Abstract
Acute stimulation of cardiac β1-adrenergic receptors (β1ARs) by norepinephrine represents the strongest endogenous mechanism for increasing cardiac function, but long-term stimulation induces cardiomyocyte apoptosis and contributes to cardiac disease. These effects have been attributed to coupling of the β1AR to the stimulatory G protein (Gs) and classical cAMP-mediated signaling. In this issue of the JCI, Noma and colleagues report that cardiomyocyte β1ARs may in addition deliver an antiapoptotic signal through transactivation of EGFRs (see the related article beginning on page 2445). Their findings provide a perspective for a novel class of receptor ligands that may direct β1AR signaling toward alternative signaling pathways.
In cardiac failure, enhanced levels of norepinephrine resulting from activation of the sympathetic nervous system lead to chronic stimulation of cardiac β-adrenergic receptors (βARs) (1). While this acutely serves to adapt cardiac output to the systemic needs, chronic stimulation of the β1 adrenergic receptor (β1AR) is clearly detrimental and contributes to cardiomyocyte hypertrophy, cell death, and progression of the disease (2–4). The deleterious consequences of β1AR stimulation are thought to be mediated by coupling of the β1AR to the stimulatory G protein (Gs) and subsequent activation of a defined set of downstream targets (Figure 1). The heart adapts to the chronically elevated norepinephrine concentrations by blunting the response to agonist stimulation, a process termed desensitization (3, 5). However, desensitization is not sufficient to compensate entirely for the chronic overstimulation of the system, and over prolonged periods of time the toxic consequences of β1AR stimulation prevail (6, 7). Mechanistically, desensitization involves a reduction in β1AR number (downregulation) and function (uncoupling). The latter occurs through phosphorylation of the third intracellular loop of β1AR and the C terminus by PKA and, more importantly, through G protein–coupled receptor kinases (GRKs) (8), followed by translocation and binding to the receptor by the multifunctional protein β-arrestin (9). Phosphorylation and subsequent desensitization of the β1AR is appreciated predominantly as a self-protective mechanism that partially decreases Gs-mediated signal transduction.
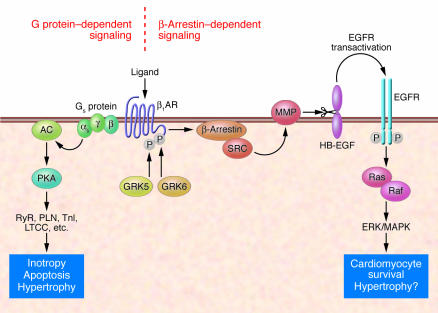
Figure 1. β1AR-mediated signal transduction in cardiomyocytes.
Classical ligand-activated βARs enhance cardiac contractility by coupling to Gs, formation of cAMP by adenylyl cyclase (AC), and PKA-dependent phosphorylation of various target proteins (e.g., ryanodine receptor [RyR]; phospholamban [PLN], troponin I [TnI], and the L-type Ca2+ channel [LTCC]). Chronic β1AR stimulation is detrimental and induces cardiomyocyte hypertrophy and apoptosis. In this issue of the JCI, Noma et al. (10) have delineated a novel signaling pathway leading to GRK- and β-arrestin–dependent Src-kinase (SRC) and MMP activation. MMP activation in turn sheds HB-EGF from the cell surface, and this serves as a ligand for cardiomyocyte EGFRs, which mediate ERK/MAPK activation. This pathway protects from β1AR-induced cardiomyocyte apoptosis but has been associated with cardiac hypertrophy.
In this issue of the JCI, Noma and coworkers (10) present exciting new evidence that may change the way we think about β1AR desensitization in the heart. Their data suggest that GRK-mediated phosphorylation of the β1AR not only serves to reduce Gs/PKA-mediated signal transduction, but in parallel, serves to initiate a powerful antiapoptotic signal by mediating transactivation of the EGFR through a β-arrestin–dependent pathway (Figure 1). This process crucially depends on two cardiac GRK isoforms — GRK5 and GRK6 — that have to date not been intensely studied with respect to their cardiac function. The data from Noma et al. provide additional evidence that β-arrestins serve as multifunctional proteins that may induce G protein–independent intracellular signaling (9).
EGFR transactivation and cardiovascular biology
Transactivation of receptor tyrosine kinases through G protein–coupled receptor (GPCR) activation was first described for Gq-coupled receptors that mediate ERK/MAPK activation in fibroblasts (11). Shedding of an extracellular domain of heparin-binding EGF (HB-EGF) that then acts as an agonist at EGFRs was identified as the underlying mechanism (Figure 1). These early studies linked two signaling paradigms that had previously been regarded as separate entities. Subsequent work has detailed the mechanism of HB-EGF shedding through membrane proteins of the ADAM (a disintegrin and metalloproteinase domain) family of metalloproteinases, which are activated upon GPCR stimulation (12). Thus, in addition to their matrix-remodeling functions, metalloproteinases may directly control EGFR signaling by proteolytically activating EGFR ligands.
Since then, GPCR-mediated transactivation has been demonstrated for a wide variety of Gq- and Gi-coupled receptors (13). Comparably less evidence has been obtained for primarily Gs-coupled receptors. For the β2AR, a PKA-induced switch from Gs to Gi coupling has been shown to mediate EGFR transactivation and subsequent ERK activation (14, 15). Remarkably, β2AR-mediated EGFR transactivation was found to be independent of metalloproteinase activation and involves βγ-subunits and c-Src (16).
Several studies have implicated signaling through EGFRs in cardiovascular biology, with marked divergence as to their presumed physiological role. Asakura et al. have identified ADAM12-mediated shedding of HB-EGF and subsequent EGFR activation as a critical step in angiotensin II type 1a receptor– (AT1AR-) and α1AR-mediated cardiac hypertrophy (17). Similar data have been obtained for α1AR-mediated vascular smooth muscle hypertrophy (18). Thus, EGFR transactivation may be viewed as being detrimental according to these studies. In contrast, mice with cardiomyocyte-restricted deletion of the EGFR ErbB2 display dilated cardiomyopathy, suggesting a cardioprotective role (19). Also, more indirect evidence based on the cardiac side effects of trastuzumab (an antibody directed against ErbB2) in breast cancer therapy suggests that EGFR activation may be required for maintaining cardiac integrity (20). However, these data have to be interpreted with caution, as the cardiotoxic side effects of trastuzumab may also be related to cellular or complement-dependent cytotoxicity initiated by binding of the antibody to cardiomyocytes. With respect to downstream signaling, it will be interesting to determine whether direct activation of the EGFR or activation via transactivation involve different downstream signaling pathways in cardiomyocytes.
Alternative signaling of the β1AR to EGFRs
The exciting data by Noma et al. (10) represent the first evidence that the β1AR may signal through the EGFR to induce a survival signal in cardiomyocytes. This study may change the linear way we think about adrenergic receptor signaling in the heart. Naturally, this raises new questions. What is the molecular chain of events that leads to metalloproteinase activation? The mechanisms by which GPCRs mediate activation of metalloproteinases are generally not well understood and may involve Gα, Gβγ, c-Src, and PKC (21). Noma et al. have convincingly demonstrated the involvement of GRK5, GRK6, Src-kinase (SRC), and β-arrestin. Subsequent work will be needed to elucidate the detailed mechanism integrating these players. It is remarkable that both GRK5 and GRK6 are needed to mediate EGFR transactivation. In this respect it will be interesting to determine whether direct phosphorylation of the β1AR by GRK5 and GRK6 is needed and, if so, what the sites of receptor phosphorylation are.
Further studies will also be needed to determine the cardiomyocyte metalloproteinase responsible for EGFR ligand shedding and whether EGFR signaling is also beneficial for the heart when chronically activated, which is presumably the case in heart failure. With respect to a role in heart failure, other groups have shown that EGFR signaling is involved in the prohypertrophic effects of angiotensin II in cardiomyocytes (17). Thus, the heart may ultimately pay a high price (in the form of hypertrophy) for decreasing cardiomyocyte apoptosis through chronic EGFR signaling. Follow-up studies in chronic models of cardiac disease will be needed to answer these questions.
Alternatively, the discrepancies between ATR- and βAR-mediated EGFR transactivation might involve spatial compartmentation of receptors and their downstream signaling or different kinetics of βAR versus ATR signals.
These studies also prompt the question: What are the downstream signaling mechanisms of β1AR-mediated EGFR transactivation besides ERK/MAPK activation? Are they different from direct activation of the EGFR? Recent evidence suggests that this might be indeed the case (21), and this could help to explain the uncertainties regarding the role of EGFR signaling in cardiomyocyte biology. In addition, it will be interesting to delineate the molecular determinants that cause different modes of receptor transactivation for the β2AR and the β1AR. Further insight into this issue might be gained from the identification of the relevant residues phosphorylated by GRK5 and GRK6 and through studies using receptor chimeras.
Therapeutic perspectives
Most importantly, the findings of Noma et al. (10) may unlock the door to novel therapeutic interventions. This possibility may seem unlikely at first glance, as both the deleterious effects through Gs as well as the protective signal through EGFR transactivation are activated through the β1AR and current cardiovascular treatment regimes heavily rely on blockade of βAR signaling. However, recent evidence indicates that GPCR ligands may target a receptor signal to specific intracellular effectors (22), and direct analysis of receptor conformational changes during activation by fluorescence resonance energy transfer microscopy has revealed marked differences for different antagonists as to the conformational change of the β1AR (23). Ultimately, this could lead to the development of ligands that may act as antagonists toward a certain (deleterious) intracellular effector while acting as agonist to another (beneficial) effector. This would allow for the development of GPCR blockers that might prove superior to currently existing substances.
The findings of Noma et al. (10) are likely to gain additional importance in disease contexts. This is because crucial elements of EGFR transactivation are upregulated during cardiac hypertrophy or failure, such as metalloproteinases, HB-EGF, EGFR, and ERK. Thus, under conditions of cardiac growth and disease, “biased” antagonists that favor signaling through EGFR transactivation may prove particularly effective.
Taken together, the results reported in this issue by Noma et al. (10) support the existence of a novel β1AR signaling pathway in the heart. Apart from their “classical” signaling properties, β1ARs are able to signal via activation of EGFRs to the Ras/Raf/MAPK pathway and thereby compensate, at least in part, for the deleterious effects caused by chronic Gs/PKA signaling.
Acknowledgments
The author is supported by the Rudolf Virchow Center/DFG Research Center for Experimental Biomedicine supported by the Deutsche Forschungsgemeinschaft, Trigen, Sanofi-Aventis, and the Bavarian Ministry of Economics. The generous support of the BMBF-Heart Failure Network (TP8) and the Interdisziplinäres Zentrum für Klinische Forschung Würzburg is gratefully acknowledged.
Footnotes
Nonstandard abbreviations used: ADAM, a disintegrin and metalloproteinase domain; β1AR, β1-adrenergic receptor; AT1AR, angiotensin II type 1A receptor; GRK, G protein–coupled receptor kinase; Gs, stimulatory G protein; HB-EGF, heparin-binding EGF.
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:2396–2398 (2007). doi:10.1172/JCI33135.
See the related article beginning on page 2445.
References
- 1.Cohn J.N., et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 2.Bristow M.R. β-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 3.Engelhardt S. β-adrenergic receptors in heart failure. Heart Fail. Clin. 2005;1:183–191. doi: 10.1016/j.hfc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Lohse M.J., Engelhardt S., Eschenhagen T. What is the role of β-adrenergic signaling in heart failure? Circ. Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 5.Bristow M.R., et al. Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N. Engl. J. Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 6.Bisognano J.D., et al. Myocardial-directed overexpression of the human β1-adrenergic receptor in transgenic mice. . J. Mol. Cell. Cardiol. 2000;32:817–830. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 7.Engelhardt S., Hein L., Wiesmann F., Lohse M.J. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. . Proc. Natl. Acad. Sci. U. S. A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockman H.A., Koch W.J., Lefkowitz R.J. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 9.Lefkowitz R.J., Rajagopal K., Whalen E.J. New roles for β-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol. Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Noma T., et al. β-Arrestin–mediated β1-adrenergic receptor transactivation of the EGFR confers cardioprotection. . J. Clin. Invest. 2007;117:2445–2458. doi:10.1172/JCI31901. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daub H., Weiss F.U., Wallasch C., Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 12.Prenzel N., et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 13.Fischer O.M., Hart S., Gschwind A., Ullrich A. EGFR signal transactivation in cancer cells. Biochem. Soc. Trans. 2003;31:1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- 14.Daaka Y., Luttrell L.M., Lefkowitz R.J. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. . Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 15.Maudsley S., et al. The β2-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. . J. Biol. Chem. 2000;275:9572–9580. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- 16.Drube S., Stirnweiss J., Valkova C., Liebmann C. Ligand-independent and EGF receptor-supported transactivation: lessons from β2-adrenergic receptor signalling. . Cell Signal. 2006;18:1633–1646. doi: 10.1016/j.cellsig.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Asakura M., et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H., Chalothorn D., Jackson L.F., Lee D.C., Faber J.E. Transactivation of epidermal growth factor receptor mediates catecholamine-induced growth of vascular smooth muscle. Circ. Res. 2004;95:989–997. doi: 10.1161/01.RES.0000147962.01036.bb. [DOI] [PubMed] [Google Scholar]
- 19.Crone S.A., et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 20.Slamon D.J., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 21.Ohtsu H., Dempsey P.J., Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am. J. Physiol. Cell Physiol. 2006;291:C1–C10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 22.Galandrin S., Bouvier M. Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol. Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- 23.Rochais F., et al. Real-time optical recording of β1-adrenergic receptor activation reveals supersensitivity of the Arg389 variant to carvedilol. . J. Clin. Invest. 2007;117:229–235. doi: 10.1172/JCI30012. [DOI] [PMC free article] [PubMed] [Google Scholar]