APH(2′′)-Ib is an enzyme responsible for high-level gentamicin resistance in E. faecium isolates. Native crystals of this enzyme have been prepared and preliminary X-ray diffraction experiments have been undertaken.
Keywords: APH(2′′)-Ib, aminoglycoside phosphotransferases, bacterial resistance
Abstract
Bacterial resistance to the aminoglycoside antibiotics is primarily the result of deactivation of the drugs. Three families of enzymes are responsible for this activity, with one such family being the aminoglycoside phosphotransferases (APHs). The gene encoding one of these enzymes, APH(2′′)-Ib, has been cloned and the protein (comprising 299 amino-acid residues) expressed in Escherichia coli, purified and crystallized in the presence of 16%(w/v) PEG 3350 and gentamicin. The crystals belong to the monoclinic space group P21, with approximate unit-cell parameters a = 79.7, b = 58.8, c = 81.4 Å, β = 98.4°, and preliminary X-ray diffraction analysis is consistent with the presence of two molecules in the asymmetric unit. Synchrotron diffraction data to approximately 2.65 Å resolution were collected from a native APH(2′′)-Ib crystal at beamline BL9-2 at SSRL (Stanford, CA, USA). Selenium-substituted crystals have also been produced and structure determination is proceeding.
1. Introduction
The aminoglycosides are potent broad-spectrum antibiotics originally isolated from soil bacteria (Greenwood, 1995 ▶). This family of compounds, which includes the clinically relevant drugs tobramycin, kanamycin, gentamicin and amikacin, consist of a central aminocyclitol ring with two or three substituted aminoglycan rings attached at different positions. These molecules are targeted to the 30S ribosome and although the exact mechanism of aminoglycoside action is not yet fully understood, it is known that drug binding decreases the dissociation rate of aminoacyl-tRNA, promoting miscoding (Karimi & Ehrenberg, 1994 ▶). It has been proposed that aminoglycoside binding could also facilitate the binding of non-cognate and near-cognate tRNAs, leading to mistranslation of the mRNA (Vakulenko & Mobashery, 2003 ▶).
Resistance to these antibiotics is now very widespread and occurs primarily through deactivation of the drug by enzymatic modification. There are three families of enzymes responsible: ATP-dependent phosphotransferases (APH), ATP-dependent adenylyltransferases (ANT) and acetyl CoA-dependent acetyltransferases (AAC), which together comprise over 75 different enzymes (Shaw et al., 1993 ▶; Smith & Baker, 2002 ▶; Vakulenko & Mobashery, 2003 ▶). These enzymes are classified firstly according to the reaction they catalyze, then by the site they modify on the aminoglycoside (given by a number in parentheses) and finally according to the aminoglycosides with which they interact (this is known as their resistance profile, specified by roman numerals at the end of the name). There are around 44 AAC, 27 APH and eight ANT enzymes known (including genetic variants with the same resistance profile) and as a whole the members of the three classes show significant familial sequence similarity.
Within the phosphotransferases, the two most important subfamilies are the APH(3′) and APH(2′′) enzymes. Two APH structures are currently known, APH(3′)-II (Nurizzo et al., 2003 ▶) and APH(3′)-IIIa (Hon et al., 1997 ▶; Burk et al., 2001 ▶; Fong & Berghuis, 2002 ▶), and have been shown to exhibit structural and functional similarity to the protein kinases. Four APH(2′′) enzymes have been identified, with resistance profiles Ia–Id (Ferretti et al., 1986 ▶; Chow et al., 1997 ▶; Tsai et al., 1998 ▶; Kao et al., 2000 ▶). Based upon detailed sequence alignments, it has been proposed that the APH(2′′) enzymes will have structures similar to the APH(3′) enzymes, given that they contain the consensus protein kinase sequence HGDxxxxN (Smith & Baker, 2002 ▶). As part of a program to investigate the molecular basis of aminoglycoside resistance, we have cloned, purified and crystallized the APH(2′′)-Ib enzyme (299 amino-acid residues; MW 35 350 Da) from Enterococcus faecium, which confers high-level gentamicin resistance (Kao et al., 2000 ▶).
2. Materials and methods
2.1. Cloning, expression and purification of APH(2")-Ib
The aph(2′′)-Ib gene, previously cloned into the pBluescript II KS(+) vector by high-fidelity Pfu Turbo polymerase (Stratagene), was PCR-amplified using two synthesized oligonucleotide primers: 2′′IbNdeI (TATCATATGGTTAACTTGGACGCTGAG; NdeI site in bold) and 2′′IbEcoRI (TATGAATTCCTGCTAAAATATAAACATC; EcoRI site in bold). The amplified DNA fragment was digested with NdeI and EcoRI and cloned into the unique NdeI–EcoRI sites of pET22b(+) (Novagen) to produce pET:APH(2′′)-Ib. After transformation into the recipient strain (Escherichia coli JM83) and selection on LB agar supplemented with 100 µg ml−1 ampicillin, the nucleotide sequence of the cloned aph(2′′)-Ib gene was verified by sequencing of both DNA strands.
Prior to protein expression, the aph(2′′)-Ib gene was subcloned into the pET42 vector using the restriction enzymes NdeI and EcoRI, effectively truncating both the N-terminal and C-terminal polyhistidine tags. For expression of the gene under the T7Lac promoter, the pET42:APH(2′′)-Ib vector harboring the aph(2′′)-Ib gene was retransformed into the recipient strain E. coli BL21(DE3). Bacteria were grown overnight in 5 ml LB broth at 310 K containing 60 µg ml−1 kanamycin. The bacterial suspension was then diluted 100-fold into fresh LB medium supplemented with 60 µg ml−1 kanamycin and grown for several hours until an OD600nm of ∼0.4 was reached. Gene expression was induced by the addition of 0.3 mM IPTG to the growth medium and cells were incubated for 3–4 h at the optimum growth temperature of 310 K (as determined from a small-scale trial). The bacteria were pelleted by centrifugation and the cells disrupted using a cell disruptor.
After centrifugation at 14 000g for 30 min, the supernatant containing the crude extract of the APH(2′′)-Ib enzyme was applied onto a 5 ml Hi-Trap Q-Sepharose ion-exchange column equilibrated with 10 mM Bis-Tris propane buffer (BTP) pH 6.5, 75 mM NaCl, 5 mM β-mercaptoethanol (BME). The column was washed with 50 ml of this buffer with a flow rate of 5 ml min−1 and the enzyme was eluted with a stepwise linear salt gradient (75–500 mM NaCl in 10 mM BTP pH 6.5, 5 mM BME). The fractions were examined by SDS–PAGE and those that contained the APH(2′′)-Ib protein (identified as a dominant band at 35 kDa) were pooled for further purification. These pooled fractions were concentrated to a final volume of 2–3 ml with a 5 kDa Vivascience 20 ml concentrator and applied onto a Superdex S-75 size-exclusion gel-filtration column equilibrated with the same buffer as above containing 150 mM NaCl. Fractions containing the APH(2′′)-Ib protein were checked using SDS–PAGE. Dynamic light-scattering experiments were carried out to measure the polydispersity of each fraction prior to crystallization. DLS was carried out using a DynaPro (model MS) molecular-sizing instrument. The APH(2′′)-Ib samples (25 µl) were centrifuged for 10 min at 13 000 rev min−1 and then transferred into a 12 µl quartz cuvette. The data were analysed using the DYNAMICS software (v.4) (Moradian-Oldak et al., 1988 ▶).
2.2. Crystallization
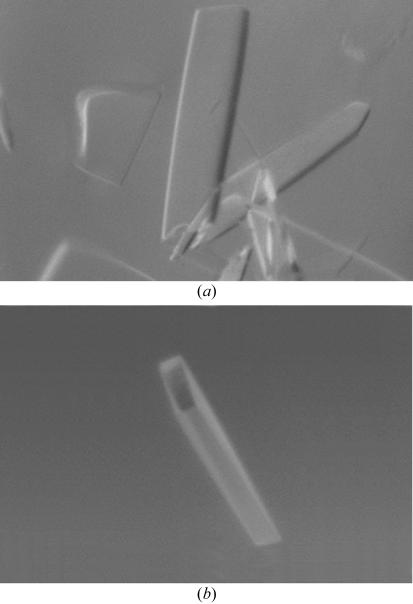
Results from dynamic light-scattering experiments indicated that the enzyme was monomeric and showed little tendency to aggregate in solution (C P/R H = 9). Fractions with similar C P/R H ratios were pooled, 1 mM gentamicin was added to the protein in 10 mM BTP pH 6.5, 150 mM NaCl, 5 mM BME and the complex was incubated overnight. The gentamicin–APH(2′′)-Ib complex was concentrated using a 5 kDa molecular-weight cutoff filter (Millipore) and initial crystallization screening was carried out using commercial sparse-matrix screens (Crystal Screens I and II, Top67, MPD and the PEG-Ion screen, Hampton Research). One condition from the PEG-Ion screen [unbuffered 20%(w/v) PEG 3350, 0.2 M ammonium chloride] gave initial crystals and further fine-screening showed that PEG 3350 without ammonium chloride gave the best quality crystals. A final drop comprising 2 µl unbuffered 12%(w/v) PEG 3350 and 2 µl gentamicin–APH(2′′)-Ib complex suspended over a reservoir containing unbuffered 16%(w/v) PEG 3350 produced large diffraction-quality crystals that were used for subsequent X-ray diffraction experiments (Fig. 1 ▶ a).
Figure 1.
(a) Typical crystals of native APH(2′′)-Ib obtained from 16%(w/v) PEG 3350. The crystals grew as thin rectangular plates. (b) The crystal used for data collection with dimensions approximately 0.20 × 0.05 × 0.01 mm.
2.3. Data collection and preliminary X-ray analysis
The APH(2′′)-Ib crystals were immersed in cryoprotectant solution [16%(w/v) PEG 3350 reservoir solution supplemented with 20% glycerol], mounted in a cryoloop, flash-frozen immediately in liquid nitrogen and stored in a sample cassette designed for use with the Stanford Automated Mounting (SAM) system (Cohen et al., 2002 ▶). The crystals were transferred to beamline BL9-2 at the Stanford Synchrotron Radiation Laboratory (SSRL) and screened for diffraction quality. X-ray diffraction data were collected from a single crystal (Fig. 1 ▶ b) maintained at 100 K with an Oxford Cryosystem, using an ADSC Quantum-315 CCD detector. A total of 180 images were collected, with an oscillation range of 1° per image, an exposure time of 30 s and a crystal-to-detector distance of 400 mm. The data were processed with the program MOSFLM (Leslie, 1992 ▶) and scaled with SCALA from the CCP4 suite of programs (Collaborative Computational Project, Number 4, 1994 ▶). Table 1 ▶ gives a summary of the data-collection statistics.
Table 1. Statistics of native data collection.
Values in parentheses refer to the data in the highest resolution shell (2.75–2.6 Å).
| Wavelength (Å) | 0.9797 |
| Space group | P21 |
| Unit-cell parameters (Å, °) | a = 79.7, b = 58.8, c = 81.4, β = 98.4 |
| Resolution range (Å) | 50.0–2.6 |
| Observed reflections | 135441 |
| Unique reflections | 23101 |
| Average redundancy | 3.7 |
| Rmerge† (%) | 0.056 (0.373) |
| 〈I/σ(I)〉 | 8.7 (2.0) |
| Completeness (%) | 99.5 (99.5) |
R
merge = 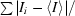
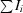 .
.
3. Results and discussion
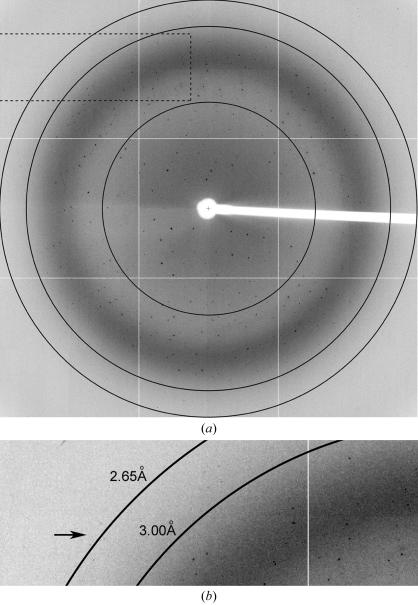
A complete X-ray data set has been collected for native APH(2′′)-Ib. The crystal diffracted to approximately 2.65 Å resolution (Fig. 2 ▶) in space group P21 (based upon systematic absences), with unit-cell parameters a = 79.7, b = 58.8, c = 81.4 Å, β = 98.4°. Calculation of the Matthews coefficient (V M; Matthews, 1968 ▶) using an estimated molecular weight of 35 kDa, gave values of 5.4 Å3 Da−1 (solvent content 77%), 2.7 Å3 Da−1 (solvent content 54%) and 1.8 Å3 Da−1 (solvent content 31%) assuming the presence of either one, two or three molecules per asymmetric unit, respectively. To determine whether non-crystallographic symmetry was present, self-rotation functions were calculated with the program MOLREP (Collaborative Computational Project, Number 4, 1994 ▶). No peaks were observed in the χ = 120° section, but the χ = 180° section showed a peak at just under 3σ which could be consistent with the presence of a non-crystallographic twofold rotation axis. Although there is no evidence that APH(2′′)-Ib functions as a dimer, the presence of two molecules in the asymmetric unit related by non-crystallographic symmetry has been observed for both APH(3′)-II (Nurizzo et al., 2003 ▶) and APH(3′)-III (Hon et al., 1997 ▶). The significance of the dimerization of the phosphotransferases is not known, since both of the APH(3′) enzymes are expected to function as monomers.
Figure 2.
(a) Diffraction image of native APH(2′′)-Ib. The resolution circles are at approximately 5.0, 3.0 and 2.65 Å resolution. (b) Close up of part of the image indicated by the dashed rectangle. Reflections near the resolution limit of 2.65 Å are indicated by an arrow.
Although the levels of amino-acid sequence identity between the members of the APH(2′′) subfamily (25–31%) and between the APH(2′′) and APH(3′) enzymes (21–23%) are somewhat low, it was thought that molecular replacement using either APH(3′)-II or APH(3′)-IIIa as a search model might give reasonable starting phases for the APH(2′′)-Ib structure. Several different models were produced from both APH(3′) structures, including partial and complete polyalanine models, separate domains and loop truncations, but no consistent rotation solution could be found. Selenomethionine-substituted APH(2′′)-Ib has now been expressed and purified. Crystals have been obtained from MES buffer pH 6.0 containing 30%(w/v) PEG 3350. Mass spectrometry has been used to verify the incorporation of selenium into the protein and additional fine-screening trials are being carried out to improve crystal quality.
Acknowledgments
This work was supported by grants from the Health Research Council of New Zealand (CAS and ENB) and the Wellcome Trust (UK). The Stanford Synchrotron Radiation Laboratory (SSRL) is funded by the Department of Energy (BES, BER) and the National Institutes of Health (NCRR, NIGMS). We gratefully acknowledge Christopher Squire and David Goldstone for help with data collection.
References
- Burk, D. L., Hon, W. C., Leung, A. K. & Berghuis, A. M. (2001). Biochemistry, 40, 8756–8764. [DOI] [PubMed] [Google Scholar]
- Chow, J. W., Zervos, M. J., Lerner, S. A., Thal, L. A., Donabedian, S. M., Jaworski, D. D., Tsai, S., Shaw, K. J. & Clewell, D. B. (1997). Antimicrob. Agents Chemother.41, 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, A. E., Ellis, P. J., Miller, M. D., Deacon, A. M. & Phizackerley, R. P. (2002). J. Appl. Cryst.35, 720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Ferretti, J. J., Gilmore, K. S. & Courvalin, P. (1986). J. Bacteriol.167, 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong, D. & Berghuis, A. M. (2002). EMBO J.21, 2323–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood, D. (1995). Antimicrobial Chemotherapy, edited by D. Greenwood, pp. 32–48. Oxford University Press.
- Hon, W. C., McKay, G. A., Thompson, P. R., Sweet, R. M., Yang, D. S. C., Wright, G. D. & Berghuis, A. M. (1997). Cell, 89, 887–895. [DOI] [PubMed] [Google Scholar]
- Kao, S. J., You, I., Clewell, D. B., Donabedian, S. M., Zervos, M. J., Petrin, J., Shaw, K. J. & Chow, J. W. (2000). Antimicrob. Agents Chemother.44, 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi, R. & Ehrenberg, M. (1994). Eur. J. Biochem.226, 355–360. [DOI] [PubMed] [Google Scholar]
- Leslie, A. G. W. (1992). Jnt. CCP4/ESF–EACBM Newsl. Protein Crystallogr.26
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Moradian-Oldak, J., Leung, W. & Fincham, A. G. (1988). J. Struct. Biol.122, 320–327. [DOI] [PubMed]
- Nurizzo, D., Shewry, S. C., Perlin, M. H., Brown, S. A., Dholakia, J. N., Fuchs, R. L., Deva, T., Baker, E. N. & Smith, C. A. (2003). J. Mol. Biol.327, 491–506. [DOI] [PubMed] [Google Scholar]
- Shaw, K. J., Rather, P. N., Hare, R. S. & Miller, G. H. (1993). Microbiol. Rev.57, 138–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. A. & Baker, E. N. (2002). Curr. Drug Targets Infect. Dis.2, 143–160. [DOI] [PubMed]
- Tsai, S., Zervos, M. J., Clewell, D. B., Donabedian, S. M., Sahm, D. F. & Chow, J. W. (1998). Antimicrob. Agents Chemother.42, 1229–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakulenko, S. B. & Mobashery, S. (2003). Clin. Microbiol. Rev.16, 430–450. [DOI] [PMC free article] [PubMed] [Google Scholar]