lack the seventh β-strand. The absence of this strand results in a deep groove, exposing the hydrophobic core of the subunit to the surface (Choudhury et al., 1999 ▶; Sauer et al., 1999 ▶). Fimbrial chaperones bind the subunits as they are translocated to the periplasm by the Sec machinery and protect them from degradation, aggregation and premature fibre formation by capping their assembly surfaces. In a mechanism called donor-strand complementation, the chaperone’s G1 β-strand is inserted into the groove on the surface of the subunit (Kuehn et al., 1991 ▶, 1993 ▶; Choudhury et al., 1999 ▶; Sauer et al., 1999 ▶). The chaperone thereby also helps the subunit to fold correctly (Barnhart et al., 2000 ▶; Sauer et al., 2000 ▶). During pilus biogenesis, the conserved N-terminal extension of one subunit replaces the chaperone’s G1 β-strand from its neighbouring subunit in a mechanism called donor-strand exchange (Choudhury et al., 1999 ▶; Sauer et al., 1999 ▶).
F4 fimbriae are expressed by enterotoxigenic Escherichia coli strains that cause diarrhoea in neonatal or recently weaned piglets (de Graaf & Mooi, 1986 ▶). In contrast to type 1 and P pili, F4 pili are not built up of a helical rod consisting of major subunits followed by a fibrillum of minor subunits and a tip adhesin. Rather, F4 pili are thin flexible structures consisting mainly of the major subunit FaeG, which also contains the adhesive properties (Gaastra & de Graaf, 1982 ▶). Mol and coworkers revealed that the F4 chaperone FaeE is a homodimer and forms heterotrimeric complexes with the F4 fimbrial subunits (Mol et al., 1994 ▶, 1995 ▶, 1996 ▶). These results cannot readily be explained in the light of current knowledge on the donor-strand complementation/donor-strand exchange mechanism. So far, only 1:1 chaperone–subunit complexes have been identified. Chaperone dimerization has been observed for PapD and SfaE. In both cases, the subunit-binding interface of the chaperone is involved in dimerization (Hung et al., 1999 ▶; Knight et al., 2002 ▶). Therefore, further research into F4 fimbrial biogenesis is necessary to clarify the molecular and structural basis of the F4 chaperone dimerization and the F4 chaperone–subunit interactions. Here, we report the crystallization of the FaeE chaperone.
2. Materials and methods
2.1. Expression and purification
2.1.1. Co-expression and purification of the FaeE–FaeG complex
The fragment of the F4 operon containing the faeE, faeF and faeG genes was amplified from the F4ad+ E. coli C1360-79 genome using PCR with the forward primer K88-4 (5′-AGT AAG CGT AAC GCA GTA ACG ACG TTT TTC-3′, based on accession No. X56003) and the reverse primer K88-2 (5′-GGG GTC GAC CTA GTA ATA AGT AAT TGC TAC GTT CAG-3′, based on accession No. V00292). The fragment was inserted into plasmid pHD40 between a blunted NcoI and a SalI restriction site. This vector is a derivative of a pBAD vector in which the araBAD promotor was replaced by the T7 promotor. The FaeF gene was cut out using BspEI and the plasmid was self-ligated using Ready-To-Go T4 DNA ligase (Amersham Biosciences). After transformation in E. coli K514, colonies were grown on Luria Broth–agar plates containing 100 µg ml−1 ampicillin. DNA-sequence analyses on the transformants were performed using an ABI PRISM 3100 Genetic Analyzer (Applied Biosciences). The plasmid used in further experiments was designed pHD147 and transformed in E. coli C43 (DE3) (Miroux & Walker, 1996 ▶). An overnight culture of E. coli C43 (DE3) (pHD147) grown at 310 K in Luria Broth (LB) medium supplemented with 100 µg ml−1 ampicillin was diluted 20 times in LB medium and incubated at 310 K. When OD600 reached 0.7, gene expression was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Cells were harvested 3 h after induction by centrifugation (10 min, 4500 rev min−1; Beckman JLA8100 rotor) and the periplasmic fraction was extracted by osmotic shock. In brief, pellets were resuspended in 20 mM N-Tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES) pH 7.0, 30% sucrose and 2.5 mM ethylenediaminetetraacetic acid (EDTA) and incubated on ice for 1 h. The suspension was centrifuged for 20 min at 10 000 rev min−1 using a Beckman JA20 rotor. The pellet was resuspended in 20 mM TES pH 7.0 and incubated on ice for 30 min. After centrifugation at 10 000 rev min−1 for 20 min, the supernatants containing the periplasmic fraction were collected. The periplasmic extract was immediately loaded onto a Poros 50HQ anion-exchange column (Applied Biosystems) equilibrated in 20 mM TES buffer pH 7.0. The column was eluted using a linear gradient to 20 mM TES pH 7.0, 1 M NaCl. Fractions containing the FaeE–FaeG complex were pooled, concentrated using Vivaspin 10 kDa cutoff concentrators (Vivascience) and loaded onto a Superdex 75 HR1030 gel-filtration column (Amersham Pharmacia) equilibrated in 20 mM TES pH 7.0, 150 mM NaCl.
2.1.2. Expression and purification of FaeE
The faeG gene was deleted from pHD147 using SalI and BstEII restriction followed by ligation. The resulting plasmid pHD163 was transformed in E. coli C43 (DE3). FaeE was expressed as described for FaeE–FaeG. The periplasmic extract was loaded onto a Poros 50HS cation-exchange column (Applied Biosystems) in 20 mM TES pH 7.0. The column was eluted using a linear gradient to 20 mM TES pH 7.0, 1 M NaCl. Fractions containing the FaeE protein were pooled, concentrated using Vivaspin 10 kDa cutoff concentrators (Vivascience) and loaded onto a Superdex 75HR1030 gel-filtration column (Amersham Pharmacia) equilibrated in 20 mM TES pH 7.0, 150 mM NaCl.
2.1.3. Expression and purification of FaeESeMet
In order to purify the selenomethionine variant of FaeE (FaeESeMet), pHD163 was transformed in the methionine auxotroph E. coli B834 (DE3) (Leahy et al., 1992 ▶). E. coli B834(DE3) (pHD163) was grown overnight at 310 K in minimal medium supplemented with 40 µg ml−1 of all amino acids, 2 µg ml−1 biotin, 2 µg ml−1 thiamine, 0.2% glucose, 2 mM MgSO4 and 100 µg ml−1 ampicillin. This culture was diluted 20 times in minimal medium supplemented with 40 µg ml−1 of all amino acids except methionine, 40 µg ml−1 selenomethionine, 2 µg ml−1 biotin, 2 µg ml−1 thiamine, 0.2% glucose, 2 mM MgSO4 and incubated at 310 K. When OD600 reached 0.7, gene expression was induced with 1 mM IPTG. Cells were harvested 3 h after induction by centrifugation (10 min, 4500 rev min−1, Beckman JLA 8100) and the periplasmic fraction was extracted by osmotic shock (see §2.1.1). Purification of FaeESeMet was identical to that of FaeE.
2.2. Crystallization and X-ray analysis
Prior to crystallization, the proteins were concentrated using the same Vivaspin concentrators as above (to 15 mg ml−1 for FaeE–FaeG and to 35 mg ml−1 for FaeE). The particle distribution of the protein samples was analysed using dynamic light scattering (DLS; RiNA, Netzwerk RNA Technologien). Crystallizations were performed using the hanging-drop vapour-diffusion method at 293 K. Crystallization drops containing 1 µl protein solution in 20 mM TES pH 7.0, 150 mM NaCl and 1 µl precipitant solution were equilibrated against 500 µl precipitant solution. Crystallization conditions were screened using the Crystal Screen and Crystal Screen 2 kits (Hampton Research). Positive conditions were further optimized. Crystallization conditions for FaeESeMet were based on the conditions established for FaeE.
X-ray data for FaeE were collected at ESRF beamline ID14-1 (Grenoble, France) and DESY/EMBL beamline BW7A (Hamburg, Germany). Data for FaeESeMet were collected at the absorption-edge, inflection-point and high-energy remote wavelengths as determined by a fluorescence scan for selenium. Data were indexed and processed using DENZO and SCALEPACK from the HKL suite (Otwinowski & Minor, 1997 ▶). TRUNCATE and MATTHEWS_COEFF from the CCP4 suite (Collaborative Computational Project, Number 4, 1994 ▶) were used to obtain structure-factor amplitudes and calculate solvent contents, respectively. Preliminary phases from the MAD data were calculated at the EMBL facility using the experimental Auto-Rickshaw automated crystal structure determination platform (Panjikar et al., 2005 ▶).
2.3. SDS–PAGE analysis
Crystals were picked from the hanging drop, washed in precipitant solution and dissolved in H2O. The remaining solution was diluted fivefold in H2O. Samples were heated at 373 K for 5 min prior to analysis on 12.5% SDS–polyacrylamide gels.
3. Results and discussion
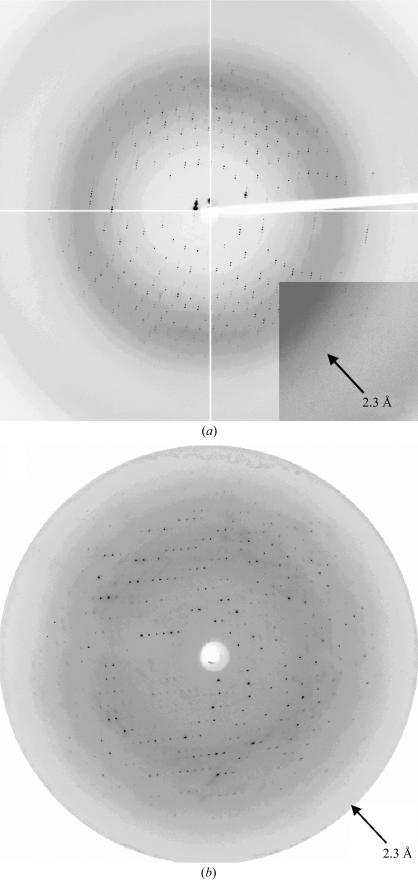
FaeE–FaeG, FaeE and FaeESeMet showed high expression levels, were purified to a high degree and had a monodisperse particle distribution according to DLS measurements. Screening for FaeE crystallization conditions using Crystal Screen 2 (35 mg ml−1 FaeE) resulted in crystal formation in condition No. 43 [0.1 M Tris pH 8.5, 50%(v/v) 2-methyl-2,4-pentanediol (MPD), 0.2 M NH4H2PO4]. Lowering the FaeE concentration to 20 mg ml−1 and changing the buffer to 0.1 M HEPES pH 7.5 resulted in larger crystals. Crystals were grown in a range of NH4H2PO4 concentrations (0.5–0.2 M) or in the absence of NH4H2PO4. Mother liquor containing 50% MPD was used as the cryoprotectant. Crystals were flash-frozen directly in the cryostream. FaeE showed two crystal forms (Figs. 1 ▶ a and 1 ▶ b), which occurred using the same condition and even in the same drop. Both crystal forms 1 and 2 belong to space group C2 and diffracted to 2.3 Å (Fig. 2 ▶ a) and 4 Å (data not shown), respectively. Single crystals of FaeESeMet (using a protein concentration of 25 mg ml−1) were obtained using one of the conditions established for FaeE. An X-ray data set was collected for a FaeESeMet crystal to 2.7 Å resolution at three wavelengths (Fig. 2 ▶ b). This crystal showed the same unit-cell parameters as FaeE crystal form 2. Data-collection statistics are summarized in Table 1 ▶.
Figure 1.
Crystals of FaeE. (a) FaeE crystal form 1, grown in 0.1 M Tris pH 8.5, 50% MPD. (b) FaeE crystal form 2, grown in 0.1 M HEPES pH 7.5, 50% MPD, 0.05 M NH4H2PO4. (c) FaeE crystal form 3, grown in 0.1 M Tris pH 8.5, 50% MPD, 0.1 M NH4H2PO4 from drops initially containing the FaeE–FaeG complex. The black bars indicate 0.1 mm.
Figure 2.
Diffraction patterns of form 1 and 2 FaeE crystals. (a) FaeE crystal form 1 exposed for 5 s on ESRF beamline ID14-1 (Grenoble, France) at a wavelength of 0.933 Å and with a detector distance of 215 mm. The arrowhead indicates a resolution of 2.3 Å. (b) FaeE crystal form 2 (FaeESeMet crystal) exposed for 50 s on the EMBL BW7A beamline at the DESY synchrotron (Hamburg, Germany) at a wavelength of 0.9789 Å and with a detector distance of 201 mm.
Table 1. Data-collection statistics and crystal parameters for the different crystal forms of FaeE.
Values in parentheses are for the highest resolution shells.
| FaeE crystal form 2 (data collected from FaeESeMet crystal) | |||||
|---|---|---|---|---|---|
| FaeE crystal form 1 | Absorption edge | Inflection point | High-energy remote | FaeE crystal form 3 | |
| Wavelength (Å) | 0.933 | 0.9789 | 0.9796 | 0.9364 | 0.9364 |
| Resolution range (Å) | 49.8–2.3 (2.4–2.3) | 39.0–2.7 (2.8–2.7) | 39.0–2.7 (2.8–2.7) | 33.0–2.7 (2.8–2.7) | 39.3–2.8 (2.9–2.8) |
| Total reflections | 639018 | 145440 | 140236 | 149032 | 111565 |
| Unique reflections | 122618 (11697) | 19280 (1903) | 19271 (1920) | 19389 (1890) | 18291 (1794) |
| Redundancy | 5.2 (4.0) | 7.5 (7.4) | 7.3 (7.2) | 7.7 (7.4) | 6.1 (6.2) |
| Mosaicity (°) | 0.3 | 0.7 | 0.7 | 0.7 | 1.4 |
| 〈I/σ(I)> | 14.56 (3.7) | 19.5 (5.4) | 19.3 (5.3) | 23.97 (4.9) | 14.91 (7.0) |
| Completeness (%) | 99.5 (95.6) | 98.0 (97.1) | 98.0 (98.2) | 98.6 (96.4) | 99.8 (100.0) |
| Rmerge (%) | 8.3 (39.0) | 7.1 (32.9) | 5.7 (29.9) | 5.3 (40.4) | 12.2 (30.2) |
| Ranom (%) | 3.7 (10.0) | 1.7 (9.9) | 2.6 (13.8) | ||
| Space group | C2 | C2 | C2 | ||
| Unit-cell parameters | |||||
| a (Å) | 195.7 | 136.4 | 109.7 | ||
| b (Å) | 78.5 | 75.7 | 78.6 | ||
| c (Å) | 184.6 | 69.4 | 87.8 | ||
| β (°) | 102.2 | 92.8 | 96.4 | ||
| Beamline | ID14-1 | BW7A | BW7A | ||
Preliminary phases from the MAD data obtained using the Auto-Rickshaw program at the EMBL facility revealed eight Se peaks in the asymmetric unit of the FaeESeMet crystals. Four selenomethionine residues are present in FaeE, indicating that the asymmetric unit contains two FaeE molecules. A Matthews coefficient calculation shows that this asymmetric unit has 65% solvent content (FaeESeMet molecular weight, 25.0 kDa; Matthews coefficient, 3.6 Å3 Da−1). The Matthews coefficient for FaeE crystal form 1 could be in the range 1.7 Å3 Da−1 (16 molecules per asymmetric unit, 29% solvent content) to 5.6 Å3 Da−1 (five molecules per asymmetric unit, 77% solvent content) (molecular weight of FaeE, 24.7 kDa). Given the proportion of the unit-cell volumes of crystal forms 1 and 2 (3.8), the asymmetric unit of crystal form 1 is likely to contain at least eight molecules (Matthews coefficient 3.5 Å3 Da−1, 65% solvent content).
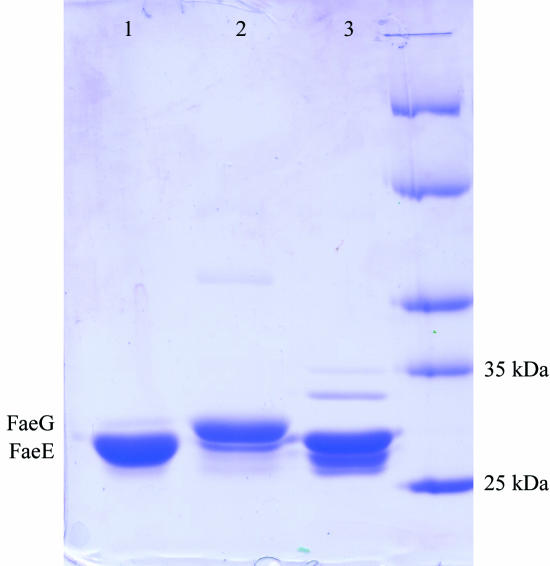
Crystal form 3 (Fig. 1 ▶ c) was obtained in solutions containing the FaeE–FaeG chaperone–adhesin complex equilibrated against a precipitant solution of 0.1 M Tris pH 8.5, 50%(v/v) 2-methyl-2,4-pentanediol (MPD) and a range of NH4H2PO4 concentrations (0.5–0.2 M). SDS–PAGE revealed that these crystals did not contain the FaeE–FaeG complex but only the FaeE chaperone (Fig. 3 ▶). Under the conditions used only FaeE crystallizes, while FaeG precipitates, probably owing to self-polymerization. X-ray data from the crystals were collected to 2.8 Å (Table 1 ▶). The Matthews coefficient for this crystal form could be in the range 3.8 Å3 Da−1 (two molecules per asymmetric unit, 67.4% solvent content) to 1.9 Å3 Da−1 (four molecules per asymmetric unit, 34.9% solvent content).
Figure 3.
SDS–PAGE analysis of the FaeE–FaeG crystallization. Lane 1, crystals grown in solutions initially containing the FaeE–FaeG complex, showing clearly that only FaeE is present. Lane 2, liquid remaining in the hanging drop after removal of the crystals, showing an excess of FaeG. Lane 3, control sample of purified FaeE.
Acknowledgments
The authors thank S. Panjikar for his excellent assistance by introducing us to the experimental Auto-Rickshaw automated crystal structure determination. JB is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (FWO-Vlaanderen). Financial support for this project was provided by the Research Council of the Vrije Universiteit Brussel (OZR846, OZR727 and OZR728) and the FWO-Vlaanderen (FWOAL241). The authors acknowledge the use of the EMBL beamline BW7A at the DESY synchrotron (Hamburg, Germay) and the use of the ESRF beamline ID14-1 (Grenoble, France).
References
- Barnhart, M. M., Pinkner, J. S., Soto, G. E., Sauer, F. G., Langermann, S., Waksman, G., Frieden, C. & Hultgren, S. J. (2000). Proc. Natl Acad. Sci. USA, 97, 7709–7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury, D., Thompson, A., Stojanoff, V., Langermann, S., Pinkner, J., Hultgren, S. J. & Knight, S. D. (1999). Science, 285, 1061–1066. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Gaastra, W. & de Graaf, F. K. (1982). Microbiol. Rev.46, 129–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graaf, F. K. de & Mooi, F. R. (1986). Adv. Microb. Physiol.28, 65–143. [DOI] [PubMed] [Google Scholar]
- Holmgren, A., Kuehn, M. J., Branden, C. I. & Hultgren, S. J. (1992). EMBO J.11, 1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren, S. J., Jones, C. H. & Normark, S. (1996). In Escherichia coli and Salmonella: Cellular and Molecular Biology, edited by F. C. Neidhardt. Washington DC: ASM Press.
- Hung, D. L., Pinkner, J. S., Knight, S. D. & Hultgren, S. J. (1999). Proc. Natl Acad. Sci. USA, 96, 8178–8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, S. D., Choudhury, D., Hultgren, S., Pinkner, J., Stojanoff, V. & Thompson, A. (2002). Acta Cryst. D58, 1016–1022. [DOI] [PubMed] [Google Scholar]
- Kuehn, M. J., Normark, S. & Hultgren, S. J. (1991). Proc. Natl Acad. Sci. USA, 88, 10586–10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn, M. J., Ogg, D. J., Kihlberg, J., Slonim, L. N., Flemmer, K., Bergfors, T. & Hultgren, S. J. (1993). Science, 262, 1234–1241. [DOI] [PubMed] [Google Scholar]
- Leahy, D. J., Hendrickson, W. A., Aukhil, I. & Erickson, H. P. (1992). Science, 258, 987–991. [DOI] [PubMed] [Google Scholar]
- Miroux, B. & Walker, J. E. (1996). J. Mol. Biol.260, 289–298. [DOI] [PubMed] [Google Scholar]
- Mol, O., Oud, R. P., de Graaf, F. K. & Oudega, B. (1995). Microb. Pathog.18, 115–128. [DOI] [PubMed] [Google Scholar]
- Mol, O., Oudhuis, W. C., Fokkema, H. & Oudega, B. (1996). Mol. Microbiol.22, 379–388. [DOI] [PubMed] [Google Scholar]
- Mol, O., Visschers, R. W., de Graaf, F. K. & Oudega, B. (1994). Mol. Microbiol.11, 391–402. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Panjikar, S., Parthasarathy, V., Lamzin, V. S., Weiss, M. S. & Tucker, P. A. (2005). Acta Cryst. D61, 449–457. [DOI] [PubMed] [Google Scholar]
- Pellecchia, M., Guntert, P., Glockshuber, R. & Wuthrich, K. (1998). Nature Struct. Biol.5, 885–890. [DOI] [PubMed] [Google Scholar]
- Sauer, F. G., Barnhart, M., Choudhury, D., Knight, S. D., Waksman, G. & Hultgren, S. J. (2000). Curr. Opin. Struct. Biol.10, 548–556. [DOI] [PubMed] [Google Scholar]
- Sauer, F. G., Futterer, K., Pinkner, J. S., Dodson, K. W., Hultgren, S. J. & Waksman, G. (1999). Science, 285, 1058–1061. [DOI] [PubMed] [Google Scholar]
- Thanassi, D. G., Saulino, E. T. & Hultgren, S. J. (1998). Curr. Opin. Microbiol.1, 223–231. [DOI] [PubMed] [Google Scholar]