l-Threonine dehydrogenase from the hyperthermophilic archaeon P. horikoshii was crystallized and preliminary X-ray crystallographic analysis was carried out.
Keywords: archaea, Pyrococcus horikoshii, hyperthermostability, threonine dehydrogenase
Abstract
Recombinant l-threonine dehydrogenase from the hyperthermophilic archaeon Pyrococcus horikoshii was prepared using an Escherichia coli expression system. The hyperthermostable l-threonine dehydrogenase consists of 348 amino acids with a molecular weight of 37.7 kDa. The enzyme was crystallized by the hanging-drop vapour-diffusion method at 277 K and preliminary X-ray crystallographic analysis was carried out. Diffraction data were collected to 2.20 Å resolution under cryogenic conditions. P. horikoshii l-threonine dehydrogenase crystals belong to space group I4122, with unit-cell parameters a = b = 143.84, c = 304.13 Å. The presence of three subunits of the enzyme per asymmetric unit was estimsted to give a Matthews coefficient (V M) of 3.5 Å3 Da−1 and a solvent content of 64.7%(v/v).
1. Introduction
The hyperthermophilic anaerobic archaeon Pyrococcus horikoshii was isolated from a hydrothermal volcanic vent in the Okinawa Trough in the Pacific Ocean (Gonzalez et al., 1998 ▶). An ORF (PH0655) in the P. horikoshii genome database has been classified as an alcohol dehydrogenase (ADH; EC 1.1.1.1; Kawarabayasi et al., 1998 ▶), with sequence homology to l-threonine dehydrogenase (TDH; EC 1.1.1.103). We have cloned PH0655 and found it to be a hyperthermophilic TDH (PhTDH; Khan et al., 1998 ▶). PhTDH requires zinc for its catalytic activity and hence was classified as an NAD+-dependent zinc-containing medium-chain enzyme. Kinetic analysis (Higashi et al., 2005 ▶) revealed that PhTDH is an ADH that can catalyze the NAD+-dependent oxidation of l-threonine to 2-amino-3-ketobutyrate (Epperly & Dekker, 1991 ▶; Johnson et al., 1998 ▶; Aoyama & Motokawa, 1981 ▶; Ray & Ray, 1985 ▶).
PhTDH purified as a single band on SDS–PAGE corresponding to a molecular weight of 37–38 kDa. This value was consistent with the molecular weight of 37 742 Da calculated on the basis of 348 amino acids from the genome-sequence data. However, the molecular weight of PhTDH determined by gel-filtration chromatography was approximately 76 kDa, suggesting that PhTDH exists as a dimer, in contrast to the tetrameric forms of the TDHs from Escherichia coli and chicken liver (Johnson et al., 1998 ▶; Aoyama & Motokawa, 1981 ▶). Homotetrameric structures of the ADHs from the hyperthermophilic archaea Sulfolobus solfataricus and Aeropyrum pernix have also been reported (Esposito et al., 2002 ▶; Guy et al., 2003 ▶). The sequence of PhTDH is homologous to those of the hyperthermophilic archaeal ADHs from S. solfataricus (27% identity; PDB code http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1jvb) and A. pernix (21% identity; PDB code http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1h2b).
The crystal structures of the ADHs from the hyperthermophilic archaea S. solfataricus and A. pernix have already been solved (Esposito et al., 2003 ▶; Guy et al., 2003 ▶). However, there is no report of any crystal structures of the TDH type of enzyme. Analysis of the structural and functional similarities between TDHs and ADHs will augment our understanding of the function of these enzymes and the structural origins of hyperthermostability in this group of archaea. We report here the crystallization and preliminary X-ray diffraction analysis of PhTDH.
2. Experimental
2.1. Expression and purification
The gene encoding PhTDH was amplified by PCR from the genomic DNA of P. horikoshii with two primers containing NdeI and BamHI restriction sites. The amplified gene was digested with NdeI and BamHI and then inserted into the pET-11a vector cut with the same restriction enzymes. The host, E. coli BL21 (DE3), was transformed using the purified plasmid for overexpression of PhTDH. The transformant cells were grown in LB medium containing ampicillin (0.05 mg ml−1) at 310 K with shaking. After an optical density of OD600 = 0.5 had been reached, production of the recombinant protein was induced by adding 1 mM isopropyl β-d-thiogalactopyranoside, followed by further cultivation for 4 h. The culture of the transformant E. coli was subjected to centrifugation at 7000g and the collected cells were frozen at 253 K. The crude enzyme was extracted from the cells by sonication in 50 mM Tris–HCl buffer pH 8.0. The extract was heated at 358 K for 30 min and then centrifuged to precipitate thermolabile proteins. The supernatant fraction was dialyzed against 50 mM Tris–HCl buffer pH 8.0 and then purified on HiTrap Q and HiLoad Superdex 200 columns (Amersham Pharmacia Biosciences, Sweden). The purity of the PhTDH fractions was confirmed by the presence of a single band on SDS–PAGE electrophoresis. The protein concentration was determined with Coomassie protein reagent (Pierce Chemical Company, USA), using bovine serum albumin as the standard protein.
2.2. Crystallization
The purified protein was dialyzed against 50 mM Tris–HCl buffer pH 7.5 and concentrated to 10 mg ml−1. Crystallization was performed using the hanging-drop vapour-diffusion method at 277 K. An Emerald BioStructures Wizard II screening kit was used for initial screening. Drops consisted of equal (1.5 µl) volumes of protein and reservoir solution. Crystals of PhTDH were obtained using a reservoir solution consisting of 0.2 M sodium chloride, 0.1 M HEPES buffer pH 7.5 and 40%(v/v) PEG 400 over a period of 5 d (Fig. 1 ▶).
Figure 1.
Crystals of PhTDH with average dimensions 0.2 × 0.1 × 0.1 mm.
2.3. Data collection and processing
X-ray diffraction measurement was carried out using the same reservoir solution, which could be used as a cryoprotectant. The crystals were scooped up in a Cryo-loop (Hampton Research, Aliso Viejo, CA, USA) and immediately flash-cooled at 100 K in a nitrogen cryostream. Diffraction data were collected using an R-AXIS VII image-plate detector and Cu Kα radiation from an FR-E rotating-anode generator (Rigaku, Tokyo, Japan). The intensity data were indexed, processed and scaled with MOSFLM v.6.2.4 (Leslie, 1992 ▶) and SCALA (Evans, 1997 ▶) from the CCP4 package (Collaborative Computational Project, Number 4, 1994 ▶).
3. Results and discussion
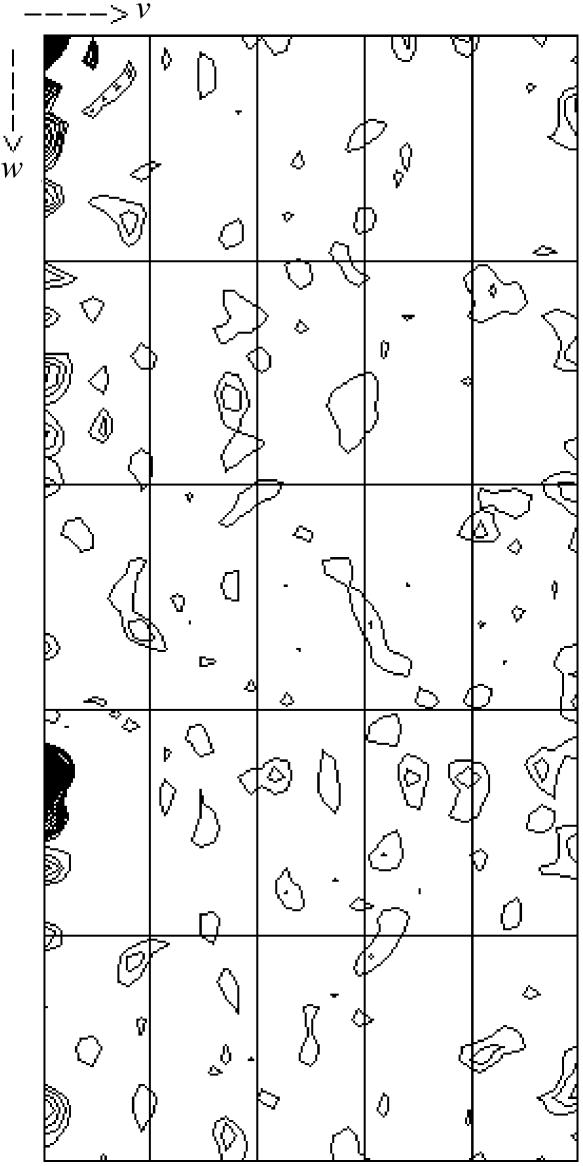
The crystal parameters and data-processing statistics are summarized in Table 1 ▶. A native data set for PhTDH was collected in the resolution range 39.5–2.20 Å with an overall R merge of 8.6% and a completeness of 100% (Table 1 ▶). The calculated Matthews coefficient, V M, of PhTDH in space group I4122 was 3.5 Å3 Da−1, with a crystal solvent content of 64.7%, for three molecules in the asymmetric unit. The presence of four molecules in the asymmetric unit would give a V M of 2.6 Å3 Da−1 and a solvent content of 52.9%. The quality and diffraction properties of the crystal were extremely dependent on the pH. Diffraction of the crystals was not observed when they were transferred to a stock solution consisting of 0.2 M sodium chloride, 40%(v/v) PEG 400 and 0.1 M HEPES buffer outside (pH 6.5–7.6) the strict crystallization pH (pH 7.5). However, diffraction was observed in the pH range 4.5–6.4 in the same stock solution. Gel-filtration experiments (Khan et al., 1998 ▶) suggest that PhTDH exists as a dimer in solution. However, the self-rotation function only showed crystallographic rotation symmetry, suggesting that the twofold axis of the dimer would have to share the crystallographic twofold axis or that the direction of the non-crystallographic twofold axis coincides with a crystallographic twofold axis. A native self-Patterson map (shown in Fig. 2 ▶) showed a strong peak at (u, v, w) = (0.0, 0.0, 0.33), suggesting non-crystallographic translational pseudosymmetry. Therefore, it is difficult to conclude on the definite content of the asymmetric unit at this stage of research. No satisfactory molecular-replacement solution has been obtained using other hyperthermophilic archaeal ADHs as models. Selenomethionine-substituted PhTDH (SeMet PhTDH) was therefore also prepared and crystallized under the same conditions as for the wild-type protein. Structure determination by MAD phasing using an SeMet PhTDH is in progress and the preliminary results strongly suggest the presence of three (not four) PhTDH molecules in the asymmetric unit.
Table 1. Data-collection statistics.
Values for the last resolution shell are given in parentheses.
| Space group | I4122 |
| Unit-cell parameters (Å) | a = b = 143.84, c = 304.13 |
| Matthews coefficient (Å 3 Da−1) | 3.5 (3 subunits per AU)/2.6 (4 subunits per AU) |
| Solvent content (%) | 64.7 (3 subunits per AU)/52.9 (4 subunits per AU) |
| Resolution range (Å) | 39.5–2.20 (2.32–2.20) |
| No. of observed reflections | 1428548 (198227) |
| Total No. of unique reflections | 80826 (11674) |
| 〈I/σ(I)〉 | 6.7 (1.7) |
| Multiplicity | 17.7 (17.0) |
| Rmerge† (%) | 8.6 (44.2) |
| Completeness (%) | 100 (100) |
R
merge = 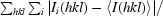
 , where I
i(hkl) is the ith intensity measurement of reflection hkl, including symmetry-related reflections, and 〈I(hkl)〉 is their average.
, where I
i(hkl) is the ith intensity measurement of reflection hkl, including symmetry-related reflections, and 〈I(hkl)〉 is their average.
Figure 2.
Native self-Patterson map. u = 0.0 section, calculated at 39.56–5.0 Å.
Acknowledgments
We wish to thank Professor Emeritus Noritake Yasuoka of Himeji Institute of Technology for fruitful discussions and helpful advice. We also thank Dr Mitsuo Ataka, our group leader, for the encouragement to carry out this work. This work was supported by the National Project on Protein Structural and Functional Analyses of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Aoyama, Y. & Motokawa, Y. (1981). J. Biol. Chem.256, 12367–12373. [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Epperly, B. R. & Dekker, E. E. (1991). J. Biol. Chem.266, 6086–6092. [PubMed] [Google Scholar]
- Esposito, L., Bruno, I., Sica, F., Raia, C. A., Giordano, A., Rossi, M., Mazzarella, L. & Zagari, A. (2003). Biochemistry, 42, 14397–14407. [DOI] [PubMed] [Google Scholar]
- Esposito, L., Sica, F., Raia, C. A., Giordano, A., Rossi, M., Mazzarella, L. & Zagari, A. (2002). J. Biol. Chem.318, 463–477. [DOI] [PubMed]
- Evans, P. R. (1997). Jnt CCP4/ESF–EACBM Newsl. Protein Crystallogr.33, 22–24.
- Gonzalez, J. M., Masuchi, Y., Robb, F. T., Ammerman, J. W., Maeder, D. L.,Yanagibayashi, M., Tamaoka, J. & Kato, C. (1998). Extremophiles, 2, 123–130. [DOI] [PubMed] [Google Scholar]
- Guy, J. E., Isupov, M. N. & Littlechild, J. A. (2003). J. Mol. Biol.29, 1041–1051. [DOI] [PubMed]
- Higashi, N., Fukada, H. & Ishikawa, K. (2005). J. Biosci. Bioeng.99, 175–180. [DOI] [PubMed]
- Johnson, A. R., Chen, Y. W. & Dekker, E. E. (1998). Arch. Biochem. Biophys.358, 211–221. [DOI] [PubMed] [Google Scholar]
- Kawarabayasi, Y. et al. (1998). DNA Res.5, 55–76. [DOI] [PubMed] [Google Scholar]
- Khan, A. R., Kosugi, Y., Ando, S., Ishida, H., Ishikawa, K., Matsui, E., Kawarabayasi, Y. & Matsui, I. (1998). Abstr. 71st Annu. Meet. Biochem. Soc. Japan, p. 1059.
- Leslie, A. G. W. (1992). Jnt CCP4/ESF–EACBM Newsl. Protein Crystallogr.26
- Ray, M. & Ray, S. (1985). J. Biol. Chem.260, 5913–5918. [PubMed] [Google Scholar]