Abstract
Since the first diabetic was treated with insulin in 1922, millions of patients have relied on frequent insulin injections and glucose monitoring to combat the disease and its complications. Improved immunosuppressive regimens in islet transplantation developed in the Edmonton protocol raised the hopes of diabetics worldwide for a complete cure and insulin independence. However, transplant success has proven to be short-lived and accompanied by significant side effects. Using a clever genetic model for conditional ablation of pancreatic β cells in vivo, Nir and colleagues show in this issue of the JCI that the immunosuppressant drugs clinically inhibit β cell proliferation in the diabetic setting (see the related article beginning on page 2553). They also demonstrate that β cells have a remarkable regenerative capacity and that normal β cell mass can recover even in the setting of hyperglycemia. Their new mouse model should aid in the development of improved immunoregulatory strategies and in the elucidation of the molecular pathways that govern β cell regeneration.
Although insulin therapy for diabetes has come a long way since the hormone’s discovery in the 1920s, insulin therapy cannot cure the disease and prevent its devastating complications in the long term. Beginning in the 1970s, whole-organ pancreas transplantation showed that replenishment of the β cell complement could fully normalize glucose homeostasis in patients. Transplantation of cadaveric islets into the liver via the portal vein was not successful until 2000, when a glucocorticoid-free immunosuppressive regimen was used and shown to result in insulin independence even in formerly brittle (that is, metabolically unstable) diabetic recipients (1). This advance increased the hopes for diabetics all over the world for a healthy life and led to dramatic policy changes in biomedical research funding. Because available organ donors would never be sufficient for the treatment of the millions of type 1 diabetics in the world, the NIH and private foundations funded major initiatives to develop new sources of β cells for transplantation, for instance through the National Institute of Diabetes and Digestive and Kidney Diseases–funded Beta Cell Biology Consortium (BCBC; http://www.betacell.org).
Multiple sources of cells have been considered as substrates for the development of this cell-based therapy. Among these are embryonic stem cells, hepatocytes, and putative resident endocrine stem cells within the pancreas; yet the success of these efforts has been limited thus far (2). Recent work has refocused the attention of diabetes researchers onto the mature β cell itself, because it was shown using genetic lineage tracing that under normal circumstances in healthy rodents, new β cells are mostly derived from existing β cells (3). Additional support for this concept came from successive labeling of proliferating cells using two thymidine analogs, which showed that adult pancreatic islets do not contain specialized progenitors that turn over rapidly, but rather that most or all β cells have the potential to proliferate (4). What these models had not established is whether β cell proliferation can also occur in the diabetic setting.
A mouse model for β cell regeneration in the diabetic state
To address this question, Nir and colleagues developed, and describe in this issue of the JCI, a new model for the conditional and controlled ablation of pancreatic β cells (5) (Figure 1). Using an elegant genetic trick, they were able to express diphtheria toxin specifically in β cells at a time of their choosing, which resulted in the elimination of β cells through apoptosis. The treated mice were severely diabetic, consistent with an 80% reduction in their β cell mass and pancreatic insulin content. Unlike commonly used chemical agents employed for the killing of β cells such as streptozotocin, there was no bystander effect of the diphtheria toxin, and the degree of β cell ablation was reproducible. The beauty of the new system is that it allowed the investigators to record the spontaneous recovery of β cell function after ablation by simply removing the inducing agent. Remarkably, within a few weeks after diphtheria toxin expression ceased, β cell mass and glucose homeostasis were normalized. This was accompanied by a dramatically increased rate of β cell proliferation. The fact that β cell mass staged a near-full recovery even in the face of severe hyperglycemia (greater than 500 mg/dl) contradicts the widely held notion that glucotoxicity is a major impediment to β cell proliferation or survival (6, 7).
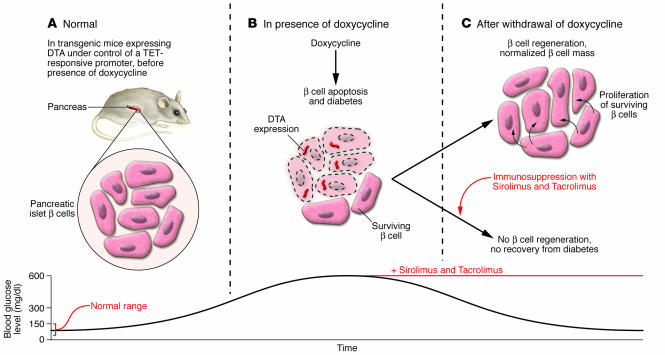
Figure 1. Immunosuppressants inhibit β cell recovery after targeted ablation.
(A) Normal β cell mass and glucose homeostasis in transgenic mice before application of doxycycline, the inducer of β cell death, in the transgenic animal model used by Nir et al. in this issue of the JCI (5). DTA, diphtheria toxin A. (B) Doxycycline treatment activates the expression of diphtheria toxin A specifically in the β cells of the pancreas, reducing β cell mass by 80% and causing hyperglycemia. (C) Regeneration of β cell mass through β cell proliferation and normalization of glucose homeostasis after doxycycline withdrawal. In the presence of Sirolimus and Tacrolimus, the immunosuppressants used in human islet transplantation, the regenerative response of the β cells is inhibited, and hyperglycemia persists.
Next, Nir and colleagues (5) addressed the question: What is the origin of the β cells that reappear even in a diabetic setting? The new β cells most likely did not arise from a reactivation of the fetal developmental program, as the neurogenin 3 gene, a marker of fetal endocrine precursors, was not reactivated in their model. Next the authors used genetic pulse-labeling of differentiated β cells, followed by labeling of those cells that had reentered the cell cycle, in order to assess the origin of the new cells. These data showed that the cells that rebuild the destroyed islets are largely if not exclusively derived from existing β cells, and not from neogenesis or from expansion of non–β cell precursors. As the authors point out, it is of course possible that in a setting of even more severe injury to the endocrine pancreas, other cells, including pancreatic duct or acinar cells or even hematopoietic progenitors, are being recruited to the β cell lineage. In this regard, the endocrine pancreas might be similar to the liver, in which hepatocytes are able to repopulate the organ under conditions of mild or medium injury, but activation of oval cells or even hematopoietic stems cells can contribute to the recovery when hepatocyte proliferation is blocked.
Immunosuppression: a necessary evil in islet transplantation
Beyond proving the regenerative capacity of the mature rodent β cell, even in the face of diabetes, what are the potential applications of this new model? Nir et al. (5) provide the first example of a practical application in which they are able to follow the regenerative response of the β cell in a precisely defined setting by evaluating this response in the presence of the immunosuppressive regimen used in human islet transplantation. Using the typical clinical doses of Sirolimus (also known as Rapamycin, a mammalian target of rapamycin inhibitor) and Tacrolimus (also known as FK506, a calcineurin inhibitor) during the recovery phase in their mice, they show that β cell regeneration is dramatically blunted in the presence of these agents. Because β cell proliferation is limited and β cell mass cannot expand, hyperglycemia persists. This raises the possibility that the immunosuppressive regimen required for the allogenic islet transplant is at the same time inhibiting the residual proliferative capacity of these transplanted human islets. Likewise, might it be possible to extend the normoglycemic life diabetics enjoy after an islet transplant using different immunosuppressive regimens?
Future directions
Using the conditional β cell ablation model described here (5), researchers will be able to test the impact of immunosuppressive drugs on β cell regeneration as a complement to testing their immunoregulatory efficacy. In addition, the new mouse model offers other clinical applications. For instance, agents and drugs suspected of being able to promote β cell expansion can be tested in a diabetic setting in vivo. Increasing existing β cell mass is a promising avenue for the treatment of type 2 diabetes, and initial positive results have been obtained with one such agent, exendin 4, an analog of glucagon-like peptide 1 (8–10). Likewise, the new model will allow researchers to uncover negative effects of commonly used drugs in β cell regeneration. Finally, these mice will also be instrumental in elucidating the molecular mechanisms that govern the proliferative response of the mature β cell.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:2380–2382 (2007). doi:10.1172/JCI33375.
See the related article beginning on page 2553.
References
- 1.Shapiro A.M., et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Bonner-Weir S., Weir G.C. New sources of pancreatic beta-cells. Nat. Biotechnol. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 3.Dor Y., Brown J., Martinez O.I., Melton D.A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 4.Teta M., Rankin M.M., Long S.Y., Stein G.M., Kushner J.A. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev. Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Nir T., Melton D.A., Dor Y. Recovery from diabetes in mice by β cell regeneration. J. Clin. Invest. 2007;117:2553–2561. doi: 10.1175/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownlee M. A radical explanation for glucose-induced β cell dysfunction. J. Clin. Invest. 2003;112:1788–1790. doi: 10.1172/JCI200320501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wajchenberg B.L. beta-cell failure in diabetes and preservation by clinical treatment. Endocr. Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 8.Brubaker P.L., Drucker D.J. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo R.A., et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 10.Kendall D.M., et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]