An X-ray crystallographic study of a LOX mutant in which Arg181 is replaced by Met was initiated in order to understand the functions of the conserved amino-acid residues around the FMN in the enzyme active site. LOX-R181M crystals belong to the tetragonal space group I422, with unit-cell parameters a = b = 192.632, c = 200.263 Å, α = β = γ = 90°.
Keywords: l-lactate oxidase
Abstract
l-Lactate oxidase (LOX) from Aerococcus viridans is a member of the α-hydroxyacid oxidase flavoenzyme family. An X-ray crystallographic study of a LOX mutant in which Arg181 is replaced by Met was initiated in order to understand the functions of the conserved amino-acid residues around the FMN in the enzyme active site. LOX-R181M crystals belong to the tetragonal space group I422, with unit-cell parameters a = b = 192.632, c = 200.263 Å, α = β = γ = 90°. There are four monomers in the asymmetric unit. Diffraction data were collected under cryogenic conditions to 2.44 Å resolution from LOX-R181M crystals at BL41XU, SPring-8.
1. Introduction
The α-hydroxyacid oxidases are a group of flavoproteins that catalyze the flavin mononucleotide (FMN) dependent oxidation of their respective substrates. These enzymes have been found to share remarkable similarities in catalytic properties and common structural motifs, making it likely that they constitute a family. The known members of this family are lactate dehydrogenase (flavocytochrome b 2; Lederer, 1991 ▶), l-lactate monooxygenase (Ghisla & Massey, 1991 ▶), glycolate oxidase (Lindqvist & Branden, 1989 ▶), l-mandelate dehydrogenase (Mitra et al., 1993 ▶) and long-chain α-hydroxyacid oxidase (Diep Lê & Lederer, 1991 ▶). Within the family, the crystal structures of glycolate oxidase (Lindqvist, 1989 ▶), flavocytochrome b 2 (Xia & Mathews, 1990 ▶) and a chimeric form of l-mandelate dehydrogenase (Sukumar et al., 2001 ▶) have been solved by X-ray diffraction studies. Comparison of these structures reveals similar protein-folding patterns, with each monomeric unit consisting of eight α-helices and eight β-strands in a typical α/β-barrel arrangement and with the FMN prosthetic group located at the C-terminal end of the β-strands.
l-Lactate oxidase (LOX) from Aerococcus viridans catalyzes the oxidation of l-lactate using molecular oxygen with the formation of pyruvate and H2O2 as products (Maeda-Yorita et al., 1995 ▶). We previously reported the crystallization of the wild-type enzyme, but the quality of the crystals was not sufficient to solve the structure (Morimoto et al., 1998 ▶). The reaction mechanisms and substrate specificities of the α-hydroxyacid oxidases are based on the interactions of FMN and the respective substrates with the adjacent amino-acid residues at the active sites. Therefore, determination of the three-dimensional structure of LOX is an important step to facilitate more detailed mechanistic studies of these flavoenzymes.
Two Arg residues in LOX, Arg181 and Arg268, are conserved in all the α-hydroxyacid oxidase family members. Based on the X-ray crystal structures of glycolate oxidase and flavocytochrome b 2, these two Arg residues are located in the vicinity of the FMN and are likely to be part of the substrate-binding site. We produced the site-directed mutant LOX-R181M, with Arg181 replaced by Met, in order to determine the effect of removing the positive charge at this position (Yorita et al., 2000 ▶). In LOX-R181M, there were only small effects on the reactivity of the reduced FMN with oxygen, but the efficiency of reduction of oxidized FMN by l-lactate was greatly reduced (Yorita et al., 2000 ▶). These results demonstrated the participation of Arg181 both in the binding of the substrate l-lactate and in influencing the properties and reactivity of the active-site FMN. Here, we describe the crystallization of LOX-R181M and preliminary X-ray diffraction results.
2. Materials and methods
2.1. Crystallization
Expression and purification of the LOX-R181M mutant has been described in previous reports (Maeda-Yorita et al., 1995 ▶; Yorita et al., 2000 ▶). The enzyme was passed through a Sephacryl S-200HR (Amersham Biosciences) column prior to crystallization and was then concentrated to 20 mg ml−1 in 50 mM Tris buffer pH 8.0 using a Centricon YM-10 (Millipore). The protein concentration was determined using a molecular-extinction coefficient at 456 nm of 11.0 × 103 M −1 cm−1; the protein has a molecular weight of 40 865 Da (Maeda-Yorita et al., 1995 ▶).
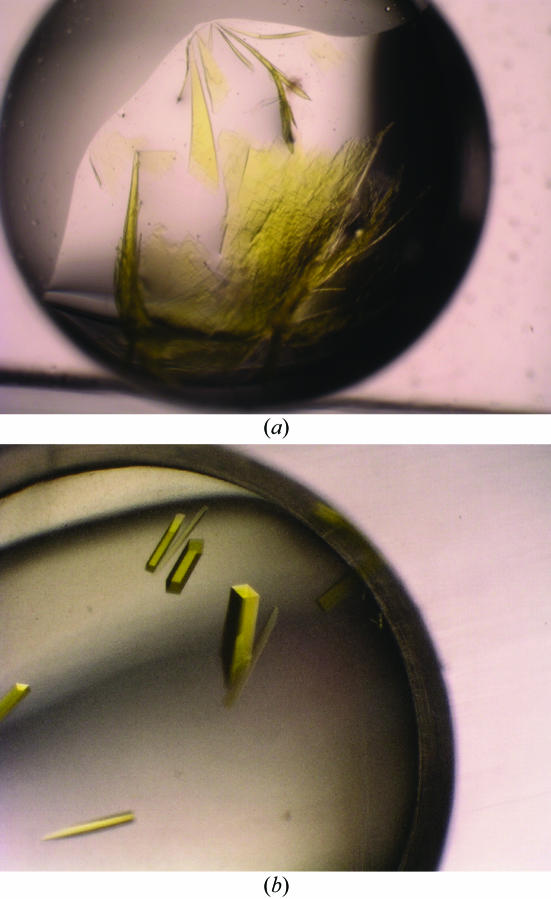
Wild-type LOX was previously crystallized at 288 K from a solution containing 10%(w/v) polyethylene glycol (PEG) 6000, 100 mM MES buffer pH 6.0 at a protein concentration of 16 mg ml−1 (Morimoto et al., 1998 ▶). We tried to crystallize LOX-R181M under the same conditions, but the result was only very thin crystals that were not suitable for diffraction experiments. We expanded crystallization trials of LOX-R181M around the original condition used to crystallize the wild-type enzyme by varying the PEG size, PEG concentration and buffer pH. One of the crystal optimization trials is shown in Fig. 1 ▶(a). In addition, we used the Additive Screen kit (Hampton Research) with either the hanging-drop or sitting-drop vapour-diffusion methods at 298 K. 1 µl of each additive solution was mixed with 4 µl reservoir solution; 2 µl protein solution and 2 µl of this pre-mixed solution were then combined to form the crystallization drop. After optimization using the additive reagents, we found benzamidine–HCl to be the most effective additive reagent. Single crystals suitable for X-ray diffraction experiments were grown at 298 K using a reservoir solution consisting of 18–20%(w/v) PEG 8000 and 50 mM Tris buffer pH 8.0 and an additive solution consisting of 2%(w/v) benzamidine–HCl. The final volume of the reservoir solution was 200 µl. In the presence of benzamidine–HCl, we obtained large single intensely yellow rod-shaped crystals with dimensions of approximately 0.3 × 0.1 × 0.1 mm (Fig. 1 ▶ b).
Figure 1.
Crystals of the LOX-R181M mutant appear yellow owing to the presence of FMN. The crystals were grown in a solution containing 20%(w/v) PEG 8000 and 50 mM Tris buffer pH 8.0 (a) in the absence and (b) in the presence of 2% benzamidine–HCl. The dimensions of the rod-shaped crystals are approximately 0.3 × 0.1 × 0.1 mm.
2.2. Data collection
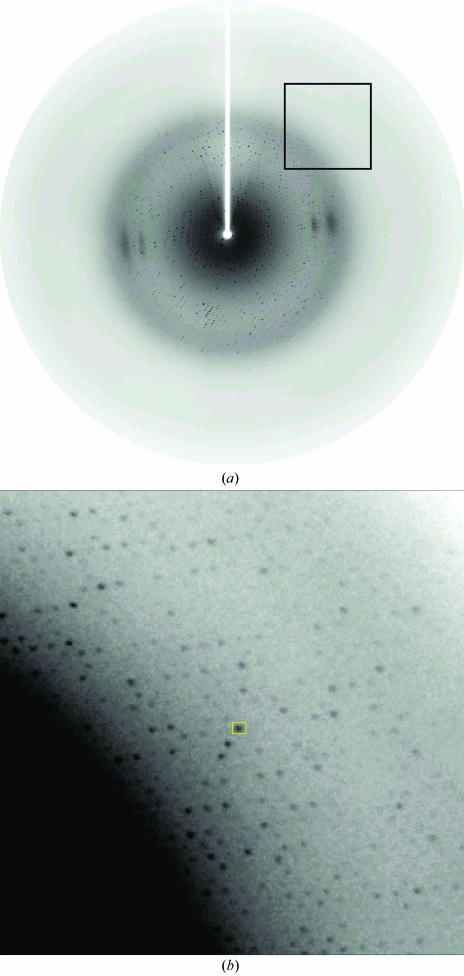
A crystal was mounted in a cryoloop and flash-frozen in a nitrogen-gas stream at 100 K. X-ray diffraction data were collected with a MAR CCD detector using synchrotron radiation at beamline BL41XU of SPring-8, Japan. The synchrotron wavelength was 1 Å and the crystal-to-detector distance was maintained at either 200 or 130 mm. A total of 180 frames were collected with 1° oscillation and 5 s exposures. A typical diffraction pattern is shown in Fig. 2 ▶ with high-angle reflections. The data were collected to 2.44 Å resolution and were processed with the program HKL2000 (Otwinowski & Minor, 1997 ▶). The data-collection statistics are given in Table 1 ▶.
Figure 2.
A typical diffraction pattern for a LOX-R181M crystal with high-angle reflections. (a) Normal size, (b) enlargement of the square in (a) near 2.5 Å resolution.
Table 1. Crystal parameters and data-collection statistics.
Values in parentheses are for the effective highest resolution shell (2.69–2.44 Å).
| Space group | I422 |
| Unit-cell parameters (Å, °) | a = 192.632, b = 192.632, c = 200.263, α = 90, β = 90, γ = 90 |
| Resolution (Å) | 2.44 |
| Crystal-to-detector distance (mm) | 130 |
| Wavelength (Å) | 1.000 |
| Exposure time (s) | 5.0 |
| No. of measurements | 1745892 |
| No. of unique reflections | 103478 |
| Redundancy | 10.8 |
| Rsym† (%) | 13.7 (32.7) |
| Completeness (%) | 94.3 (100.0) |
| I/σ(I) | 3.6 (2.2) |
R
sym = 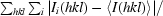
 , where I
i(hkl) are the intensities of symmetry-related reflections and 〈I(hkl)〉 is the average intensity over all symmetry equivalents.
, where I
i(hkl) are the intensities of symmetry-related reflections and 〈I(hkl)〉 is the average intensity over all symmetry equivalents.
3. Results and discussion
We have successfully obtained large diffracting crystals of LOX-R181M and collected a complete data set under cryogenic conditions using the SPring-8 synchrotron facility. Since LOX-R181M crystals grow in the presence of benzamidine, we were able to concentrate the crystallization drop by evaporation and then use this solution as a cryoprotectant. After the LOX-R181M crystals had grown to full size, the hanging- or sitting-drop well in the crystallization box was opened and the drop solution was allowed to evaporate gradually. At 5 min intervals during the evaporation procedure, a small amount of the solution was scooped into a cryo-mounting loop and the diffraction pattern was checked for ice rings. This evaporation procedure was successful in cryoprotecting the LOX-R181M crystal for the diffraction experiment.
Crystals of the LOX-R181M mutant belong to the tetragonal space group I422, with unit-cell parameters a = b = 192.632, c = 200.263 Å. Considering the molecular weight of the LOX-R181M mutant (40 842 Da), we assume there to be four LOX-R181M monomers in an asymmetric unit and thus 64 monomers in a unit cell, resulting in a Matthews coefficient (V M) of 2.81 Å3 Da−1 and a solvent content of 56.1%. These values are in the normal range for globular protein crystals (Matthews, 1968 ▶; Kantardjieff & Rupp, 2003 ▶).
Glycolate oxidase (GLO) crystallized in space group I422, in which the crystal packing of GLO yields an octamer (Lindqvist & Branden, 1989 ▶). Molecular-exclusion chromatography of lactate oxidase using Sephadex G-100 provided the basis for the report that wild-type LOX was a tetramer in 50 mM potassium phosphate buffer pH 7.0 in the presence of 0.2 M potassium chloride (Duncan et al., 1989 ▶). Thus, our LOX-R181M crystals may contain four monomers in the asymmetric unit.
Molecular-replacement analysis of LOX-R181M using the program CNS (Brünger et al., 1998 ▶) with the glycolate oxidase molecule (PDB code http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1gox; Lindqvist, 1989 ▶) as a search model is now under way to solve the structure. Crystallization trials of wild-type LOX and new LOX mutants are also in progress with and without substrate or substrate analogues using the same methods that were successful for the R181M mutant.
Acknowledgments
This research was partly supported by a Grant-in-Aid (to YM) for the National Project on Protein Structural and Function Analysis from the Ministry of Education, Culture, Sports, Science and Technology of Japan, for which the authors are greatly appreciative.
References
- Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst. D54, 905–921. [DOI] [PubMed] [Google Scholar]
- Diep Lê, K. H. & Lederer, F. (1991). J. Biol. Chem.266, 20877–20881. [PubMed] [Google Scholar]
- Duncan, J. D., Wallis, J. O. & Azari, M. R. (1989). Biochem. Biophys. Res. Commun.164, 919–926. [DOI] [PubMed] [Google Scholar]
- Ghisla, S. & Massey, V. (1991). Chemistry and Biochemistry of the Flavoenzymes, Vol. 2, edited by F. Muller, pp. 243–289. Boca Raton, FL, USA: CRC Press.
- Kantardjieff, K. A. & Rupp, B. (2003). Protein Sci.12, 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer, F. (1991). Chemistry and Biochemistry of the Flavoenzymes, Vol. 2, edited by F. Muller, pp. 153–242. Boca Raton, FL, USA: CRC Press.
- Lindqvist, Y. (1989). J. Mol. Biol.209, 151–166. [DOI] [PubMed] [Google Scholar]
- Lindqvist, Y. & Branden, C. I. (1989). J. Mol. Biol.264, 3624–3628. [PubMed]
- Maeda-Yorita, K., Aki, K., Sagai, H., Misaki, H. & Massey, V. (1995). Biochimie, 77, 631–642. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Mitra, B., Gerlt, J. A., Babbitt, P. C., Koo, C. W., Kenyon, G. L., Joseph, D. & Petsko, G. A. (1993). Biochemistry, 32, 12959–12967. [DOI] [PubMed] [Google Scholar]
- Morimoto, Y., Yorita, K., Aki, K., Misaki, H. & Massey, V. (1998). Biochimie, 80, 309–312. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Sukumar, N., Xu, Y., Gatti, D. L, Mitra, B. & Mathews, F. S. (2001) Biochemistry, 40, 9870–9878. [DOI] [PubMed]
- Xia, Z. X. & Mathews, F. S. (1990). J. Mol. Biol.212, 837–863. [DOI] [PubMed] [Google Scholar]
- Yorita, K., Matsuoka, T., Misaki, H. & Massey, V. (2000). Proc. Natl Acad. Sci. USA, 97, 13039–13044. [DOI] [PMC free article] [PubMed] [Google Scholar]