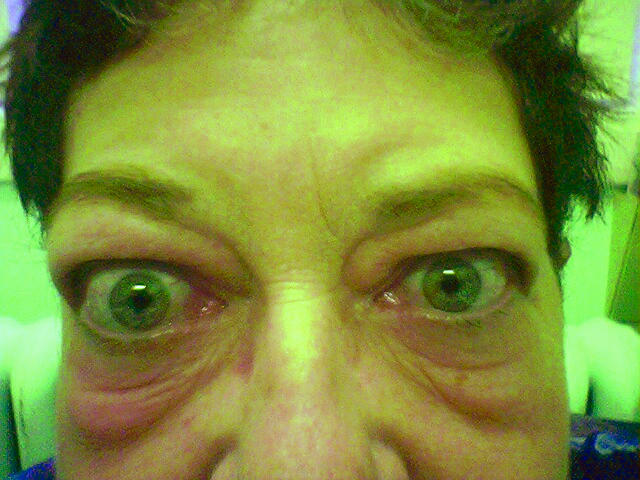
Thyroid eye disease can lead to impaired social and physical functioning. A patient’s quality of life is greatly affected, and physicians often feel powerless to control this progressive disease. Patients with thyroid eye disease (also known as Graves ophthalmopathy [GO]) frequently experience cosmetic disfigurement and functional disability. Pain, proptosis, ocular injection, swelling of the eyelids, diplopia, and, less often, blindness could result from this condition. Family physicians need to be aware of the condition’s psychosocial effect on patients and of their own crucial role during the disease process. Early detection and early treatment are important to change the course of the disease.
Case description
A mildly overweight 57-year-old ex-smoker taking no prescription medications presented to clinic complaining of her “bulging eyes.” She estimated that the bulging had been progressing for approximately 1 year, the right eye more so than the left. There was no complaint of headache or pressure behind the eyes, but she reported periorbital puffiness, eye irritation, and diplopia. There were no symptoms of hyperthyroidism; in fact, she suffered from the opposite, with increased fatigue, loss of energy, depression, and weight gain. She was very unhappy with her appearance and fearful about the possibility of progressive disfigurement. The thyrotropin (TSH) level had been normal in October 2003, but the patient stated she had a cousin who required a thyroidectomy. On examination, vision was 20/40 bilaterally with slightly decreased extraocular movement in upward gaze, pupils equal and reactive, fundi normal, and bilateral exophthalmos (R > L) The thyroid was slightly enlarged but no nodules were palpated, and there was no sign of myxedema Because of the asymmetric presentation, the patient was referred to the urgent eye clinic and the ophthalmologist’s report stated that the findings were consistent with GO. The TSH level was elevated at 9.39 mU/L (normal range 0.35 to 5.0 mU/L) and 11.36 mU/L on repeat bloodwork. Thyroid scan reported low uptake (5.2%) and multinodular goitre, suggestive of hypothyroidism, so levothyroxine was started. Orbital radiographs were normal. Computed tomography and magnetic resonance imaging of the head and orbits were performed to rule out a mass; results indicated enlargement of extraocular muscles with exophthalmos R > L, consistent with thyroid ophthalmopathy. The patient was referred to Endocrinology while continuing to have regular follow-up appointments with her family physician to address the psychosocial aspects of the disease and to later monitor the side effects related to treatment.
Graves ophthalmopathy
Thyroid eye disease is usually associated with Graves disease but can also be associated with Hashimoto thyroiditis.1 Graves ophthalmopathy is an autoimmune inflammatory disorder that can be extremely unpleasant, can be cosmetically distressing, and, occasionally, can threaten sight. Graves ophthalmopathy is clinically apparent in 25% to 50% of people with Graves disease. However, more than 70% of the remaining patients can be demonstrated to have ophthalmopathy by magnetic resonance imaging of the orbits.2
Pathophysiology
Hyperthyroidism in Graves disease is due to the binding of stimulatory autoantibodies to the thyrotropin receptor (TSH-R) on thyroid follicular cells, which leads to excessive production of thyroid hormone.3 The pathophysiology of GO is less well understood. One proposed hypothesis is an immune response to a TSH receptor–like protein in orbital connective tissue, initiating cytokine formation, fibroblast production, increased extraocular muscle (EOM) volume, and fluid accumulation.3 There is a close relationship between the level of TSH-R antibody and the severity of GO.2
During the active stage, histologic examination of the orbital adipose tissue and EOM reveals a predominantly T cell inflammatory infiltrate, fibroblast proliferation, and widespread deposition of glycosaminoglycans (GAG), which strongly supports a role of cellular immunity in the pathogenesis of thyroid eye disease.3 Gross enlargement of the muscle bodies in GO increases intraorbital pressure, which might be relieved by forward protrusion of the globe. In a sense, proptosis could provide natural orbital decompression. Enlargement of the muscles at the apex of the orbit might result in compressive optic neuropathy and vision loss. Later, the inactive stage is characterized by widespread fibrosis and scarring, which might lead to permanent proptosis and diplopia.3
Smoking
Patients with GO are 4 times more likely to be smokers or former smokers. The more cigarettes smoked daily, the greater the risk of developing GO, and smoking cessation seems to reduce this risk.4 The mechanisms involved in this association are unclear. It has been theorized that the link might be a result of increased orbital GAG synthesis in response to relative hypoxia.5
Diagnosis
The diagnosis of GO is made on clinical grounds. Specialized diagnostic tests are not required for bilateral eye disease in the presence of autoimmune thyroid disease. Hashimoto screen, thyroid-specific bloodwork (TSH, free triidothyronine [free T3], free thyroxine [free T4]), and a thyroid scan should be done to detect thyroid dysfunction. Antibody titres against TSH-R, thyroglobulin, or thyroperoxidase can help confirm a diagnosis of GO.1 True unilateral ophthalmopathy is rare and might be a result of the disease progressing more rapidly in 1 eye. It must be distinguished from other causes of unilateral eye disease, such as orbital tumours and orbital cellulitis. Orbital imaging is indicated when the patient is euthyroid, when the diagnosis is in doubt, and when optic neuropathy is suspected. A computed tomographic scan of the head and especially of the orbits shows enlarged muscle bellies and provides anatomic detail with an excellent view of the bone and sinuses.
Clinical findings
The symptoms of GO depend on the intensity of acute inflammation and its related severity. Common signs and symptoms are found in Table 1. The initial inflammatory phase might be progressive and last for 6 to 24months or, occasionally, for up to 5years, before a plateau phase is reached, usually lasting 1 to 3years. This is followed by resolution of inflammation and the final inactive burned-out stage.2
Table 1.
Signs and symptoms of Graves ophthalmopathy
| Symptoms |
| Eye irritation |
| Gritty sensation in the eye |
| Dryness |
| Epiphora (excessive lacrimation) |
| Visual blurring |
| Diplopia |
| Discomfort behind the eye |
| Pain on eye movement |
| Photophobia |
| Vision loss |
| Signs |
| Periorbital and eyelid edema |
| Conjunctival erythema |
| Chemosis (conjunctival edema) |
| Punctate epithelial erosions |
| Superior limbic keratoconjunctivitis |
| Upper eyelid retraction |
| Lower lid retraction exaggerated by exophthalmos leads to scleral show |
| Lagophthalmos (failure of the eyelids to protect the eye) |
| Proptosis |
| Ophthalmoplegia (paralysis of the eye muscles) |
| Restrictive hypotropia and esotropia |
| Loss of colour vision and perception (optic neuropathy) |
| Papilledema (optic neuropathy) |
Early transient diplopia is common and often aggravated in the morning, presumably secondary to tissue fluid accumulation during the night. Proptosis and EOM dysfunction, especially when the patient gazes upward, might produce disabling diplopia. An inability to close the eyelids could lead to corneal ulceration and vision loss. A change in colour vision might be the first sign of optic neuropathy, which occurs in 5% to 10% of patients with GO.6 This presentation warrants an urgent review by an ophthalmologist, as irreversible vision loss occurs in 30% of cases.1
Treatment
Medical treatment of GO has progressed little in the past 25 years and remains inadequate. Thyroid eye disease should be managed under the combined care of endocrinology and ophthalmology to provide accurate assessment of disease activity and response to treatment. Patients should be referred promptly, as the medical treatment of thyroid eye disease is more likely to be effective when given while the eye tissue is acutely inflamed. The primary care physician is an essential member of the management team and provides ongoing support, initiates discussions regarding the choice of therapy, monitors thyroid function, and adjusts medications.
Most patients have mild disease that requires only symptomatic measures, such as eye lubricants, avoidance of bright light and dust, sleeping with the head raised, and cessation of smoking. Restoration of euthyroidism in patients tends to stabilize or improve the coexisting ophthalmopathy and is an important part in the overall care of the patient.6
A sizable minority of patients (10% to 35%) require medical treatment and, when specific therapy for GO is indicated, there remains a lack of consensus on the best approach.4 Suggested management parameters vary depending on disease severity and activity. Mild disease requires only supportive management; moderately severe active disease can be treated with oral prednisolone 60–100 mg daily tapered over several months or with orbital radiotherapy; moderately severe inactive disease can require rehabilitative surgery, including orbital decompression, eye muscle surgery, and eyelid surgery (in that order when indicated); severe disease (optic neuropathy) can be treated with intravenous methylprednisolone or with orbital decompression.2 Immunosuppressive therapies, including oral or intravenous corticosteroids, cyclosporine, octreotide, and orbital irradiation, are the mainstays of medical treatment with less than acceptable side-effect profiles.3 Surgical intervention is necessary for those with severe proptosis, optic neuropathy, serious diplopia, or persistent widened lid aperture.7
Conclusion
Current therapies are less than satisfactory, with only two thirds of patients responding favourably, regardless of the treatment, because of residual ocular deformity.2 The effect of the disease on patients’ overall well-being or health-related quality of life has only recently been studied and suggests that the disease affects patients’ self-confidence and frustration levels, and triggers anger and concern about others’ negative appraisals of them. The study indicates the disease affects personal relationships, social interaction, and group activities.8 Patients with GO might experience a great deal of distress from the psychosocial effects of facial disfigurement, daily discomfort, and fear of vision loss. Regular follow-up with a family physician is important in order to address the risk of depression and to help the patient cope with the negative side effects associated with medical treatment. Early identification and early intervention are key to potentially salvaging a patient’s sight.
EDITOR’S KEY POINTS
The diagnosis of Graves ophthalmopathy is made on clinical grounds. Specialized tests are not required for bilateral eye disease with existing autoimmune thyroid disease.
Urgent review by an ophthalmologist is warranted if a change in colour vision (an early sign of optic neuropathy) occurs.
Because restoration of euthyroidism tends to stabilize or improve Graves ophthalmopathy, early identification and early intervention are important.
POINTS DE REPÈRE DU RÉDACTEUR
Le diagnostic de l’ophtalmopathie de Graves se fonde sur des motifs cliniques. Il n’est pas nécessaire de procéder à des tests spécialisés pour une maladie ophtalmologique bilatérale accompagnée d’une maladie thyroïdienne autoimmunitaire existante.
Il y a lieu de faire voir le patient sans délai par un ophtalmologiste s’il se produit un changement dans la vision de la couleur (un signe avant-coureur d’une neuropathie optique).
Parce que la restauration de l’euthyroïdie a tendance à stabiliser ou à améliorer l’ophtalmopathie de Graves, il est important de l’identifier et d’intervenir rapidement.
References
- 1.Boulos PR, Hardy I. Thyroid-associated orbitopathy: a clinicopathologic and therapeutic review. Curr Opin Ophthalmol. 2004;15(5):389–400. doi: 10.1097/01.icu.0000139992.15463.1b. [DOI] [PubMed] [Google Scholar]
- 2.El-Kaissi S, Frauman AG, Wall JR. Thyroid-associated ophthalmopathy: a practical guide to classification, natural history and management. Intern Med J. 2004;34(8):482–91. doi: 10.1111/j.1445-5994.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- 3.Bahn RS, Heufelder AE. Pathogenesis of Graves’ ophthalmopathy. New Engl J Med. 1993;329(20):1468–75. doi: 10.1056/NEJM199311113292007. [DOI] [PubMed] [Google Scholar]
- 4.Cawood T, Moriarty P, O’Shea D. Recent developments in thyroid eye disease. BMJ. 2004;329(7462):385–90. doi: 10.1136/bmj.329.7462.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev. 2003;24(6):802–35. doi: 10.1210/er.2002-0020. [DOI] [PubMed] [Google Scholar]
- 6.Wiersinga WM, Prummel MF. An evidence-based approach to the treatment of Graves’ ophthalmopathy. Endocrinol Metab Clin North Am. 2000;29(2):297–319. doi: 10.1016/s0889-8529(05)70133-x. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;168(5):575–85. [PMC free article] [PubMed] [Google Scholar]
- 8.Terwee CB, Dekker FW, Prummel MF, Wiersinga WM. Graves’ ophthalmopathy through the eyes of the patient: a state of the art on health-related quality of life assessment. Orbit. 2001;20(4):281–90. doi: 10.1076/orbi.20.4.281.2608. [DOI] [PubMed] [Google Scholar]


