Abstract
Deleterious effects on the heart from chronic stimulation of β-adrenergic receptors (βARs), members of the 7 transmembrane receptor family, have classically been shown to result from Gs-dependent adenylyl cyclase activation. Here, we identify a new signaling mechanism using both in vitro and in vivo systems whereby β-arrestins mediate β1AR signaling to the EGFR. This β-arrestin–dependent transactivation of the EGFR, which is independent of G protein activation, requires the G protein–coupled receptor kinases 5 and 6. In mice undergoing chronic sympathetic stimulation, this novel signaling pathway is shown to promote activation of cardioprotective pathways that counteract the effects of catecholamine toxicity. These findings suggest that drugs that act as classical antagonists for G protein signaling, but also stimulate signaling via β-arrestin–mediated cytoprotective pathways, would represent a novel class of agents that could be developed for multiple members of the 7 transmembrane receptor family.
Introduction
β-Adrenergic receptors (βARs) belong to the family of 7 transmembrane receptors (7TMRs) (1) and mediate the powerful regulatory effects on cardiac function of the catecholamine neurotransmitters epinephrine and norepinephrine. β1ARs constitute more than 70% of the cardiac βARs. Catecholamine stimulation of β1ARs results in activation of heterotrimeric G proteins followed by rapid phosphorylation of the receptor, resulting in desensitization (2). Homologous desensitization of β1ARs is brought about by phosphorylation of the receptor by G protein–coupled receptor kinases (GRKs), leading to the recruitment of β-arrestin, which then sterically interdicts further coupling to G proteins (3) and targets the receptor for internalization (3). In addition to β-arrestin’s role in terminating G protein signaling, recent studies demonstrate that β-arrestins also function as adapter molecules, allowing for the assembly of multiprotein signaling complexes such as ERKs and tyrosine kinases (4, 5). For the angiotensin II type 1A receptor (AT1AR), this second wave of β-arrestin–mediated signaling has recently been demonstrated to be independent of G protein signaling (6) and to require the activity of GRKs 5 and 6 (7).
The signaling mechanisms that underlie the activation of the mitogenic ERK growth response by 7TMRs are complex and likely result from both classical G protein–regulated effectors such as PKA and PKC and non–G protein–mediated crosstalk, such as EGFR transactivation (8). The current paradigm of transactivation involves agonist stimulation of a 7TMR, which through a number of undefined steps leads to MMP-mediated cleavage and extracellular shedding of heparin-binding EGF (HB-EGF), resulting in EGFR activation (9–11). It has become increasingly apparent that several distinct mechanisms can be involved in linking 7TMR stimulation to the transactivation of EGFRs and induction of cellular signaling. While these heterogeneous pathways may require direct 7TMR/EGFR interactions, they can also be dependent or independent of G protein activation (8, 12). Although previous studies have shown that recruitment of Gβγ subunits (13) and increases in intracellular calcium (14) precede EGFR transactivation, the precise events that immediately follow 7TMR ligand binding to trigger EGFR activation remain unknown. To better understand the molecular mechanisms that underlie transactivation of the EGFR in the heart, we used WT and mutant β1ARs in a variety of in vitro and in vivo model systems.
Results
Stimulation of β1ARs induces EGFR transactivation.
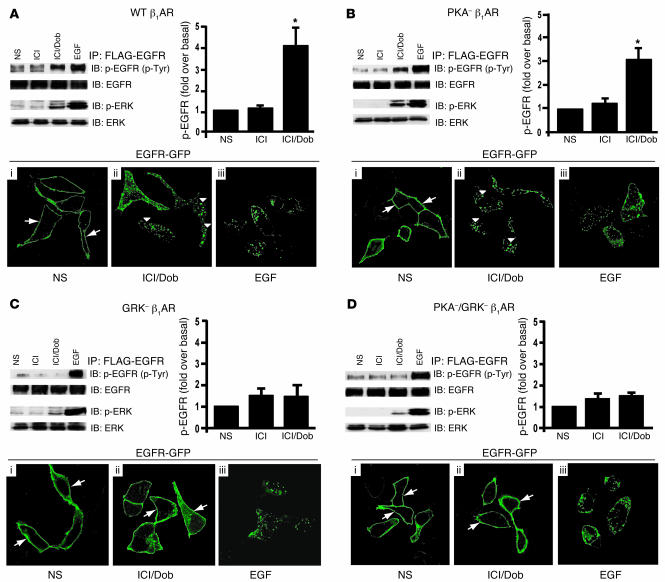
We tested whether β1AR stimulation could mediate EGFR transactivation and induce activation of downstream signaling pathways. Cells stably expressing WT β1ARs were transfected with FLAG-EGFR and stimulated with either the β1AR-specific agonist dobutamine (Dob) or EGF, following pretreatment with the β2AR-specific antagonist ICI-118,551 (ICI) to block endogenous β2ARs. Stimulation with Dob resulted in a significant increase in phosphorylation of the EGFR along with activation of ERK, which was somewhat less marked than direct stimulation with EGF ligand (Figure 1A, top panel). To test whether activation of β1ARs leads to internalization of EGFRs, cells stably expressing WT β1ARs were transfected with GFP-tagged EGFR (EGFR-GFP) and stimulated with Dob or EGF. Maximal EGFR internalization was visualized by confocal microscopy 30 minutes following Dob or EGFR stimulation (15, 16). In the absence of agonist, EGFR had a uniform membrane distribution (Figure 1A, i, arrows). In contrast, Dob stimulation resulted in internalization of EGFRs as visualized by marked redistribution into cellular aggregates (Figure 1A, ii, arrowheads). Dob-induced EGFR internalization was qualitatively similar to that evoked by EGF treatment (Figure 1A, iii).
Figure 1. β1AR-mediated transactivation of EGFR requires GRK phosphorylation sites.
HEK293 cells stably expressing WT β1AR (A), PKA– β1AR (B), GRK– β1AR (C), or PKA– GRK– β1AR (D) and transfected with FLAG-EGFR were treated with ICI and Dob (as described in Methods) and compared with cells with no stimulation (NS) or EGF stimulation. WT β1AR (A) and PKA– β1AR (B) induced increases in phospho-EGFR and phospho-ERK1/2 after 5 minutes in response to treatment with Dob, while GRK– β1AR (C) and PKA–/GRK– β1AR (D) lacked this effect; *P < 0.05 versus NS. EGFR internalization following Dob or EGF stimulation for 30 minutes was visualized using confocal microscopic analysis of HEK293 cells stably expressing the above β1AR mutants and transfected with EGFR-GFP. In the absence of agonist, EGFR-GFP was visualized on the membrane in each stable cell line (A–D, i, arrows), while EGF stimulation resulted in redistribution of EGFR-GFP into cellular aggregates (A–D, iii). Treatment of either WT β1AR or PKA– β1AR cells with Dob resulted in a similar redistribution of EGFR into cellular aggregates (A and B, ii, arrowheads), an effect that was absent in GRK– β1AR and PKA–/GRK– β1AR cells, where EGFR-GFP remained on the membrane (C and D, ii, arrows). Original magnification, ×100.
Phosphorylation of β1AR by GRK is necessary for EGFR transactivation.
To test whether GRK-mediated phosphorylation of the β1AR following agonist stimulation is a required step in EGFR transactivation, we used the mouse WT β1AR and 3 phosphorylation site–deficient mutants that we previously cloned and characterized (17). The 3 phosphorylation-defective mutants contain alanine for serine or threonine substitutions at: (a) the 4 putative PKA phosphorylation sites in the third intracellular loop and proximal carboxy tail (PKA– β1AR); (b) the 14 putative GRK phosphorylation sites within the carboxyl tail (GRK– β1AR); and (c) both sets of phosphorylation site substitutions (PKA–/GRK– β1AR) (17). To investigate whether GRK phosphorylation of the β1AR is required for EGFR transactivation, we stimulated HEK293 cells stably expressing PKA– β1AR (receptors with only GRK phosphorylation sites) with agonist and measured EGFR activation. Dob stimulation resulted in robust phosphorylation of the EGFR and ERK activation (Figure 1B), albeit somewhat less than direct EGF stimulation. Agonist stimulation of PKA– β1ARs also triggered robust EGFR internalization, as shown by the coalescence of GFP fluorescence aggregates within the cell (Figure 1B, ii, arrowheads), which again was comparable to direct EGF stimulation (Figure 1B, iii).
To determine whether GRK phosphorylation sites are essential for EGFR transactivation, cells expressing GRK– β1AR (receptors with only PKA phosphorylation sites) were transfected with FLAG-EGFR. Dob stimulation of GRK– β1AR showed no significant EGFR transactivation, minimal ERK activation (Figure 1C), and absence of EGFR internalization (Figure 1C, ii). As expected, β1ARs lacking both PKA and GRK phosphorylation sites also failed to trigger EGFR transactivation (Figure 1D). Additionally, using U2S sarcoma cells expressing endogenous β1ARs, HEK293 cells transfected with β1ARs, and freshly isolated cardiomyocytes, we show strong EGFR phosphorylation and/or ERK activation upon treatment with Dob, an effect that was blocked by pretreatment with the specific EGFR antagonist AG 1478 (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI31901DS1).
Taken together, these results demonstrate that the EGFR is transactivated in response to β1AR agonist stimulation in several cell types and that the mechanism for transactivation requires phosphorylation of the β1AR on consensus GRK phosphorylation sites, a process known to promote β-arrestin recruitment and activation (18).
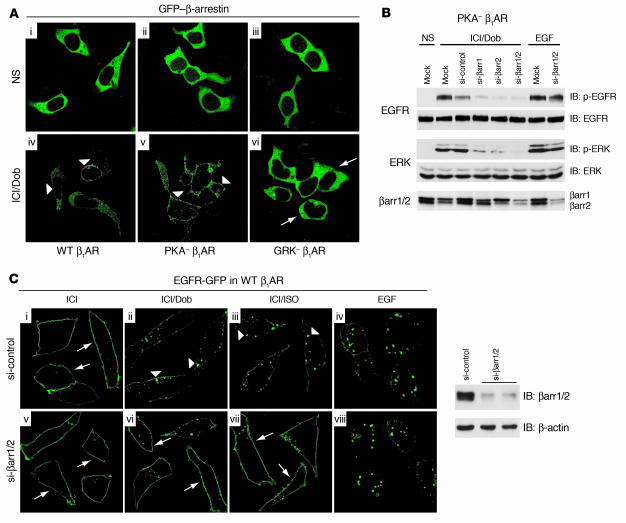
Both β-arrestin1 and β-arrestin2 are required for β1AR transactivation of EGFR.
Previous studies have shown that recruitment of activated c-Src to the β2AR complex by β-arrestin is needed for ERK activation (19). To test the possible role of β-arrestin in EGFR transactivation, HEK293 cells stably expressing either WT β1AR or β1AR mutants were transfected with GFP–β-arrestin and stimulated with Dob. Robust membrane translocation of GFP–β-arrestin was seen in cells expressing WT β1ARs and PKA– β1ARs (Figure 2A, iv and v, arrowheads) but was completely absent in cells expressing GRK– β1ARs (Figure 2A, vi, arrows).
Figure 2. β-Arrestin is required for β1AR-mediated EGFR transactivation.
(A) HEK293 cells stably expressing WT β1AR, PKA– β1AR, or GRK– β1AR were transfected with GFP–β-arrestin. In the absence of agonist, GFP–β-arrestin (green) had a cytosolic distribution (i–iii). Ten minutes of agonist stimulation (Dob) resulted in redistribution of GFP–β-arrestin to the membrane in cells expressing WT β1AR and PKA– β1AR (iv and v, arrowheads), whereas no redistribution was observed in cells expressing GRK– β1AR (vi, arrows). Original magnification, ×100. (B) HEK293 cells stably expressing PKA– β1AR were transfected with FLAG-EGFR alone (Mock) or with siRNAs targeting β-arrestin1 (si-βarr1), β-arrestin2, β-arrestin1/2, or scrambled siRNA (si-control). Reduced Dob-stimulated phospho-EGFR and phospho-ERK1/2 were observed in cells transfected with siRNA targeting β-arrestin. (C) HEK293 cells stably expressing WT β1AR were transfected with EGFR-GFP and si-control or si-βarr1/2 to knock down expression (right panel). In the absence of agonist, EGFR-GFP was located at the membrane (i and v, arrows), while EGF stimulation induced EGFR-GFP redistribution into aggregates (iv and viii). Treatment of si-control–transfected cells with Dob or ISO also resulted in redistribution of EGFR into aggregates (ii and iii, arrowheads), an effect that was diminished in si-βarr1/2–transfected cells, where EGFR-GFP remained at the membrane (vi and vii, arrows). Original magnification, ×150.
To directly determine the requirement of β-arrestin1 or β-arrestin2 for β1AR-mediated EGFR transactivation, we used siRNA that specifically targets either β-arrestin1 or β-arrestin2 or both (20). Dob stimulation of PKA– β1ARs resulted in intense phosphorylation of the EGFR associated with significant ERK activation in mock- and scrambled siRNA–transfected cells (Figure 2B). In contrast, transactivation of the EGFR and associated ERK activation were significantly blocked in the presence of siRNA targeting either β-arrestin1 or β-arrestin2 or both (Figure 2B). Immunoblotting for β-arrestin showed selective and effective depletion of specific β-arrestins in the presence of their respective siRNAs, consistent with previous studies (Figure 2B, lower panels) (20). Similar data was obtained using WT β1ARs (Supplemental Figure 1). Moreover, siRNA targeting of β-arrestin1/2 blocked EGFR internalization following either Dob or isoproterenol (ISO) stimulation but not EGF stimulation (Figure 2C, vi and vii, arrows).
Last, consistent with our siRNA experiments, transactivation of the EGFR was also completely blocked in WT β1AR– or PKA– β1AR–expressing cells in the presence of dominant-negative β-arrestin (βArrV43D) (Supplemental Figure 1).
Inability of a phosphorylation-independent β-arrestin mutant to rescue GRK– β1AR–mediated EGFR transactivation.
In the cytosol, β-arrestins are constitutively phosphorylated proteins and become dephosphorylated at the plasma membrane upon binding of activated 7TMRs. β-Arrestin dephosphorylation promotes the recruitment of endocytic proteins such as the clathrin adapter protein, AP2, as well as clathrin itself, which are required for receptor internalization (21, 22). To test whether targeting of β-arrestin to activated 7TMRs in the plasma membrane can rescue EGFR transactivation by the GRK– β1AR mutant, we used a “phosphorylation-independent” β-arrestin mutant (βarrR169E-YFP tagged). βarrR169E binds with high affinity to agonist-occupied receptors without requiring receptor phosphorylation and functions as a constitutively active molecule (23, 24). HEK293 cells stably expressing GRK– β1AR were transfected with βarrR169E-YFP along with FLAG-EGFR. Dob stimulation of GRK– β1ARs resulted in substantial recruitment of βarrR169E-YFP to GRK– β1ARs as assessed by immunoblotting for β-arrestin coimmunoprecipitating with the receptor (Figure 3A, upper panel). Moreover, translocation of βarrR169E-YFP to agonist-stimulated GRK– β1ARs resulted in robust AP-2 recruitment to the receptor, confirming that this β-arrestin mutant functions as a constitutive active β-arrestin for GRK- β1ARs (Figure 3A, lower panel).
Figure 3. GRK5 and -6 are required for β-arrestin–mediated EGFR transactivation.
(A) Cells stably expressing WT β1AR and GRK– β1AR transfected with constitutively active β-arrestin, rβarrR169E, both respond to Dob stimulation by increasing association of β-arrestin and AP2, the clathrin adapter protein, which is involved in βAR internalization. (B) GRK– β1AR cells were transfected with EGFR-GFP, localized to the plasma membrane (i, arrows), and rβarrR169E-YFP, localized in the cytosol (ii, arrowheads). EGF stimulation induced redistribution of EGFR-GFP from plasma membrane to aggregates (vii, arrows), with no effect on rβarrR169E-YFP (viii, arrowheads). Conversely, Dob stimulation resulted in redistribution of rβarrR169E-YFP from the cytosol to the plasma membrane (v, arrowheads) with no change in EGFR-GFP distribution (iv, arrows). Original magnification, ×100. (C) Transfection of GRK– β1AR cells with constitutively active rβarrR169E did not restore EGFR transactivation in response to Dob stimulation. (D) HEK293 cells transiently expressing PKA– β1AR were transfected with FLAG-EGFR alone (Mock) or with siRNAs targeting ubiquitous GRKs (si-GRK2, si-GRK3, si-GRK5, and si-GRK6) or a scrambled siRNA sequence (si-control). (E and F) Summary of 6 independent experiments showing significant inhibition of EGFR transactivation (E) and ERK1/2 activation (F) upon Dob stimulation in the cells transfected with siRNA targeting GRK5 or -6; *P < 0.001 versus Dob-stimulated Mock, si-control, si-GRK2, and si-GRK3.
We next carried out confocal microscopy experiments to test whether the recruitment of constitutively active β-arrestin to the agonist-stimulated GRK– β1AR supports transactivation. HEK293 cells stably expressing GRK– β1AR were cotransfected with βarrR169E-YFP and EGFR-GFP and stimulated with Dob. EGFR was visualized in green, and β-arrestin–YFP was visualized using a red filter to distinguish the YFP fluorescence of βarrR169E from the GFP fluorescence of EGFR. In the absence of Dob, the majority of βarrR169E-YFP was localized in the cytosol (Figure 3B, ii, arrowheads), with EGFR on the membrane (Figure 3B, i, arrows). However, Dob stimulation of GRK– β1AR was unable to induce EGFR internalization (Figure 3B, iv, arrows) or EGFR phosphorylation (Figure 3C), despite the prominent membrane translocation of β-arrestin (Figure 3B, v, arrowheads). In contrast, EGF stimulation caused EGFR internalization (Figure 3B, vii, arrows) with no discernible membrane recruitment of β-arrestin (Figure 3B, viii, arrowheads). These data suggest that receptor phosphorylation by GRK is critical for β1AR-mediated EGFR transactivation.
Selective role for GRK5 and -6 in promoting β1AR-mediated EGFR transactivation.
Since EGFR transactivation was not rescued by targeting β-arrestin to the GRK– β1AR, we next sought to determine the role for the various GRKs in the transactivation process. GRKs have been divided into 3 subfamilies based on their sequence similarity and distribution. GRK1 and -7 are exclusively expressed in the retina, GRK2 and -3 interact with Gβγ subunits through their pleckstrin homology domain; and GRK4, -5, and -6 are membrane associated (25). Since GRK2, -3, -5, and -6 are ubiquitously expressed in mammalian tissues, we investigated their role in transactivation. HEK293 cells were transfected with PKA– β1AR alone or together with siRNAs targeting GRK2, -3, -5, or -6. Dob stimulation resulted in robust phosphorylation of EGFR associated with significant ERK activation in the setting of siRNA knockdown of GRK2 and -3 expression (Figure 3, D–F). In contrast, β1AR-mediated EGFR transactivation and downstream ERK activation were almost completely blocked in the presence of siRNA targeting GRK5 or -6 (Figure 3, D–F). Similarly, GRK5 or -6 knockdown blocked WT β1AR-mediated EGFR phosphorylation and ERK activation, which was insensitive to Gi inhibition by pertussis toxin or PKA inhibition by H89 (Supplemental Figure 2).
A number of studies have shown that AT1R stimulation can transactivate EGFRs by either G protein–dependent or G protein–independent pathways (12, 26, 27). However, under the conditions of our cell culture system, angiotensin II stimulation (1 μM) in cells stably expressing AT1Rs did not induce EGFR phosphorylation or ERK1/2 phosphorylation (Supplemental Figure 2). These data are consistent with the known heterogeneity of cell culture systems for studying complex signaling pathways (11).
Molecular mechanism for β1AR-mediated EGFR transactivation.
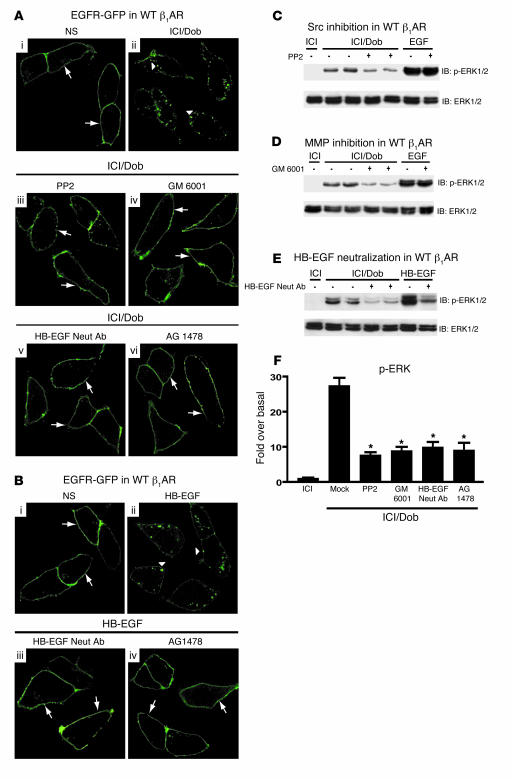
Our studies show a requirement for GRK5/6 and β-arrestin in β1AR-mediated EGFR transactivation. Since β-arrestin is known to interact with the cytosolic tyrosine kinase Src (19), we tested whether pharmacological inhibition of Src would block this process. HEK293 cells stably expressing β1ARs were transfected with EGFR-GFP and pretreated with or without PP2 prior to Dob stimulation. In the absence of PP2, EGFR-GFP was localized in punctate aggregates in the cytosol (Figure 4A, ii, arrowheads). However, in the presence of PP2, Dob failed to induce EGFR internalization (Figure 4A, iii, arrows).
Figure 4. Src, MMP, and HB-EGF are downstream from β-arrestin in β1AR-mediated EGFR transactivation.
(A) HEK293 cells stably expressing WT β1AR were transfected with EGFR-GFP. In the absence of agonist, EGFR-GFP was located at the membrane (i, arrows), while Dob stimulation induced EGFR-GFP redistribution into aggregates (ii, arrowheads). Pretreatment with inhibitors of Src (PP2), MMP (GM 6001), HB-EGF (HB-EGF neutralizing antibody [Neut Ab]), or EGFR (AG 1478) prevented the redistribution of EGFR-GFP into aggregates following Dob stimulation (iii–vi, arrows). (B) Treatment with HB-EGF (1 ng/ml) induced EGFR-GFP internalization (ii, arrowheads) compared with control (i, arrows), while pretreatment with either HB-EGF neutralizing antibody or AG 1478 abrogated this effect (iii and iv, arrows). Original magnification (A and B), ×150. ERK activation was blocked by PP2 (C), GM 6001 (D), and HB-EGF neutralizing antibody (E). (F) Quantification of phospho-ERK response. *P < 0.001 versus Mock (Dob stimulation alone); n ≥ 9 each condition.
Recent studies have suggested that Src is upstream of MMP cleavage of HB-EGF (28); thus, we tested whether MMP inhibition would abrogate β1AR-mediated transactivation. β1AR and EGFR-GFP–expressing HEK293 cells were pretreated with the MMP inhibitor GM 6001 prior to Dob stimulation. Similar to PP2 inhibition, pretreatment with GM 6001 prevented β1AR-stimulated EGFR internalization (Figure 4A, iv, arrows).
EGFR transactivation involves the autocrine/paracrine release of the potent EGFR ligand HB-EGF, which binds to the EGFR and promotes its activation by inducing dimerization and autophosphorylation (10). Accordingly, we next tested whether an inhibitor of HB-EGF would also block the β1AR-mediated transactivation process. Pretreatment of β1AR/EGFR-expressing HEK293 cells with a neutralizing antibody to HB-EGF completely abrogated β1AR-stimulated EGFR internalization (Figure 4A, v, arrows). Inhibition of EGFR activity with AG 1478 also prevented β1AR-induced EGFR internalization (Figure 4A, vi, arrows). We also tested the ability of HB-EGF neutralizing antibody and AG 1478 to prevent direct stimulation of EGFR by authentic HB-EGF and found that both reagents blocked EGFR internalization (Figure 4B, iii and iv, arrows) as compared with HB-EGF (Figure 4B, ii, arrowheads).
To test whether the activation of downstream signaling by β1AR-mediated EGFR transactivation also requires Src, MMP activity, and HB-EGF shedding, we performed experiments with the above inhibitors and measured ERK phosphorylation. Dob-stimulated ERK phosphorylation was significantly inhibited by treatment with PP2 (72%), GM 6001 (68%), and the HB-EGF neutralizing antibody (64%) (Figure 4, C–F). Taken together, these data demonstrate that the molecular mechanisms responsible for β1AR-mediated transactivation downstream of GRK5/6 and β-arrestin include Src, MMP activation, and HB-EGF stimulation of EGFR, consistent with their known role in EGFR transactivation by other 7TMRs.
Transactivation of β1ARs in vivo requires both β-arrestin and GRK5/6.
To determine whether this newly defined β-arrestin and GRK5/6-dependent signaling pathway can be recapitulated in the heart in vivo, we used mice individually lacking the genes encoding β-arrestin2, GRK5, or GRK6. Mice were pretreated with ICI and then challenged with Dob or EGF. Myocardial lysates were immunoblotted for phospho-ERK. Consistent with our cell culture experiments, Dob-mediated ERK activation was completely blocked in the β-arrestin2–knockout mice compared with their WT littermate controls (Figure 5A). Moreover, ERK activation was also completely blocked in GRK5- and -6–knockout mice (Figure 5, B and C). In the same hearts following ICI and Dob treatment, EGFR was immunoprecipitated from the myocardial lysates and immunoblotted for phosphotyrosine. Although detection of phosphorylated EGFR from myocardial lysates was less consistent than that from cell culture lysates, Dob-mediated EGFR phosphorylation was blocked in hearts from β-arrestin2–, GRK5-, and GRK6-knockout mice (Supplemental Figure 3). These data demonstrate that in vivo, the mechanism for β1AR-mediated EGFR transactivation requires GRK5/6 and β-arrestin recruitment to agonist-stimulated β1ARs.
Figure 5. In vivo β1AR-mediated EGFR transactivation requires GRK5, GRK6, and β-arrestin2.
β-Arrestin2–knockout mice (βarr2 KO; A), GRK5 KO mice (B), or GRK6 KO mice (C) and their WT littermate controls were injected with Dob following ICI pretreatment or with EGF alone. Myocardial lysates were immunoblotted with anti–phospho- and anti–total ERK1/2 antibodies. Accompanying histograms show summary data of 6 independent experiments depicting the fold increase in ERK1/2 phosphorylation following Dob treatment; *P < 0.05 versus ICI. Dob-mediated ERK1/2 phosphorylation was completely blocked in βarr2 KO, GRK5 KO, and GRK6 KO mice compared with their WT littermate controls.
β1AR-mediated EGFR transactivation occurs in the heart in vivo.
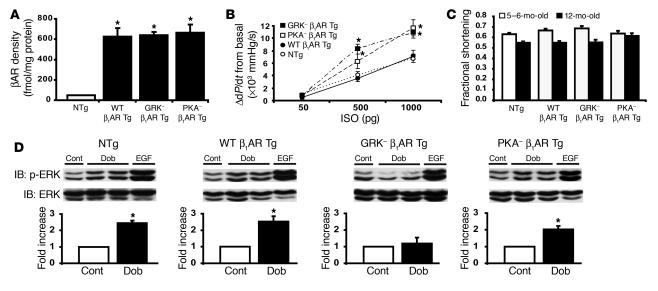
While the EGFR family appears to play a critical role in normal heart development (29–31), emerging evidence indicates that transactivation of the EGFR by HB-EGF may also be an important molecular mechanism for normal heart function (32) and may be perturbed during the development of pathological cardiac hypertrophy (33). Since stimulation of β-arrestin–mediated signaling has been shown to activate a number of cellular signaling pathways (18), we tested whether β-arrestin–dependent β1AR-mediated transactivation in the heart plays an important homeostatic role under normal and stress conditions. To address this question, we generated Tg mice with cardiac-specific overexpression of the mouse WT β1AR (WT β1AR Tg) and the 2 mutant β1ARs lacking either GRK phosphorylation sites (GRK– β1AR Tg) or PKA phosphorylation sites (PKA– β1AR Tg) used in our cell culture experiments (Supplemental Figure 4). βAR expression evaluated by [125I]cyanopindolol radioligand binding on cardiac membranes from LVs revealed an approximately 14-fold increase in receptor levels in all Tg lines compared with non-Tg (NTg) mice (Figure 6A). We next performed in vivo hemodynamic studies to determine the level of agonist-stimulated βAR responsiveness (i.e., in vivo G protein coupling) in the 3 lines of Tg mice. As expected, ISO-stimulated cardiac contractility was significantly enhanced in the GRK– and PKA– β1AR Tg mice compared with NTg or WT β1AR Tg mice (Figure 6B) (as measured by difference from basal LV dP/dtmax or absolute change in LV dP/dtmax), indicating a state of diminished βAR desensitization. In vivo, diminished βAR desensitization in the heart is observed in genetically engineered mice containing a 50% reduction in GRK2 levels (34) and in mice overexpressing an inhibitor peptide for GRK2 (35).
Figure 6. Cardiac characteristics of Tg mice overexpressing mouse WT β1AR, GRK– β1AR, or PKA– β1AR.
(A) Myocardial expression levels of βAR were equivalent in WT β1AR Tg (n = 6), GRK– β1AR Tg (n = 5), and PKA– β1AR Tg (n = 7) mice and approximately 14-fold greater than in their NTg littermates (n = 15); *P < 0.05 versus NTg littermates. (B) In vivo hemodynamics show βAR responsiveness as monitored by the increase in LV contractility (LV dP/dtmax) in WT β1AR (filled circles; n = 11), GRK– β1AR (filled squares; n = 5), PKA– β1AR (open squares; n = 7) Tg mice and NTg littermates (open circles; n = 18). Both GRK– β1AR and PKA– β1AR Tg mice showed enhanced contractile response; *P < 0.05 versus NTg littermates. (C) Conscious echocardiography in 5- to 6- and 12-month-old mice indicated no significant differences in fractional shortening among NTg and Tg mice at each age in sedentary conditions. (D) Immunoblotting of LV lysates of NTg and the 3 lines of β1AR Tg mice given i.p. injections of Dob (1 mg/kg, 10 minutes) or EGF (30 μg/kg, 15 minutes) revealed increased ERK1/2 phosphorylation in NTg, WT β1AR Tg, and PKA– β1AR Tg mice. Tg overexpression of GRK– β1AR prevented Dob-mediated ERK1/2 activation in the heart. Histograms depict the summaries of fold increase in phospho-ERK1/2 in response to Dob stimulation (n = 4–8); *P < 0.05 versus control (Cont).
Cardiac function was preserved in all 3 lines of Tg mice at 5–6 months and 12 months of age (Figure 6C and Supplemental Table 1). These data are consistent with the concept that homologous and heterologous desensitization of the receptor require phosphorylation of specific GRK residues in the C-terminal tail and PKA residues in the third intracellular loop. Interestingly, overexpression of mouse β1ARs had little untoward effects on cardiac function under normal conditions, a finding that is in contrast to Tg overexpression of human β1ARs in mice (36).
Our cell culture data (Figures 1–4) show that β1ARs lacking GRK phosphorylation sites cannot stimulate β-arrestin–mediated EGFR transactivation. To determine whether this is also true in vivo, we administered Dob to normal and Tg mice by i.p. injection, and the hearts were removed 10 minutes later for biochemical assay. We found a 2- to 3-fold increase in activation of ERK pathways in the heart in NTg, WT β1AR Tg, and PKA– β1AR Tg mice, with virtually no activation in the hearts of GRK– β1AR Tg mice (Figure 6D).
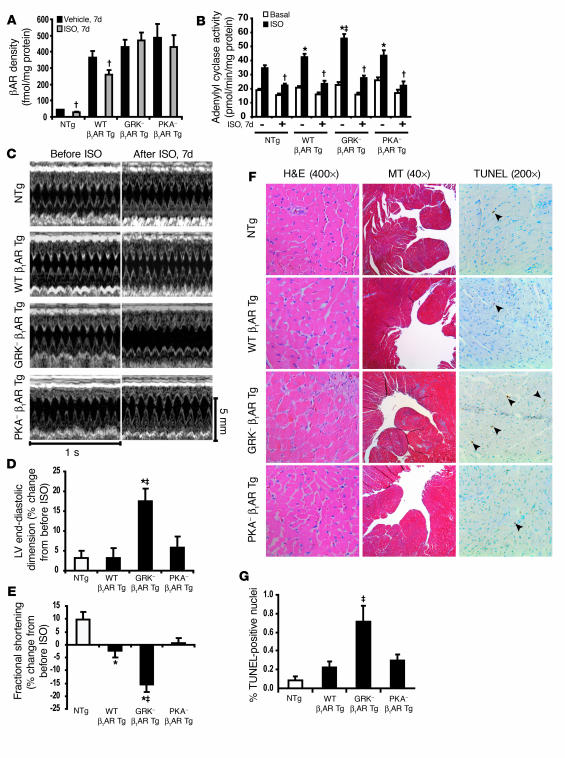
To evaluate the level of βAR–G protein coupling and second messenger generation in the hearts of the Tg mice after chronic catecholamine stimulation, we measured βAR density and adenylyl cyclase activity in crude myocardial membrane preparations after 7 days of ISO administration. Following chronic ISO treatment, βAR density was equally reduced in WT β1AR Tg mice and NTg littermates; however, neither the GRK– nor the PKA– β1AR Tg hearts showed βAR downregulation (Figure 7A). Despite the absence of βAR downregulation in the GRK– and PKA– β1AR Tg mice, β1ARs were markedly desensitized in both of these lines, as shown by blunted ISO-stimulated adenylyl cyclase activity (Figure 7B). Taken together, these data suggest that while either GRK- or PKA-mediated phosphorylation of the β1AR is sufficient to impair G protein coupling, both are required for agonist-dependent receptor downregulation.
Figure 7. Deterioration of cardiac function in GRK– β1AR Tg mice following chronic ISO treatment.
(A) WT β1AR Tg and NTg mice showed βAR downregulation in the LV membrane fraction following chronic ISO treatment (1 week), whereas GRK– β1AR Tg and PKA– β1AR Tg mice did not; †P < 0.01 versus vehicle in corresponding group. (B) Adenylyl cyclase activity following acute ISO stimulation was enhanced in all vehicle-treated mice but desensitized in all chronic ISO-treated NTg and the 3 lines of β1AR Tg mice; *P < 0.05 versus vehicle-treated NTg ISO; ‡P < 0.05 versus vehicle-treated WT β1AR Tg and PKA– β1AR Tg ISO; †P < 0.01 versus vehicle-treated ISO in each corresponding group. (C) Representative M-mode echocardiography before and after chronic ISO treatment in β1AR-Tg mice and NTg littermates. Percent changes from pre-ISO treatment in LV end-diastolic dimension (D) and fractional shortening (E) indicate significant LV dilatation and decreased fractional shortening in GRK– β1AR Tg mice following chronic ISO treatment; ‡P < 0.01 versus all Tg groups; *P < 0.05 versus NTg littermates. (F) Representative H&E, Masson trichrome (MT), and TUNEL staining following chronic ISO treatment reveals increased interstitial fibrosis (blue stain, MT panels) and apoptosis (arrows, TUNEL panels) in GRK– β1AR Tg mice. (G) Percentage of TUNEL-positive nuclei following chronic ISO treatment in NTg and the 3 lines of β1AR Tg mice (n = 5 each); ‡P < 0.05 versus all groups.
Cardioprotective role of β1AR-mediated EGFR transactivation in vivo.
Since heart failure is a condition associated with excess sympathetic stimulation, and β-arrestin–mediated signaling has been linked to the activation of protective signaling pathways (18), we tested whether β1AR-mediated EGFR transactivation would confer cardioprotection under conditions of chronic catecholamine stimulation. Remarkably, GRK– β1AR Tg mice showed significant deterioration in cardiac function with marked LV dilatation and reduced fractional shortening after 7 days of treatment with the βAR agonist ISO (Figure 7, C–E, and Supplemental Table 2). In contrast, ISO had minimal or no deleterious effect on NTg, WT β1AR Tg, or PKA– β1AR Tg mice, despite the fact that both WT β1AR Tg and PKA– β1AR Tg mice showed enhanced agonist-stimulated second messenger signaling at baseline (Figure 7B). Histological analysis of hearts from GRK– β1AR Tg mice following chronic ISO treatment revealed increased interstitial fibrosis and apoptotic nuclei compared with the WT β1AR– and PKA– β1AR–overexpressing mice (Figure 7, F and G). Taken together, these findings suggest that phosphorylation of β1AR by GRK may induce activation of cytoprotective signaling pathways that are independent of classic G protein–dependent signaling. Indeed, while all 3 Tg lines showed increased G protein signaling in the heart (as measured by enhanced adenylyl cyclase activity), only the hearts containing GRK– β1ARs that were unable to stimulate EGFR transactivation responded to catecholamines with a deterioration in cardiac function and greater myocyte apoptosis.
To explore whether the mechanism for the deleterious effect of catecholamines in the GRK– β1AR Tg mice is due to an inability to transactivate the EGFR in response to βAR stimulation, we treated normal mice with the pharmacological EGFR inhibitor erlotinib. Acutely, erlotinib treatment in vivo blocked myocardial ERK signaling by both β1AR-mediated transactivation and direct EGF stimulation in WT mice (Figure 8A) and only in hearts of WT β1AR Tg and PKA– β1AR Tg mice (Supplemental Figure 4). We then measured cardiac function in normal C57BL/6 mice following a 2-week treatment with both erlotinib (20 mg/kg, i.p. daily) and ISO. Cardiac function significantly deteriorated in mice treated with both ISO and erlotinib, as shown by the significant increase in LV diastolic dimension and reduction in fractional shortening (Figure 8, B and C). Moreover, erlotinib-induced cardiac dysfunction was associated with an approximately 3-fold increase in apoptosis compared with ISO-treated mice without the EGFR inhibitor (Figure 8, D and E). These data support our hypothesis that β-arrestin–mediated transactivation of the EGFR confers cardioprotection under conditions of catecholamine excess.
Figure 8. Pharmacological inhibition of EGFR causes dilated cardiomyopathy following chronic ISO treatment.
(A) NTg mice were pretreated for 1 hour with erlotinib (20 mg/kg; EGFR antagonist) or DMSO (10%; control) i.p., followed by i.p. injection of Dob (1 mg/kg, 10 minutes) or EGF (30 μg/kg, 15 minutes). Immunoblotting of the cardiac lysates revealed increases in Dob- and EGF-stimulated ERK1/2 phosphorylation, which was blocked by erlotinib pretreatment; *P < 0.05 versus control (n = 4–6 each). Serial echocardiographic parameters, LV end-diastolic dimension (B) and fractional shortening (C), following chronic ISO treatment in conjunction with erlotinib (20 mg/kg/d) indicated that erlotinib treatment mimics the cardiac phenotype observed in chronic ISO-treated GRK– β1AR Tg mice (Figure 6); *P < 0.05 versus each group at each time point. (D) Representative TUNEL staining following chronic ISO with or without erlotinib shows increased apoptosis (arrowheads) in LV sections (×200) from NTg mice undergoing chronic ISO with erlotinib treatment, as described above. (E) Percent TUNEL-positive nuclei in LV sections from NTg mice undergoing the following chronic treatments: vehicle plus DMSO (n = 6), vehicle plus erlotinib (n = 8), ISO plus DMSO (n = 7), and ISO plus erlotinib (n = 9); *P < 0.05 versus DMSO in same group.
Discussion
We have identified a new signaling pathway that uses β-arrestin and the EGFR to activate mitogenic and cell survival signaling pathways. We show the essential role of β-arrestin and GRK5/6 in this process. Moreover, we demonstrate in vivo that β1AR transactivation of cardiac EGFRs has a cardioprotective role in the face of chronic catecholamine stimulation.
Until recently, negative regulation of G protein signaling, i.e., desensitization, was the only known role for β-arrestins in 7TMR function. However, more recently they have also been shown to function to bring activated receptors to clathrin-coated pits for endocytosis (18) and to serve as ligand-activated scaffolds to initiate 7TMR signaling to MAPK and Akt cell-survival pathways (18).
Diverse pathways by which 7TMRs activate MAPKs continue to be discovered and constitute ever more complex signaling networks. Until quite recently, all these pathways were thought to be mediated by heterotrimeric G proteins (8). Such pathways generally, but not invariably, involve activation of second messenger kinases PKA and PKC. One category of mechanisms includes those that lead to transactivation of the EGFR, which then mediates ERK activation via the canonical Ras pathway. Multiple mechanisms connecting 7TMRs to EGFR transactivation have been described. Two examples are Src-mediated autocrine release of EGFR ligands such as HB-EGF (10) and formation of 7TMR-EGFR signaling complexes (5). Moreover, in addition to ERK activation, transactivation of receptor tyrosine kinases can lead to the recruitment of scaffold proteins, such as Shc, Grb2, and Sos, leading to a complex signaling network (37, 38).
In addition to multiple G protein–dependent pathways of ERK activation, recently it has been found that numerous 7TMRs can also utilize a novel mechanism by which β-arrestins, after translocation to the plasma membrane by receptor activation, organize and scaffold an active signaling complex including Src, RAF, MEK, and ERK (4, 19, 39). This pathway has been extensively investigated for the AT1AR (6, 40). Its kinetics, as well as the cellular localization of the activated ERK, are quite distinct from those of ERK phosphorylation stimulated by the angiotensin receptor through conventional PKC-mediated processes. To date, this β-arrestin mechanism has not been suggested to be EGFR dependent. Moreover, we examined angiotensin receptor activation of ERK in the same HEK293 cells used in previously published studies (6, 7) and found no effect of EGFR inhibitors. Thus, the β1AR-stimulated, β-arrestin–mediated EGFR transactivation mechanism defined here appears to be novel and distinct from that previously defined for β-arrestin–mediated ERK activation by several 7TMRs. It should also be emphasized that neither this nor several previous studies have found any evidence that β-arrestins function downstream of EGFR in the activation of ERK (18). While activation of c-Src by β-arrestins has been previously documented in several systems (19, 41), in this study we identify the precise molecular mechanism by which β-arrestins transactivate EGFRs following β1AR stimulation. Based on the data from our studies we would propose the following steps in this pathway. In our model (Figure 9), the events that follow ligand action of the β1AR are in sequential order: β1AR conformational change, GRK5/6-mediated receptor phosphorylation, and β-arrestin recruitment and activation. β-Arrestin recruits Src to the activated receptor. This leads to MMP activation with release of HB-EGF, which via autocrine stimulation promotes EGFR dimerization and autophosphorylation (10, 42). While the precise mechanism by which Src activates MMPs is not known, it is clear that Src activity is required for HB-EGF shedding, as we in this study and others have shown (43).
Figure 9. Signal transduction pathway of β1AR–stimulated EGFR transactivation.
Ligand stimulation of the β1AR leads to GRK5/6-mediated receptor phosphorylation and β-arrestin recruitment. β-Arrestin recruits Src, which leads to MMP activation to promote HB-EGF shedding. HB-EGF binds with the EGFR to induce dimerization and autophosphorylation and subsequent downstream signaling.
The transactivation process requires both β-arrestin1 and β-arrestin2, since elimination of either isoform in cells by siRNA, or in mice by gene targeting, abrogates EGFR activation by β1AR stimulation. This dual requirement might reflect distinct roles in the process played by each isoform or perhaps the need for heterodimerization of β-arrestin1 and –2, as recently demonstrated (44). In the case of the previously defined mechanism for β-arrestin scaffolding of ERK, various patterns of β-arrestin requirement have been defined, involving either β-arrestin1 or –2 alone or both (20, 45). Indeed, for the β2AR, Shenoy et al, recently demonstrated β-arrestin–mediated activation of ERK signaling by the β2AR that occurs independent of G protein (45), but which appears to be distinct from the pathway we define here for the β1AR.
The importance of GRK5 or -6 for transactivation is highlighted by both our in vitro and in vivo studies on hearts of GRK5- and GRK6-knockout mice. One possible mechanism for this finding is that phosphorylation of the β1AR at preferred residues on the C-terminal tail enables the receptor to interact with and activate β-arrestin in a conformation-specific fashion. The requirement for a precise receptor–β-arrestin interaction suggests that a GRK5/6-dependent receptor conformation allows for docking of β-arrestin in a 3-dimensional structure that permits activation of downstream target proteins, including possibly activation of the MMPs. This concept is supported by our data showing that (a) the non-GRK phosphorylatable receptor GRK– β1AR is unable to recruit β-arrestin and mediate EGFR transactivation; and (b) the simple targeting of a phosphorylation-independent, constitutively active β-arrestin to β1ARs lacking GRK phosphorylation sites, while adequate to recruit AP2, is insufficient to induce βAR-mediated EGFR transactivation. Our data also suggest that there are distinct receptor–β-arrestin interactions that favor desensitization, e.g., following GRK2 phosphorylation, while other receptor–β-arrestin interactions favor G protein–independent signaling, e.g., following GRK5/6 phosphorylation. These data are also consistent with our recent studies of the β2AR (45), AT1AR (7), and the V2 vasopressin receptor (46).
We have previously shown that overexpression of a GRK2 inhibitor peptide preserves βAR responsiveness and improves survival in a number of experimental models of heart failure (1). At first glance these results may seem surprising, since enhancing catecholamine sensitivity should result in enhanced toxicity. However, our current data show that reduction of GRK2 levels by siRNA in vitro does not prevent β1AR-mediated EGFR transactivation. This would be expected to enhance cell survival pathways in vivo and may thus explain the salutary effects seen with GRK2 inhibition in the heart.
The importance of β-arrestin–mediated EGFR signaling in the heart is highlighted by our in vivo studies, which demonstrate that the loss of β1AR-mediated EGFR transactivation in Tg mice results in enhanced apoptosis and cardiac deterioration. Moreover, the loss of EGFR activity in NTg animals by administration of the highly selective EGFR inhibitor (47) similarly leads to marked cardiovascular deterioration in the face of chronic catecholamine administration. Apoptotic heart cell death has been implicated in the overall process of myocardial remodeling and the transition from cardiac hypertrophy to chronic heart failure (48), which is due in part to chronic β1AR stimulation (49, 50). We have shown here that β1AR-mediated ERK and Akt activation in the heart, via β1AR-mediated EGFR transactivation, correlates with improved tolerance of catecholamine-induced cardiomyocyte toxicity.
It is interesting that our WT β1AR Tg mice did not show deterioration with age, in contrast to the results reported by Engelhardt et al. (36). While the reason for this difference is not clear, one possibility maybe that we overexpressed the mouse β1AR gene, whereas the previously published β1AR Tg used the human β1AR. It is also interesting that overexpression of the mouse WT β1AR did not lead to increased responsiveness to catecholamine stimulation (Figure 6B) despite overexpression of 600 fmol/mg protein of receptor. One explanation may be that with marked overexpression there is a sizable fraction of receptor that is constitutively desensitized. While it appears that either set of phosphorylation sites (PKA or GRK) is sufficient for receptor desensitization, both sets are required for receptor downregulation (Figure 7, A and B). The mechanism for this finding is unknown but may be due to differences in receptor trafficking, adapter protein binding, or possibly receptor/β-arrestin ubiquitination (51).
Currently, controversy exists as to the physiological role 7TMR-mediated EGFR transactivation plays in the heart. On one hand there is evidence to show that angiotensin II–stimulated EGFR transactivation can contribute to pathological hypertrophic signaling in the heart (33, 52, 53). However, it is also known that the EGFR family member HER2 plays a protective role in the development of dilated cardiomyopathy (29). Moreover, the precise role for the different ErbB receptors in βAR-mediated transactivation is an important question that will require additional investigation. Here we show how β-arrestin–mediated transactivation of the EGFR can confer cardioprotection in the face of chronic β1AR stimulation. Further investigation into the precise molecular mechanisms by which the EGFR signals in the cardiomyocyte will lead to a better understanding of the physiological importance of these processes.
Our study has important therapeutic implications for the development of novel drugs for patients with heart failure. Currently, beta blockers are considered cornerstone therapies for the treatment of heart failure, in an effort to reverse the pathological remodeling that results from heightened activity of the sympathetic nervous system. Beta blockers have been developed based on a signaling paradigm in which stimulation of the receptor by agonist (e.g., norepinephrine) leads to activation of a heterotrimeric G protein, which then leads to second messenger–stimulated signaling (e.g., via cAMP). Beta blockers competitively antagonize these cardiotoxic actions of catecholamines. However, our data show that the β-arrestin mechanism not only serves to desensitize G protein signaling but also leads to signaling in its own right by activating critical prosurvival signaling pathways such as ERK, MAPK, and Akt. These findings support the emerging concept that there exist unique ligand-receptor conformations that preferentially allow binding of β-arrestin to promote downstream signaling in the absence of G protein activation. Our data further suggest that it should be possible to develop novel receptor blocking drugs, such as beta blockers, that are able to occupy the receptor and hence act as classical antagonists for G protein signaling, while simultaneously activating signaling down this newly discovered β1AR/β-arrestin/EGFR pathway. We postulate that such “super” receptor blockers may represent an entirely novel class of agents that will share with standard beta blockers the ability to block the deleterious effects of β1AR overstimulation, while simultaneously being able to activate cell protective pathways through a β-arrestin–dependent mechanism.
In conclusion, we have identified a new signaling mechanism for the β1AR, that requires GRK5/6, involves the recruitment of β-arrestin, and leads to the transactivation of the EGFR. Activation of this β1AR/EGFR transactivation pathway provides cardioprotection in vivo under conditions of catecholamine toxicity, such as those observed in chronic heart failure. Novel ligands that occupy the receptor and block agonist interaction, thus acting as classical antagonists for G protein signaling, while at the same time activating signaling through newly discovered β-arrestin mediated pathways, would potentially have the unique ability to stimulate cardioprotective signaling.
Methods
Cell culture.
HEK293 and U2S sarcoma cells were maintained as previously described (54). The cells were transfected with cDNA encoding 2 μg FLAG-EGFR, EGFR-GFP, β-arrestin–GFP, β-arrestin1 V53D, or rβarrR169E-YFP with Lipofectamine reagent (Invitrogen). Transfected cells were incubated overnight in serum-free medium supplemented with 0.1% BSA, 10 mM HEPES (pH 7.4), and 1% penicillin prior to stimulation. Under serum starvation conditions, cells were preincubated with ICI (10 μM; β2AR antagonist; Sigma-Aldrich) and either PP2 (5 μM; Src inhibitor; Calbiochem), GM 6001 (25 μM; MMP inhibitor; Calbiochem), HB-EGF neutralizing antibody (10 μg/ml; R&D Systems), or AG 1478 (1 μM; EGFR inhibitor; Calbiochem) for 30 minutes, followed by stimulation with Dob (1 μM; Sigma-Aldrich), ISO (1 μM; Sigma-Aldrich), EGF (10 ng/ml; Roche Diagnostics), or HB-EGF (1 ng/ml; R&D Systems) for 5 minutes (immunoblotting) or 20–30 minutes (confocal microscopy). Cell lines stably expressing WTβ1AR, PKA– β1AR, GRK– β1AR, and PKA–/GRK– β1AR have been previously described (17).
Immunoprecipitation, immunoblotting, and detection.
Following stimulation, cells were washed once with PBS, solubilized in 1 ml of lysis buffer (5 mM HEPES, 250 mM NaCl, 10% glycerol, 0.5% Nonidet P-40, 2 mM EDTA, and protease inhibitors) as previously described (54). Prior to immunoprecipitation, 25 μl of whole cell lysates was aliquoted into a separate tube for protein estimation and analysis of total cellular phospho-ERK1/2. Immunoprecipitation of the FLAG epitope was carried out as previously described (17). Immunoprecipitates were resolved by SDS-PAGE and transferred to nitrocellulose membrane or PVDF (Bio-Rad) for immunoblotting. Anti-phosphotyrosine (PY20) (BD Biosciences) was used to detect tyrosine phosphorylation of EGFR at 1:3,000, and phospho-ERK1/2 (Cell Signaling) was also used at 1:3,000. Immunoblotting for total EGFR (Upstate) and total ERK (Santa Cruz Biotechnology Inc.) was carried out at 1:2,000. EGFR from the myocardial lysates was immunoprecipitated using anti-EGFR antibody (Upstate) and immunoblotted for phospho-EGFR using anti–phospho-EGFR (Tyr845) (Cell Signaling Technology) at 1:1,000. Detection was carried out using ECL (Amersham Biosciences). Densitometric analysis was performed using Bio-Rad Fluoro-S Multi-Image software. β-Arrestin immunoblotting was carried out using A1CT rabbit polyclonal antibody at a dilution of 1:3,000 as previously described (6). Anti-GRK–specific antibodies (Santa Cruz Biotechnology Inc.) were used to detect GRK2, -3, -5, and -6 as described previously (46). β-Actin and FLAG immunoblotting were carried out using monoclonal antibodies at dilutions of 1:3,000 each (Sigma-Aldrich).
Immunoblotting for myocardial phospho-ERK and phospho-Akt was performed as previously described (55). Hearts were homogenized with NP-40 lysis buffer containing 137 mM NaCl, 20 mM Tris pH 7.4, 1% NP-40, 20% glycerol, 10 mM PMSF, 1 mM Na3VO4, 10 mM NaF, 2.5 μg/ml aprotinin, and 2.5 μg/ml leupeptin.
Adult myocyte isolation.
Adult murine myocytes were isolated from WT male and female C57BL/6 mice as described previously (55). Following isolation, myocytes were resuspended in Medium 199 (Invitrogen) and seeded in 24-well cell culture plates at 2,000–3,000 myocytes per well. Adult murine myocytes were incubated for 20 minutes with 10 μM ICI (β2AR antagonist) with or without 1 μM AG 1478 (EGFR antagonist). Following pretreatment, myocytes were treated with 1 μM ISO for 5 minutes and compared with no stimulation or 10 ng/ml EGF stimulation. Analysis of phospho- and total ERK levels were carried out as described above.
Confocal laser microscopy.
Confocal microscopy was performed as previously described (54). Briefly, HEK293 cells stably expressing FLAG-tagged WT β1AR and β1AR mutants were transfected with cDNA encoding fluorescently labeled EGFR (EGFR-GFP) (2 μg). Following transfection, cells were trypsinized and plated onto 35-mm glass-bottomed culture dishes (MatTek Corp.). Following stimulation, cells were washed once with PBS and fixed in 4% paraformaldehyde for 30 minutes. EGFR internalization following Dob, ISO, HB-EGF, or EGF stimulation was visualized by green fluorescence using a single sequential line of excitation filters. EGFR-GFP internalization and βarrR169E-YFP recruitment was visualized using a combination of excitation 488 nm and emission filters between 499 and 520 nm. YFP visualization was carried out with an LSM META (Zeiss) (520–552 nm).
siRNA experiments targeting β-arrestins and GRKs.
siRNA targeting β-arrestin1, -2, or both were generated by BLAST search algorithm (http://www.ncbi.nlm.nih.gov/blast/) for a unique 21-nt sequence for β-arrestins from the National Center for Biotechnology Information. The sequence of the 21-nt siRNAs has been previously described (20, 40). The sequence of siRNAs targeting GRK2, -3, -5, or -6 have been previously described (46). 30%–40% confluent HEK293 cells stably expressing either PKA– β1AR or WT β1AR in 6-well dishes were transfected with 0.2 μg of FLAG-EGFR and 3.5 μg of siRNA using the GeneSilencer Transfection reagent (Gene Therapy Systems) as previously described (46). For confocal microscopy experiments with β-arrestin knockdown, WT β1AR cells underwent transfection with 1 μg EGFR-GFP and 3.5 μg of siRNA targeting both β-arrestins or 3.5 μg of control siRNA and were replated onto 35-mm glass-plated culture dishes after 60 hours. For GRK-knockdown experiments, HEK293 cells were transfected with 0.2 μg of FLAG-EGFR, 4 μg of siRNA targeting GRK2, -3, -5, or -6, and an appropriate amount of plasmid cDNA encoding either WT β1AR or PKA– β1AR. The expression of the receptors in transient transfection was 475–600 fmol/mg protein. All assays were performed 60–72 hours after siRNA transfection. Cells were serum starved for 12 hours before stimulation.
Histological analysis.
Heart specimens were fixed with 10% neutral buffered formalin, embedded in paraffin, and sectioned at 5-μm thickness. Sections were stained with H&E and Masson trichrome. DNA fragmentation was detected in situ using TUNEL (55). In brief, deparaffinized sections were incubated with proteinase K, and DNA fragments were labeled with fluorescein-conjugated dUTP using TdT (Roche Diagnostics). The total number of nuclei was determined by manual counting of DAPI-stained nuclei in 6 random fields per section (original magnification, ×200). All TUNEL-positive nuclei were counted in each section.
Membrane fractionation, βAR radioligand binding, and adenylyl cyclase activity.
Plasma membrane and cytosolic fractions from frozen LVs were separated by centrifugation at 37,000 g as previously described (55). Receptor binding with 20 μg of protein from plasma membrane was performed using [125I]cyanopindolol (350 pM) as described previously (55). Receptor density (fmol) was normalized to milligrams of membrane protein. Adenylyl cyclase assays were performed using 20 μg of membrane fraction as described previously (56). Generated cAMP was quantified using a liquid scintillation counter (MINAXI-4000; Packard Instrument Co., PerkinElmer).
Generation of Tg mice.
The FLAG-tagged mouse WT β1AR and 2 different mutants lacking either the putative GRK phosphorylation sites (GRK– β1AR) or the putative PKA phosphorylation sites (PKA– β1AR) were directionally subcloned into a vector downstream of α–myosin heavy chain gene promoter and upstream of the SV40 polyadenylation site (Supplemental Figure 3). The GRK– β1AR mutant was generated by mutating the serine/threonine amino acids residues in the carboxyl tail to alanine at positions 412, 417, 426, 427, 437, 443, 444, 445, 448, 450, 451, 461, 462, and 464, and the PKA– β1AR mutant was generated by mutating the serine residues in the third intracellular loop to alanine at positions 295, 296, 301, and 401 (17). Tg founders were identified by Southern blot analysis of tail DNA using the SV40 poly(A) as a probe. Tg founder mice were backcrossed to C57BL/6 mice for at least 7 generations before being used in experiments. Mice were screened by PCR with sense primer 5′-CATGGGTGTGTTCACGCTC-3′, located in mouse β1AR coding sequence, and an antisense primer, 5′-CCTCTACAGATGTGATATGGC-3′, located in the SV40 poly(A) site. βAR radioligand binding assays were carried out on multiple generations to analyze and confirm the receptor expression. Animals studies were approved by the Institutional Review Board at Duke University Medical Center.
Echocardiography.
Echocardiography was performed on conscious mice with either an HDI 5000 (Philips) or a Vevo770 (VisualSonics) echocardiograph (55).
Treatment protocol for mice.
C57BL/6 mice or β-arrestin2–knockout, GRK5-knockout, or GRK6-knockout mice received ICI (5 mg) by i.p. injection. After 5 minutes of ICI pretreatment, mice were administered either saline, the β1AR-specific agonist Dob (1 mg/kg, i.p.), or EGF (300 μg/kg, i.v.). Five minutes after Dob or EGF administration, hearts were excised and flash frozen in liquid N2 for biochemical assays.
Mini-osmotic pumps were implanted as described previously (57). ISO was dissolved in 0.002% ascorbic acid, and pumps (Alzet model 1007D; DURECT) were filled to deliver at the rate of 3 mg/kg/d over a period of 7 days. In control mice, vehicle (0.002% ascorbic acid) was used. For erlotinib treatment, erlotinib (20 mg/kg/d, i.p.) was given to mice the day before implantation of ISO pumps and continued for 2 weeks. Erlotinib was dissolved in DMSO and diluted to 10% DMSO with 0.9% saline. Erlotinib is a highly selective EGFR inhibitor with an IC50 of 2 nM for EGFR compared with ErbB2 (IC50 350 nM) (58). Control mice were injected with an equal volume of 10% DMSO. Echocardiography was performed on mice before and after ISO treatment.
Statistics.
Data are expressed as mean ± SEM. Statistical significance was determined using 1-way ANOVA (with Bonferroni correction for multiple comparisons). A P value of less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by NIH grants HL56687 and HL075443 to H.A. Rockman and HL23457 to R.J. Lefkowitz and a grant from the U.S.-Israel Binational Science Foundation to L. Barki-Harrington and H.A. Rockman. We thank Keshava Rajagopal for his insightful discussions and critique of the work.
Footnotes
Nonstandard abbreviations used: βAR, β-adrenergic receptor; AT1AR, angiotensin II type 1A receptor; Dob, dobutamine; GRK, G protein–coupled receptor kinase; HB-EGF, heparin-binding EGF; ICI, ICI-118,551; ISO, isoproterenol; 7TMR, 7 transmembrane receptor.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:2445–2458 (2007). doi:10.1172/JCI31901
Liza Barki-Harrington’s present address is: Department of Biology, Faculty of Sciences, University of Haifa, Mt. Carmel, Haifa, Israel.
Takahisa Noma, Anthony Lemaire, and Sathyamangla V. Naga Prasad contributed equally to this work.
See the related Commentary beginning on page 2396.
References
- 1.Rockman H.A., Koch W.J., Lefkowitz R.J. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 2.Koch W.J., Lefkowitz R.J., Rockman H.A. Functional consequences of altering myocardial adrenergic receptor signaling. Annu. Rev. Physiol. 2000;62:237–260. doi: 10.1146/annurev.physiol.62.1.237. [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz R.J. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. . J. Biol. Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 4.Luttrell L.M., et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maudsley S., et al. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J. Biol. Chem. 2000;275:9572–9580. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- 6.Wei H., et al. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J., et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luttrell L.M. Composition and function of G protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity. . J. Mol. Neurosci. 2005;26:253–264. doi: 10.1385/JMN:26:2-3:253. [DOI] [PubMed] [Google Scholar]
- 9.Daub H., Weiss F.U., Wallasch C., Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 10.Prenzel N., et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 11.Shah B.H., Catt K.J. Matrix metalloproteinase-dependent EGF receptor activation in hypertension and left ventricular hypertrophy. Trends Endocrinol. Metab. 2004;15:241–243. doi: 10.1016/j.tem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Werry T.D., Sexton P.M., Christopoulos A. “Ins and outs” of seven-transmembrane receptor signalling to ERK. Trends Endocrinol. Metab. 2005;16:26–33. doi: 10.1016/j.tem.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Luttrell L.M., Della Rocca G.J., van Biesen T., Luttrell D.K., Lefkowitz R.J. Gbetagamma subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J. Biol. Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 14.Zwick E., et al. Critical role of calcium-dependent epidermal growth factor receptor transactivation in PC12 cell membrane depolarization and bradykinin signaling. J. Biol. Chem. 1997;272:24767–24770. doi: 10.1074/jbc.272.40.24767. [DOI] [PubMed] [Google Scholar]
- 15.Huang F., Kirkpatrick D., Jiang X., Gygi S., Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol. Cell. 2006;21:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Tanos B., Pendergast A.M. Abl tyrosine kinase regulates endocytosis of the epidermal growth factor receptor. J. Biol. Chem. 2006;281:32714–32723. doi: 10.1074/jbc.M603126200. [DOI] [PubMed] [Google Scholar]
- 17.Rapacciuolo A., et al. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J. Biol. Chem. 2003;278:35403–35411. doi: 10.1074/jbc.M305675200. [DOI] [PubMed] [Google Scholar]
- 18.Lefkowitz R.J., Shenoy S.K. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 19.Luttrell L.M., et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 20.Ahn S., Wei H., Garrison T.R., Lefkowitz R.J. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. J. Biol. Chem. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- 21.Laporte S.A., et al. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl. Acad. Sci. U. S. A. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naga Prasad S.V., et al. Phosphoinositide 3-kinase regulates beta2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/beta-arrestin complex. J. Cell Biol. 2002;158:563–575. doi: 10.1083/jcb.200202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovoor A., Celver J., Abdryashitov R.I., Chavkin C., Gurevich V.V. Targeted construction of phosphorylation-independent beta-arrestin mutants with constitutive activity in cells. J. Biol. Chem. 1999;274:6831–6834. doi: 10.1074/jbc.274.11.6831. [DOI] [PubMed] [Google Scholar]
- 24.Violin J.D., Ren X.R., Lefkowitz R.J. G-protein-coupled receptor kinase specificity for beta-arrestin recruitment to the beta2-adrenergic receptor revealed by fluorescence resonance energy transfer. J. Biol. Chem. 2006;281:20577–20588. doi: 10.1074/jbc.M513605200. [DOI] [PubMed] [Google Scholar]
- 25.Pitcher J.A., Freedman N.J., Lefkowitz R.J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 26.Eguchi S., Frank G.D., Mifune M., Inagami T. Metalloprotease-dependent ErbB ligand shedding in mediating EGFR transactivation and vascular remodelling. Biochem. Soc. Trans. 2003;31:1198–1202. doi: 10.1042/bst0311198. [DOI] [PubMed] [Google Scholar]
- 27.Thomas W.G., Qian H., Smith N.J. When 6 is 9: ‘uncoupled’ AT1 receptors turn signalling on its head. Cell. Mol. Life Sci. 2004;61:2687–2694. doi: 10.1007/s00018-004-4245-2. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter G. EGF receptor transactivation mediated by the proteolytic production of EGF-like agonists. Sci. STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.15.pe1. [DOI] [PubMed] [Google Scholar]
- 29.Crone S.A., et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 30.Gassmann M., et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 31.Lee K.F., et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 32.Iwamoto R., et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3221–3226. doi: 10.1073/pnas.0537588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshioka J., et al. Cardiomyocyte hypertrophy and degradation of connexin43 through spatially restricted autocrine/paracrine heparin-binding EGF. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10622–10627. doi: 10.1073/pnas.0501198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rockman H.A., et al. Control of myocardial contractile function by the level of beta-adrenergic receptor kinase 1 in gene-targeted mice. J. Biol. Chem. 1998;273:18180–18184. doi: 10.1074/jbc.273.29.18180. [DOI] [PubMed] [Google Scholar]
- 35.Koch W.J., et al. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 36.Engelhardt S., Hein L., Wiesmann F., Lohse M.J. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luttrell L.M. ‘Location, location, location’: activation and targeting of MAP kinases by G protein-coupled receptors. J. Mol. Endocrinol. 2003;30:117–126. doi: 10.1677/jme.0.0300117. [DOI] [PubMed] [Google Scholar]
- 38.Natarajan K., Berk B.C. Crosstalk coregulation mechanisms of G protein-coupled receptors and receptor tyrosine kinases. Methods Mol. Biol. 2006;332:51–77. doi: 10.1385/1-59745-048-0:51. [DOI] [PubMed] [Google Scholar]
- 39.DeFea K.A., et al. beta-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn S., Shenoy S.K., Wei H., Lefkowitz R.J. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J. Biol. Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 41.Luttrell D.K., Luttrell L.M. Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene. 2004;23:7969–7978. doi: 10.1038/sj.onc.1208162. [DOI] [PubMed] [Google Scholar]
- 42.Fischer O.M., Hart S., Gschwind A., Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc. Trans. 2003;31:1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- 43.Pierce K.L., et al. Epidermal growth factor (EGF) receptor-dependent ERK activation by G protein-coupled receptors: a co-culture system for identifying intermediates upstream and downstream of heparin-binding EGF shedding. J. Biol. Chem. 2001;276:23155–23160. doi: 10.1074/jbc.M101303200. [DOI] [PubMed] [Google Scholar]
- 44.Storez H., et al. Homo- and hetero-oligomerization of beta-arrestins in living cells. J. Biol. Chem. 2005;280:40210–40215. doi: 10.1074/jbc.M508001200. [DOI] [PubMed] [Google Scholar]
- 45.Shenoy S.K., et al. β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J. Biol. Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 46.Ren X.R., et al. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fabian M.A., et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 48.Kang P.M., Izumo S. Apoptosis and heart failure: a critical review of the literature. Circ. Res. 2000;86:1107–1113. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 49.Communal C., Singh K., Sawyer D.B., Colucci W.S. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 50.Zhu W.Z., et al. Linkage of β1-adrenergic stimulation to apoptotic heart cell death through protein kinase A–independent activation of Ca2+/calmodulin kinase II. . J. Clin. Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shenoy S.K. Seven-transmembrane receptors and ubiquitination. Circ. Res. 2007;100:1142–1154. doi: 10.1161/01.RES.0000261939.88744.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas W.G., et al. Adenoviral-directed expression of the type 1A angiotensin receptor promotes cardiomyocyte hypertrophy via transactivation of the epidermal growth factor receptor. Circ. Res. 2002;90:135–142. doi: 10.1161/hh0202.104109. [DOI] [PubMed] [Google Scholar]
- 53.Asakura M., et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 54.Naga Prasad S.V., Jayatilleke A., Madamanchi A., Rockman H.A. Protein kinase activity of phosphoinositide 3-kinase regulates beta-adrenergic receptor endocytosis. Nat. Cell Biol. 2005;7:785–796. doi: 10.1038/ncb1278. [DOI] [PubMed] [Google Scholar]
- 55.Perrino C., et al. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J. Clin. Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Esposito G., et al. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 57.Nienaber J.J., et al. Inhibition of receptor-localized PI3K preserves cardiac β-adrenergic receptor function and ameliorates pressure overload heart failure. J. Clin. Invest. 2003;112:1067–1079. doi: 10.1172/JCI18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rabindran S.K. Antitumor activity of HER-2 inhibitors. Cancer Lett. 2005;227:9–23. doi: 10.1016/j.canlet.2004.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.