Abstract
Decades of research in the area of developmental psychobiology have shown that early life experience alters behavioral and brain development, which canalizes development to suit different environments. Recent methodological advances have begun to identify the mechanisms by which early life experiences cause these diverse adult outcomes. Here we present four different research programs that demonstrate the intricacies of early environmental influences on behavioral and brain development in both pathological and normal development. First, an animal model of schizophrenia is presented that suggests prenatal immune stimulation influences the postpubertal emergence of psychosis-related behavior in mice. Second, we describe a research program on infant rats that demonstrates how early odor learning has unique characteristics due to the unique functioning of the infant limbic system. Third, we present work on the rodent Octodon degus, which shows that early paternal and/or maternal deprivation alters development of limbic system synaptic density that corresponds to heightened emotionality. Fourth, ajuvenile model of stress is presented that suggests this developmental period is important in determining adulthood emotional well being. The approach of each research program is strikingly different, yet all succeed in delineating a specific aspect of early development and its effects on infant and adult outcome that expands our understanding of the developmental impact of infant experiences on emotional and limbic system development. Together, these research programs suggest that the developing organism’s developmental trajectory is influenced by environmental factors beginning in the fetus and extending through adolescence, although the specific timing and nature of the environmental influence has unique impact on adult mental health.
Keywords: development, learning, stress, juvenile, infant, schizophrenia, limbicsystem, amygdala, olfaction, maternal deprivation
INTRODUCTION
Over half a century ago, developmental psychobiologists demonstrated the profound, enduring effects of early life experience on behavioral development using a rodent model (Denenberg, 1963; Greenbough, 1975; Levine, 1962; Turkewitz, Gordon, & Birch, 1965). This work clearly demonstrated the interplay between an organism’s genes and the environment, which produces a bidirectional cascading interaction between genes and environment (Schneirla, 1966). This work was quickly replicated in nonhuman primate models (Harlow & Harlow, 1965; Levine ;Suomi, Harlow, & Domek, 1970) and applied to humans (Ainsworth, 1969; Bowlby, 1965). This research has profoundly influenced how we think about child development. It is now clear that early life experiences canalize behavioral development and initiate a developmental trajectory that predisposes one to physical and psychiatric health or illness. A theoretical framework that guides much developmental work is the assumption that early life events enable the infant to form characteristics that will be valuable for the environment it is likely to encounter in adulthood. This process must occur within a behavioral system that is designed specifically to enable the infant to cope with the unique environmental demands of infancy. Thus, while the infant must modify its behavior to cope with the demands of infancy, such as learning about its caregiver and finding the nipple for nursing, the brain is being organized for an environment that the infant has not yet encountered. Thus far, behavioral literature suggests that a stressful early life prepares infants for a stressful adult life. However, early life experiences can also increase the probability of diseases that are not adaptive and do not prepare the infant for adult life, such as the long-term disability caused by schizophrenia.
The overriding theoretical commonality of both pathological and adaptive outcomes is that complex interactions between genes and the environment at specific times in development result in specific outcomes. Remarkably, this is the same framework that guided developmental research decades ago, although current methodological techniques have begun to identify specific genetic and neural mechanisms that produce specific behavioral endpoints. Particularly, the development of new and powerful techniques to monitor physiological, cellular, and molecular events has begun to highlight mechanisms of early experiential effects. These advances have permitted the integration of our existing behavioral understanding with anatomical, physiological, cellular, and molecular processes that underlie behavioral development. Understanding the mechanisms that “decide” and control developmental trajectory is necessary for successful childrearing, as well as therapeutic intervention to ameliorate or attenuate mistakes in gene expression or a harsh environment.
Unique problems are encountered by researchers when assessing infant development and adult outcome. The infant brain is not an immature version of the adult brain; rather it is designed to function within the unique ecological niche of infancy. For example, while the infant must have a neurobehavioral repertoire to optimize finding a nipple with milk, the adult must have a neurobehavioral repertoire optimizing foraging for food. While both systems have the similar end result of procuring food, striking differences are found with respect to sensory information integration with past experiences and learning, as well as the motor output.
Here, we present four different research programs that demonstrate the intricacies of early environmental influences on behavioral and brain development in both pathological and normal development. The approach of each research program is strikingly different, yet all succeed in delineating a specific aspect of early development and its effects on infant and adult outcome that expand our understanding of developmental impact of infant experiences on emotional and limbic system development.
PRENATAL IMMUNOLOGICAL PRECIPITATION OF MAL-NEURODEVELOPMENT: RELEVANCE FOR SCHIZOPHRENIA AND BEYOND
Research from the laboratory of Joram Feldon at the Swiss Federal Institute of Technology Zurich assesse: environmental effects on development during the fetal period, eloquently illustrating that early life in utero represents a period of unique vulnerability with potentially longlasting effects on mental health. Disturbances directed at the maternal host can lead to direct physiological changes in the fetal environment when the fetus is undergoing rapid development, notably in the central nervous system. This is supported by epidemiological studies showing that maternal bacterial and viral infections during pregnancy are linked to a number of neuropsychiatric disorders with a presumed neurodevelopmental origin, including schizophrenia (Brown et al., 2004a; Mednick, Machon, Huttunen, & Bonett, 1988; O’Callaghan et al., 1994), autism (Miller et al., 2005; Rodier & Hyman, 1998) and mental retardation (Rantakallio & von Wendt, 1985; Revello & Gerna, 2004). Although the precise mechanisms underlying these epidemiological associations remain to be fully worked out, one favored hypothesis suggests that the maternal immune response to the infectious process may be the critical causative event, as the precise identity of the infectious agents do not seem to matter (Brown et al., 2004a,b; Buka et al., 2001; O’Callaghan et al., 1994; Patterson, 2002; Pearce, 2001).
The Cytokine Hypothesis of the Maternal Infection Model of Schizophrenia
Recently, several research groups, including Feldon and colleagues at ETH Zurich, have brought this epidemiologically motivated hypothesis into the realm of laboratory experimentation by establishing an animal model of prenatal maternal infection in mice for the evaluation of adult brain and behavioral pathology with an initial focus on schizophrenia (Meyer, Feldon, Schedlowski, & Yee, 2005, 2006a; Meyer et al., 2006b, Meyer, Schwendener, Feldon, & Yee, 2006c; Ozawa et al., 2006; Shi, Fatemi, Sidwell, & Patterson, 2003; Zuckerman, Rehavi, Nachman, & Weiner, 2003; Zuckerman & Weiner, 2003, 2005). A single intravenous injection of the inflammatory agent PolyI:C (polyriboinosinic-polyribocytidilic acid) to pregnant dams was used to induce an acute immune response in the maternal host in activating the release of a host of immunologically relevant cytokines.
Cytokines are low molecular-weight proteins, which function as the main chemical mediators of the host’s immune response against various infectious agents (Borish and Steinke, 2003; Curfs, Meis, & Hoogkamp-Korstanje, 1997). Besides their crucial roles in the recruitment and proliferation of T- and B-lymphocytes in the periphery, cytokines also modulate the survival and differentiation of neurons (Ling, Potter, Lipton, & Carvey, 1998; Marx, Jarskog, Lauder, Lieberman, & Gilmore, 2001; Potter, Ling, & Carvey, 1999) as well as the growth and organizational complexity of dendrites (Gilmore, Jarskog, Vadlamudi, & Lauder, 2004). Alterations in cytokine levels are expected to exert multiple effects on the developing central nervous system. Amongst the diverse repertoire of cytokine responses associated with an infection, elevation of proinflammatory cytokines in the maternal host, and subsequently in the fetal environment, has been considered as the key trigger for malneurodevelopment in the developing fetus (Brown et al., 2004b; Buka et al., 2001; Gilmore & Jarskog, 1997; Patterson, 2002;Pearce, 2001). The faulty development of the central nervous system initiated in utero may remain relatively silent and only lead to the overt psychopathological traits in later life. This is a hallmark of the ontogenesis of several psychiatric disorders, including schizophrenia, of which early life events as well as genetic predisposition are of etiological relevance (Bracha, Torrey, Bigelow, Lohr, & Linington, 1991; Bracha, Torrey, Gottesman, Bigelow, & Cunniff, 1992; Gilmore et al., 1996; Jakob & Beckman, 1986; Murray, Jones, & O’Callaghan, 1991).
Maternal Immune Challenge in Pregnancy Leads to Multiple Psychopathology
There is considerable experimental evidence in animals supporting a causal link between prenatal immune challenge and the postpubertal emergence of multiple brain and behavioral abnormalities. The multiplicity of psychopathology observed in the adult offspring readily suggests that the perturbations caused by maternal immune challenge are widespread, and fundamental to a range of normal psychological functions. Characterization at the behavioral phenotypic levels thus demands the use of multiple behavioral paradigms, as important factors such as the intensity and timing of the immune challenge (Meyer et al., 2005, 2006a,b, c) may not reveal their impacts unless a sufficient comprehensive portrayal of the adult psychopathology is achieved (to be discussed later).
Amongst the multitude of behavioral, cognitive and psychopharmacological deficits revealed, many could be related to the endophenotypes of schizophrenia and related disorders. Psychosis-like abnormalities have been detected in adult offspring following prenatal exposure to bacterial endotoxin (Borrell, Vela, Arévalo-Martin, Molina-Holgado, & Guaza, 2002;Fortier, Joober, Luheshi, & Boksa, 2004; Golan, Lev, Hallak, Sorokin, & Huleihel, 2005), human influenza virus (Shi et al., 2003), or the viral mimic PolyI:C (Meyer et al., 2005, 2006a,b,c; Ozawa et al., 2006; Zuckerman et al., 2003; Zuckerman & Weiner, 2003, 2005). The wide spectrum of behavioral and cognitive dysfunctions observed in offspring subjected to prenatal immune activation included deficits in exploratory behavior and social interaction (Meyer et al., 2005, 2006b; Shi et al., 2003), deficiency in sensorimotor gating in the form of reduced prepulse inhibition (Borrell et al., 2002; Meyer et al., 2005; Ozawa et al.; Shi et al., 2003), impaired selective learning (Meyer et al., 2005, 2006a, c; Zuckerman et al., 2003; Zuckerman & Weiner, 2003,2005), perseverative behavior (Meyer et al., 2006b), as well as working memory deficits in the watermaze (Meyer et al., 2005) and impaired cognitive performance in novel object recognition tasks (Ozawa et al., 2006). Adult animals subjected to prenatal immune activation also display an enhanced locomotor reaction to indirect dopamine-receptor agonists such as D-amphetamine and methamphetamine (Meyer et al., 2005; Ozawa et al., 2006; Zuckerman et al., 2003) and the noncompetitive NMDA-receptor antagonist, dizocilpine (MK-801) (Meyer, Schwendener, Feldon, Yee, unpublished observation; Zuckerman & Weiner, 2003).
The responsiveness of these various behavioral deficits to known antipsychotic drug (typical and atypical neuroleptics) treatment has also been examined. Normalization of behavioral and cognitive performance in prenatally immune challenged offspring has been demonstrated in the paradigms of prepulse inhibition (Borrell et al., 2002;Shi et al., 2003), latent inhibition (Zuckerman et al., 2003;Zuckerman & Weiner, 2003,2005), and novel object recognition task (Ozawa et al., 2006). These findings have added to the predictive and construct validity of the model.
Notably the maternal infection model captures the expectation of the neurodevelopmental perspective of schizophrenia in that the underlying pathophysiological and neuropathological mechanisms are progressive in nature. The affected subjects do not exhibit the full range of functional impairments until after puberty (Meyer et al., 2006c; Ozawa et al., 2006; Zuckerman et al., 2003; Zuckerman & Weiner, 2003) even though signs of neuroanatomical changes could be identified before adulthood (Fatemi et al., 1999, 2002; Meyer et al., 2006c). The model thus offers the possibile identification of prodromal psychosis and the investigation of possible prevention interventions (Remington & Shammi, 2004), highlighting the potential of cytokine-oriented intervention as preventive treatment.
The Psychopathological Profile Depends on the Precise Time of Maternal Infection
The impact of maternal infection on neurodevelopment is expected to vary during the course of fetal life. First, the physiological changes experienced by the mother during the course of pregnancy can influence the precise pattern of immune responses (Entrican, 2002; Sargent, 1993). Second, it is expected that the vulnerability of the fetus to inflammation-mediated malneurodevelopment critically depends on the precise stage of prenatal development. In relation to schizophrenia, there is a debate over the suggestion that the second trimester of human pregnancy confers the maximal risk for schizophrenia in the resultant offspring (reviewed in Cannon & Clarke, 2005; Machón, Mednick, & Huttunen, 1995; McDonald & Murray, 2000; McGrath, Castle, & Murray, 1995). Although there is support that maternal infection subsequent to the second trimester is not involved ( Machón et al.; McGrath et al., 1995), several studies have questioned if the second trimester is critical (e.g., Crow & Done, 1992; Mino, Oshima, Tsuda, & Okagami, 2000; Morgan et al., 1997; Selten & Slaets, 1994). Indeed, serologic evidence of prenatal infuenza in the etiology of schizophrenia has indicated that maternal infection particularly in the first trimester is associated with a higher incidence of schizophrenia in the offspring (Brown et al., 2004a). Hence, maternal infections over a more extended period, from early- to mid-pregnancy, can increase the risk of schizophrenia.
Experiments have been conducted in Feldon’s laboratory to address this issue by comparing the phenotypic profiles of offspring having experienced maternal PolyI: C-induced immune activation on gestation days (GD) 6, 9, 13, and 17 (Meyer et al., 2006a,b). In one study, they demonstrated a loss of latent inhibition in adult mice following prenatal immune challenge on GD 6 and GD 9; this effect was marginal in mice treated on GD 13, and was absent in mice treated on GD 17. Hence, the vulnerability of latent inhibition impairment appears to decrease as a function of gestation days. On the other hand, the expression of the US-preexposure effect was abolished by the prenatal immunological manipulation regardless of the precise time of maternal immune challenge (Meyer et al., 2006a). In another study, Meyer et al. (2006b) added to their initial findings by demonstrating a functional double dissociation between prenatal immunological stimulation on GD 9 and on GD17: the former suppressed spatial exploration, while the latter led to response perseveration in discrimination reversal (Meyer et al., 2006b). Maternal immune activation in early, mid, and late pregnancy can indeed lead to divergent behavioral dysfunction. However, the characterization should not be considered as “all-or-none”: a distinct pattern of psychopathology can be expected depending on the time of maternal immune activation. A similar conclusion can be derived from analyses at the immunological and anatomical levels (see Meyer et al., 2006b). However, the data are in support for the general impression that comparison between immune activations at early/mid and at late pregnancy yielded qualitatively more distinct patterns of pathology than the comparison between early and mid-pregnancy treatment. The conclusion based on the PolyI:C maternal infection model indicates that early/mid and late gestation periods in mice correspond to two windows with differing vulnerability to adult behavioral dysfunction, brain neuropathology in early adolescence, and of the acute cytokine responses in the fetal brain.
Feldon and colleagues also raised the interesting hypothesis that the behavioral contrast between early/mid and late pregnancy immune activation may be related to differing symptom clusters of schizophrenia. It is known that schizophrenia is associated with a myriad of psychological and cognitive abnormalities. Symptoms tend to segregate into clusters (e.g., the positive-negative symptoms dichotomy in schizophrenia), which appear to follow separate developmental courses (Gross, 1997; Murray, O’Callaghan, Castle, & Lewis, 1992; Sporn et al., 2004). They also respond differentially to distinct classes of pharmacotherapy (Kapur and Remington, 2001; Maguire, 2002; Miller, 2004), and are suggested to be associated with dysfunctions localized to discrete brain regions (Liddle et al., 1992). The possibility that these symptom clusters or even subtypes of schizophrenia may correspond to distinct neurodevelopmental disturbances has been suggested based on genetic and epidemiological evidence (e.g., Cannon et al., 2003; Stöber, Franzek, Beckmann, & Schmidtke, 2002).
One hypothesis emerging from this large collection of data set postulates that the distinction between early/mid and late pregnancy immune challenge may capture the positive-negative dichotomy of schizophrenia. Experimental data available so far readily indicate that prenatal immune challenge at early/mid pregnancy in mice leads to a variety of abnormalities associated with positive symptoms of schizophrenia, including increased sensitivity to acute dopaminergic stimulation (Meyer et al., 2005), loss of latent inhibition (Meyer et al., 2005,2006a), and deficits in sensorimotor gating (Meyer et al., 2005; Meyer, Yee, Feldon, unpublished observation), while prenatal immune activation on late gestation particularly leads to the emergence of behavioral, cognitive, and pharmacological dysfunctions associated with negative symptoms such as perseverative behavior (Meyer et al., 2006b), and increased sensitivity to NMDA-receptor antagonism (Meyer, Feldon, Yee, unpublished observation; see also Zuckerman & Weiner, 2005). Further examination and evaluation of the structural and functional consequences of in utero immune challenge at different times of gestation may provide important insight into the fundamental neuroimmunological mechanisms underlying the segregation of positive and negative symptoms in schizophrenia from a neurodevelopmental point of view.
Maternal–Infant Interaction in Early Postnatal (Preweaning) Life in the Infection Model of Schizophrenia
Feldon and colleagues have also extended their studies to examine the possible contributions of early postnatal mother–infant interaction following prenatal maternal infection. Until now, the putative roles of postnatal maternal factors have received little attention in the causal relationship between prenatal immune challenge and the post-pubertal emergence of psychosis-related abnormalities.
It is known that immunological stimulation is strongly associated with the activation of the stress response axes, including the hypothalamic-pituitary-adrenal (HPA) axis (Goebel et al., 2002; Haddad, Saade, & Safieh-Garabedian, 2002; Webster & Sternberg, 2004), and maternal physiological and/or psychological stress during pregnancy has been shown to alter postpartum maternal behavior (Brayden, Altemeier, Tucker, Dietrich, & Vietze, 1992; Meek, Dittel, Sheehan, Chan, & Kjolhaug, 2001; Patin et al., 2002). However, it has not yet been shown whether gestational immunological stress is associated with alterations in postpartum maternal factors that may result in an unfavorable rearing environment for neonatal development. Since the rodent and primate brain undergoes significant development and maturation in early postnatal life, disruption of the intricate mother–infant relationship as a result of gestational immune challenge may confer additional risk for the early-life organism to develop psychosis-related behavior in later life. Indeed, human adoption studies have demonstrated that adoptees with a high-genetic risk for schizophrenia are significantly more sensitive to adverse rearing patterns in adoptive families than are adoptees at low risk (Mäki et al., 2005; Tienari et al., 2004; Wahlberg et al., 1997), who therefore are more prone to the emergence of psychosis in later life. This is in full agreement with the hypothesis that the early external environmental factors in combination with genetic predisposition determine the eventual expression of the diseased phenotype (Cardno et al., 1999; Franzek & Beckmann, 1996; Petronis, 2004; Tsuang, 2000).
In the first attempt to dissect the prenatal and postnatal maternal contributions in the infection model of schizophrenia and related disorders in mice, Feldon and colleagues used a crossfostering design to directly compare the relative impact of mid-pregnancy inflammatory events and postnatal maternal factors on the emergence of selective learning deficits in juvenile and adult offspring (Meyer et al., 2006c). The authors found that prenatal immunological stimulation led to the postpubertal emergence of selective learning deficits regardless of the postnatal adoption procedure, that is, latent inhibition deficiency was equally observed in prenatally immunechallenged offspring that were adopted by control or immune-stimulated surrogate mothers. This readily suggested that the adoption of prenatally immune challenged neonates by control surrogate rearing mothers did not confer any protective effects against the subsequent emergence of selective learning deficits. Hence, inflammation-mediated disruption of fetal brain development as such is sufficient to induce significant adult psychopathology (Brown et al., 2004a,b; Gilmore & Jarskog 1997; Patterson, 2002; Pearce, 2001).
In addition, Meyer et al. (2006c) went on to show that control neonates born to nonchallenged dams but raised by immune-challenged surrogate mothers displayed learning disabilities (in the form of impaired aversive conditioning) upon reaching the juvenile and preadolescent stage of development. This suggests that immunologically induced stress (e.g., activation of the HPA axis and subsequent sickness behavior) experienced during pregnancy may affect maternal postpartum factors in such a way that neonatal adoption by immune-challenged surrogate mothers is sufficient to induce maturationindependent learning disabilities in the fostered offspring. Since the association of the adoption by immunechallenged surrogate mothers and the emergence of learning impairments may have important implications in the area of developmental psychobiology, further effort is warranted to investigate more thoroughly the neurodevelopmental and neurophysiological consequences of this crossfostering procedure. One clear approach would be to obtain direct measures of postpartum maternal behavior and physiology following immunological stress during pregnancy in order to identify the critical pathological factors in postnatal mother–infant interaction.
According to the analysis of the relative contributions of pre- and postnatal factors by Feldon and colleagues, inflammation-mediated events occurring during prenatal fetal brain development may be critical in precipitating adult deficit in selective learning (perhaps more closely related to positive symptomatology), while postnatal maternal factors may act in precipitating general learning disabilities, reflecting a more global deterioration of brain function (and perhaps more closely related to negative symptomatology).
Summary
An integrative summary of the data from the laboratories of Feldon and others is schematized in Figure 1, which also includes specific working hypotheses addressed by on-going research. The prenatal immune activation in rodents represents a novel and powerful class of environmental-neurodevelopmental animal models to study the disease process of schizophrenia and related disorders. It enjoys a high level of face, construct, and predictive validity, with intrinsic epidemiological and immunological relevance known to the disease. Its formulation is also unique in not being based on any specific neurological features of the disease, and thus does not rely on any specific presumption of the disease’s neural substrate. The fact that the critical experimental manipulation is conducted prenatally allows a multifaceted, longitudinal monitoring of the disease process as it unfolds during the course of neurodevelopment from childhood to adulthood, and the concomitant evaluation of the influence of external environmental factors. It is a clear illustration of how early life experience in the form of a single welldefined biologically relevant manipulation can, over development of time, cumulate into specific psychopathology and pathophysiology in adulthood experienced during pregnancy may affect maternal postpartum factors and thus interfere with normal maternal–infant interactions in early postnatal life. This may modulate postnatal brain maturation processes and additionally induce behavioral and cognitive dysfunctions in later life.
FIGURE 1.
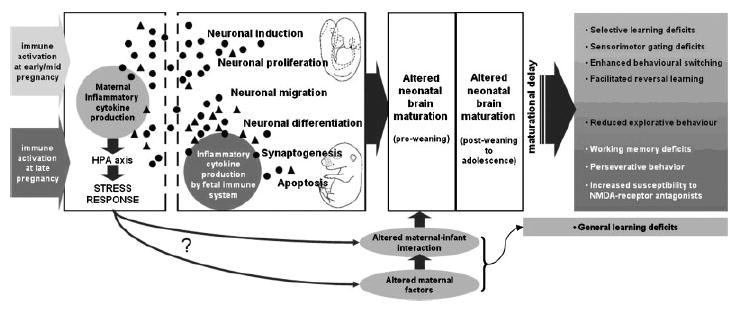
The diagram illustrates the hypothesized early prenatal and postnatal factors involved in the precipitation of brain and behavioral pathology following prenatal immune challenge. Exposure to viral and bacterial pathogens rapidly results in the production of pro-(●) and anti-inflammatory (▲) cytokines in the maternal host. The specificity of the pathogen-induced cytokine production is critically influenced by the genetic background (not shown) as well as the gestational stage. Immunological stimulation is also strongly associated with the activation of the stress response axes such as the hypothalamic-pituitary-adrenal (HPA) axis. Trans-placental transfer of maternally produced cytokines and/or production of cytokines at the maternal-fetal interface lead to an elevation of these molecules in the fetal environment, including the fetal brain. The fetal system at late but not early/mid gestation contributes to this process by endogenously synthesizing and secreting specific cytokines following maternal immune activation. Once in the fetal brain, cytokines affect ongoing neurodevelopmental processes depending on the fetal developmental stage and cytokine specificity. Disturbance of normal fetal brain development by cytokine-associated inflammatory events results in altered neonatal brain maturation and ultimately leads to the emergence of adult psychopathology. The precise pattern of brain and behavioral pathology emerging in adulthood is also critically determined by the time of prenatal immune challenge, that is, prenatal immune activation at early/mid gestation precipitates behavioral dysfunctions associated with (but not limited to) the positive symptoms of schizophrenia, whereas prenatal immune activation at late gestation triggers behavioral pathology associated with the negative symptoms of the disease. Immunologically induced stress (e.g., via activation of the HPA axis).
RESEARCH CORTICAL AND LIMBIC SENSORY PROCESSING DURING ONTOGENY
Recent work in Regina Sullivan’s lab suggests that an infant’s unique processing of sensory information simultaneously shapes both the infant’s behavior to its present environment, and also sends the limbic system on a trajectory to prepare it for adult life (Sullivan et al., 2000; Morriceau & Sullivan 2006). A major focus of this work has been an exploration of the experience-dependent emergence of amygdala (and more generalized limbic system) function on behavior in mother–infant interactions. The limbic system integrates physiological, emotional, and memorial components of an individual’s response to its sensory environment. As in other brain systems, limbic system function, and thus this integration, is shaped by early experience. A number of labs have examined the development of limbic system function through, for example, description of the ontogeny of hippocampal synaptic plasticity, the ontogeny of fear or stress responses, or the ontogeny of associative memory—all approaches primarily representative of limbic system intrinsic activity and output.
A new collaborative effort between the laboratories of Donald Wilson and Regina Sullivan at the University of Oklahoma, however, has focused on understanding ontogeny of limbic system input, with a goal of understanding how limitations or unique characteristics of neonatal sensory processing may shape limbic system function and development. In rodents, the single largest sensory input to the limbic system is olfaction, with the olfactory cortex, amygdala, and hippocampus only two to three synapses from the nose. The results to date suggest that while sensory input can drive limbic system neurons early in development, the dramatic change in temporal structure of these responses with age may influence processing by limbic circuits.
Odors are transduced by a large array of olfactory receptor neurons in the nose, each of which appears to express a single member of the large (1,000 member) family of G-protein coupled olfactory receptor genes (Buck, 1996). Most naturally occurring odors are complex mixtures composed of many different molecules, each activating different olfactory receptors. Thus, we have no individual receptor for “coffee” but rather synthesize the multiple molecular features of that stimulus into a unitary percept, or odor object. This process has been hypothesized to be similar to visual object or face recognition (Wilson & Stevenson, 2003). Processing of odor feature information is refined by the local circuits of the olfactory bulb, while synthesis is believed to occur primarily within the primary olfactory (piriform) cortex.
While there is a strong spatial dimension to odor feature encoding within the olfactory bulb (Leon & Johnson, 2003), the temporal characteristic of neural firing and subthreshold activity may also serve as an important factor in encoding odor identity. As in many sensory systems, neural activity within the olfactory bulb and cortex is oscillatory, in part due to the cyclic nature of respiration, but also due to local inhibitory-excitatory circuit interactions that create medium (10–40 Hz) and high frequency (40–90 Hz) oscillations (Freeman, 2000). The role of these oscillations in olfaction is not known, but evidence suggests they may (1) serve as a source of synchrony to finely control timing of action potentials in disparate neurons (Laurent et al., 2001), (2) serve as a mechanism to enhance synaptic plasticity within the olfactory system or downstream of the olfactory system, and thus store memories of familiar odor input patterns (Haberly, 2001), and/or (3) reflect higher order feedback to the olfactory system, signaling important or learned stimuli (Kay, Lancaster, & Freeman, 1996).
Of importance to the developmental discussion here is the fact that expression of high-frequency oscillations (gamma frequency oscillations) are heavily dependent on local GABAergic interneurons, especially within the olfactory bulb. Despite the early emergence and critical role of olfaction in the neonatal rodent, these interneurons—granule cells—do not develop until relatively late postnatally (peak neurogenesis at around postnatal day 21; Brunjes & Frazier, 1986). Given the hypothesized functions of circuit oscillations, we predicted that in very young rat pups with very few granule cells, olfactory system temporal processing of odors should be impaired, with concomitant impairment in odor discrimination/ perception. In fact, rat pups during the first postnatal week had no detectable odor-evoked gamma frequency oscillations in either their olfactory bulb or piriform cortex (Figure 2 Fletcher, Smith, Best, & Wilson, 2005). However, odor discrimination of molecularly dissimilar (e.g., banana and peppermint) or similar (ethyl butyrate and ethyl proprionate) was comparable between 1-week and 3-week old pups (Fletcher et al., 2001, 2005.). These results suggest that high-frequency oscillations are not critical for odor discrimination per se. However, we are currently examining how the emergence of mature temporal response patterns over early development influences synaptic plasticity within the olfactory system and its direct targets (e.g., the amygdala).
FIGURE 2.
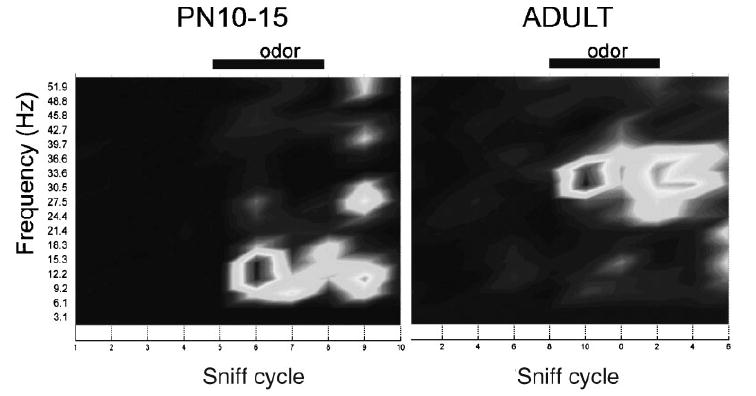
Gray-scale images of odor-evoked local field potential (LFP) oscillatory activity in the basolateral amygdala of immature and adult rats. Light gray and white regions represent high power in an FFT analysis of LFP activity within specific frequency bands. In young rats, odor stimulation evokes strong, low frequency activity with minimal high-frequency components. In contrast, odors evoke strong high-frequency activity (low gamma/high beta) in adults. These results are similar to those observed in olfactory bulb and piriform cortex (Fletcher et al., 2005), and could have an important impact on local circuit processing of odors and their associations (see text).
A second process involved in olfactory perception is habituation—the ability to filter out background and/or stable inputs while remaining response to new, dynamic stimuli. In olfaction, habituation may also be important for figure-ground separation where a target odor presented against a background is perceived as that target alone, and not a mixture of the two. Work in our lab has demonstrated that odor habituation is largely a cortical phenomenon, mediated by depression of cortical afferent synapses (Best & Wilson, 2004; Wilson, 1998). Pharmacological blockade of this synaptic depression prevents adaptation of cortical odor responses (Best & Wilson), and habituation of both odor-evoked reflexes (Best, Thompson, Fletcher, & Wilson, 2005) and odor investigation (Yadon & Wilson, 2005).
Given that synaptic activity plays a critical role in shaping the development of local circuits in the brain (e.g., Hubel & Wiesel, 1970), the ability to filter relatively unimportant information may be just as critical during early development as it is in mature organisms. Our recent work has demonstrated that habituation of an odor-evoked reflex is expressed normally by at least the first postnatal week in rats (Fletcher & Wilson, 2001), as is the synaptic mechanisms of cortical adaptation (Thompson, Best, & Wilson, 2005).
Together, these two series of studies suggest that understanding limbic system function during development, and the effects of early experience on later limbic system function must take into account the unique features of sensory input occurring at that time. Thus, while both a neonate and an adolescent may share some similar olfactory processing abilities, the fine structure of sensory input to the limbic system is not the same in these two organisms. How these differences in sensory input limit or extend immediate and eventual limbic system function are currently being explored.
EARLY EMOTIONAL MEMORIES ARE STORED IN THE BRAIN
Research from the laboratory of Katharina Braun at the University of Magdeburg, Germany, suggests that deprivation from parental care can dramatically alter emotional development. Whereas the basic wiring of the mammalian central nervous system is genetically preprogrammed, its fine tuning throughout the phases of infancy and childhood is highly dependent on experience. Experience that is encountered during time windows of elevated synaptic plasticity “imprint” on this highly adaptive brain substrate and leave their “footprints,” that is, “carve” templates into limbic synaptic wiring patterns, which will determine emotional as well as cognitive “grammars” and strategies and thereby optimize or limit cognitive as well as emotional capacity throughout life.
Although the impact of early environment on the development of behavior has been acknowledged during the past decades and has been studied extensively by the different research disciplines of psychology, neurobiology, psychiatry, and neurology, and although our insight into the molecular and genetic principles of brain development has increased exponentially in the past decade through the experimental work in the field of neurobiology, these different sets of knowledge have not yet been conceptually linked. Only the understanding of the interplay between early experience and the neuron’s molecular machinery will guide us to understand that early childhood presents itself as an investment opportunity for our society, and that it takes a well-functioning family and educational system to grow a brain.
The critical impact of the early socio-emotional environment on behavioral development is well documented by observations from clinical studies, which showed that disturbances or interruptions of the child–parent interaction lead to behavioral disturbances, including the so-called hospitalism syndrome, and later can result in severe and permanent deficits in speech behavior, personality development, intellectual and social capacity, and mental disturbances. Brain developmental studies indicate that juvenile emotional experience interferes with the establishment and maintenance of neuronal networks in the developing limbic system.
One important aim of brain development is to learn how to attend effectively to relevant environmental information (to “essentializa”) and to simultaneously discard all unimportant data—distinguishing the relevant from the superfluous. Moreover, the brain has to develop an internal emotional judgment system which controls not only perceptions, but which is also essential for higher cognitive tasks. The development of higher cognitive and emotional functions is tightly linked also with respect to their functional representation in the same brain regions, and thereby determine together the way in which the more mature or adult brain handles cognitive tasks.
Brain development is driven by a highly complex interaction between genetic disposition and experienceinduced adaptation of neuronal networks. Experimental research in animal models reveals that the enrichment and complexity of the environment eventually determines the complexity of the cellular signal transduction systems in the brain. Pre- and early postnatal experience and learning events, such as the formation of an attachment to a caregive are processed by neurons and their synaptic networks during developmental periods of highly elevated plasticity, during which the neurons (and glia) run their genetic and molecular machinery at high-activity levels. Thus, early experience and learning events interfere with a much more dramatic influence on the establishment, refinement, and functional maturation of neuronal and synaptic networks than learning and memory formation in the more mature, adult brain.
Impact of Early Emotional Experience on Synaptic Reorganization of Limbic Brain Regions
The balance of the intricate and only poorly understood interaction between genetic and epigenetic factors shifts during maturation of functional brain systems. Starting at birth, the sensory, motor and in particular, the emotional experiences are gaining more influence over the cellular genetic and molecular machinery and thereby refine and optimize the respective brain systems, and shape the individual’s behavior for coping with the environment in later life.
Although studies in animal models and in humans have revealed convincing evidence for the critical impact of neonatal and juvenile emotional experiences, the systematic experimental analysis has mainly focused on changes of endocrine function and behavior, whereas comparably little is known about brain functional changes in response to emotional experience during childhood and adolescence. The early studies of Scheibel, Lindsay, Tomiyasu, and Scheibel (1975, 1976), Rosenzweig and Greenough and colleagues (Rosenzweig & Bennett, 1996; Turner & Greenough, 1985) have shown that the degree of sensory and motor experience is related to synaptic densities in the sensory and motor cortex areas. For instance, neurons in the visual cortex of rats which have been raised in an impoverished environment show reduced dendritic arborization and synaptic density, compared to control animals, which were raised in an enriched environment. Similar results were obtained in the somatosensory and motor cortex of monkeys, which during their first 6 months of life had been raised in environments of different complexity: those animals who had been raised in an impoverished environment displayed reduced synaptic density (Bryan & Riesen, 1989).
These studies remained restricted to the analysis of developing sensory and motor cortical areas in relation to sensory and motor activation, and only recently, the impact of neonatal emotional experience and learning on synaptic development in the limbic system has been discovered. Experimental analysis in an animal model for filial imprinting (Lorenz, 1935; Hess, 1959), that is, the very first neonatal emotional learning event in vertebrates including human and nonhuman primates, which results in the formation of an emotional bond to the mother or caregiver, has revealed dramatic changes of synaptic connectivity in prefrontal forebrain regions in relation to this learning process. Domestic chicks, which were imprinted on an artificial acoustic or visual stimulus representing the mother, showed increasedsynaptic densities in an associative forebrain region (Horn, Bradley, & McCabe, 1985; Horn, Nicols, & Brown, 2001) but decreaseddensities of excitatory spine synapses in two other higher associative forebrain regions (Bock & Braun, 1998,1999a,b;Wallhausser & Scheich, 1987). The reduction of spine synapses which was observed after acoustic filial imprinting critically depends on the activation of glutamatergic NMDA receptors (Bock, Schnabel, & Braun, 1997; Bock & Braun, 1999a), and appears to reflect a synaptic network which responds with a potentiated response upon recall of the learned, emotionally relevant stimulus. In fact, the successfully imprinted animals respond to the presentation of the learned imprinting stimulus with enhanced electrical (Bredenkotter & Braun, 1997; Brown & Horn, 1994) and metabolical (Bock, Wolf, & Braun, 1996; Maier & Scheich, 1983; Wallhausser & Scheich) activity, which is accompanied by increased release of glutamate (Gruss & Braun, 1996) when the animals are exposed to the learned acoustic imprinting stimulus. Interestingly, this almost 45% decrease of excitatory spine synapses in the prefrontal forebrain regions of chicks only occurs if the animal is given the opportunity to associate the acoustic stimulus with a positive emotional situation, that is, when it learns that this (initially irrelevant and emotionally neutral) stimulus represents its mother. Passive acoustic stimulation during which the chick is not allowed to display a behavioral approach response does not alter spine densities, indicating the critical importance of the emotional component in this learning-related synaptic reorganization.
Regressive synaptic changes might be a typical characteristic for juvenile imprinting-like learning as opposed to adult learning, where increased numbers of synapses have been observed (Kleim et al., 1998; Moser, Trommald, & Andersen, 1994; O’Malley, O’Connell, Murphy, & Regan, 2000). Similar reductions in dendritic spine density have been observed during and after sexual (Bischof & Rollenhagen, 1999) and song imprinting (Nixdorf-Bergweiler, Wallhausser-Franke, & DeVoogd, 1995) in song birds, and in mynah birds who were trained to imitate human speech (Rausch & Scheich, 1982). The “trick” used by the juvenile brain to achieve high-speed “turbo” learning appears to be a synaptic selection process, similar to what has been described for the activity-dependent maturation of sensory cortical regions (Goodman & Shatz, 1993; Singer, 1995) and the innervation of skeletal muscle fibers (Changeux & Danchin, 1976). In the course of synaptic selection the initial synaptic composition at birth, and the abundance of synaptic connections which is built up during late prenatal and early postnatal periods is selectively pruned during the learning process, and thereby the network is selectively tuned to the specific emotional experience, which the animal has encountered during infancy. It is tempting to speculate that this experience-expectant synaptic reorganization process results in emotional “templates,” which are continuously refined and adapted in response to emotional experience until adolescence. Neuroanatomical investigations of Huttenlocher (Huttenlocher, 1979; Huttenlocher & Dabholkar, 1997) and Rakic and colleagues (Rakic, Bourgeois, & Goldman-Rakic, 1994) in human and nonhuman primates have clearly shown that each cortical region has specific time windows of synaptic proliferation and of synaptic pruning, which most likely reflects synaptic reorganization in the course of experience-dependent or experience-expectant competitive synaptic selection. It appears likely that this is also the case for limbic regions which are involved in emotional modulation such as the anterior cingulate and orbitofrontal cortex, nucleus accumbens, and the amygdala. The biological advantage and the outcome of this synaptic competition mechanism is the optimal adaptation of functional brain systems to a given environment which thereby increases the chance for survival and reproduction.
The disadvantage of experience-driven synaptic reorganization lies in the enhanced vulnerability of brain systems during developmental time periods of pronounced neuronal plasticity, during which adverse (or the complete lack of) environmental influences, equally can “imprint” in the brain and most likely result in retarded, malfunctioning, and “defective” synaptic wiring patterns. Early traumatic experience, such as repeated exposure to brief separation from the mother or both parents, repeated (unpleasant and mildly painful) injections, handling, chronic social isolation, or the loss of one parent have also been shown to induce dramatic region-specific synaptic changes in the developing brain, in particular in limbic regions.
Investigations in young Octodon degus demonstrated that repeated separation from the parents, daily saline injections or handling, and deprivation from paternal care alters limbic neuronal connections. Octodon degus, a semiprecocial lagomorph/rodent that is becoming increasingly popular as a laboratory animal, displays the same principal brain anatomy as common laboratory rodents. Compared to laboratory rats or mice this species displays closer similarities to human and nonhuman primate behavior and development, such as the presence of cortisol in the blood and the maturity of their sensory systems, which allows them to perceive and respond to familiar and novel stimuli from their environment immediately after birth. Similar to human babies (DeCasper & Fifer, 1980), the newborn degu pups learn to recognize and to respond to their mothers’ vocalizations within the first days of life, and this vocal communication appears to be an important component for the establishment and maintenance of the emotional attachment to the parents. Whereas common laboratory rodents appear not to exhibit a filial attachment of the kind shown, for example, by young primates, in the biparental species Octodon degus the pups have been shown to develop a strong attachment to both parents.
Functional imaging studies in 1-week-old degus during acute separation stress identified the brain areas which are responsive to this type of acute stress. After a 45-min period of separation from the family and the home cage the stressed animals showed a decreased metabolic activity in most limbic cortical and subcortical regions, as well as in some sensory cortex areas (Braun & Bock, 2003). It remains to be determined whether this acute downregulation of brain activity becomes chronic if the juvenile animals repeatedly or chronically have to cope with separation stress or social deprivation. This idea would be in line with the hypothesis of a dysmaturational pathogenesis for hypofrontality, which has been detected in Romanian orphans using the PET technique (Chugani et al., 2001), as well as in patients suffering from attention deficit hyperactivity syndrome, schizophrenia, or criminal aggression (Brower & Price, 2001; Manoach, 2003; Raine, Buchsbaum, & LaCasse, 1997; Rubia et al., 1999).
On the cellular and synaptic level it appears likely that this reduction of brain activity during stressful experience or as a result of deprivation reflects metabolic mechanisms which underlie experience-dependent (in this case stressdependent) synaptic reorganization. In line with this idea we found in 21- and 45-day-old degus, which were repeatedly exposed to daily parental separation during the first three weeks of life significantly elevated (up to 143%) densities of dendritic spines in the anterior cingulate cortex ( Helmeke, Ovtscharoff, Poeggel, & Braun, 2001a ; Helmeke, Poeggel, & Braun, 2001b; Poeggel et al., 2003a). The repeatedly stressed animals also showed significantly elevated spine densities on the hippocampal CA1 pyramidal neurons (up to 109% on the distal apical segments and up to 106% on the basal segment), whereas significantly reduced spine densities were observed on the granule cell dendrites in the dentate gyrus (down to 92%) and on the apical dendrites in the medial nucleus of the amygdala (down to 95%) (Poeggel et al., 2003a). Studies in laboratory rats which experienced maternal separation between postnatal days 14–16 confirmed the findings in degus, and in addition revealed a possible link between the synaptic changes and endocrine function (Bock, Gruss, Becker, & Braun, 2005). When rats are exposed to maternal separation during the stress hyporesponsive period of the hypothalamic-pituitary-adrenal (HPA) axis no synaptic changes were observed on layer II/III pyramidal neurons in the anterior cingulate cortex. Thus, the magnitude and direction of such stress-induced synaptic changes appears not only to depend on the brain region and cell type, but also on the time during which stress is induced. Rats which experienced maternal separation between postnatal days 3–5 ended up with fewer dendritic spines in the anterior cingulate cortex, as opposed to the animals which experienced the same amount of maternal separation at days 14–16 (Bock et al.). Chronic social isolation after weaning resulted in reduced numbers of perforated synapses and smaller synaptic contact zones in the dorso-medial amygdala (Ichikawa, Mastuoka, & Mori, 1993) of rats, whereas an increased density of dendritic spines was found in the preoptic region of deprived rats (Sanches-Tosccano, Sanchez, & Garzon, 1991). Furthermore, rats which were exposed to stress in utero during the last week of gestation (i.e., the pregnant dams were exposed to different types of stress) showed changes in the density of dendritic spines in the anterior cingulate and orbitofrontal cortex compared to control animals (Murmu et al., 2006).
On the neurochemical level, neonatal and juvenile exposure to stress and social deprivation induces changes in the balance of neurotransmitters in the limbic system, in particular of catecholaminergic systems (Holson, Ali, & Scallet, 1988), which play a major role in the modulation of emotional responses. Avariety of studies have revealed that the activation of the HPA-axis, as it occurs, for instance, during the separation from the mother or both parents (Gruss, Westphal, Luley, & Braun, 2006; Lehmann & Feldon, 2000) and other juvenile and adult stress paradigms (Kudielka & Kirschbaum, 2005), modulate monoaminergic systems in adult animals (Fuchs & Flugge, 2002) and also during development. For instance, rats which have been raised in social isolation display dramatically decreased dopamine metabolites (Miura, Qiao, & Ohta, 2002). This is in line with anatomical findings in degus and gerbils which were raised under socially deprived conditions and which as adolescents showed reduced densities of dopaminergic fiber innervation in some subregions of the prefrontal cortex (Braun, Lange, Metzger, & Poeggel, 2000; Winterfeld, Teuchert-Noodt, & Dawirs, 1998). In the ventrolateral orbital prefrontal cortex and agranular insular cortex of deprived animals significantly increased density of tyrosine hydroxylase-immunoreactive fibers were found (up to 172% in the ventrolateral orbital prefrontal cortex and up to 143% in the agranular insular cortex). The lateral orbital prefrontal cortex showed increased 5-hydroxytryptamine-positive fiber densities (up to 118%) (Poeggel, Nowicki, & Braun, 2003b). The neonatally stressed and later socially deprived animals also showed increased tyrosine hydroxylase (TH)-immunoreactive fiber densities (representing dopaminergic and noradrenergic fibers, respectively) in the core (up to 115%) and shell region (up to 113%) of the nucleus accumbens, whereas decreased fiber densities (down to 84%) were observed in the hilus of the dentate gyrus. In the stratum granulosum and subgranular layer the fiber densities increased up to 168% and 127%, respectively. Serotonergic innervation in the deprived animals was increased (up to 126%) in the core region of the nucleus accumbens, in the central nucleus of the amygdala (up to 112%) and in the outer subregion of the dentate gyrus stratum moleculare (up to 149%), whereas decreased fiber densities were detected in the dentate subgranular layer (down to 86%) and in the stratum lacunosum of the hippocampal CA1 region (down to 86%) (Gos et al., 2006). Monkeys which have been raised under conditions of maternal deprivation showed significantly reduced density of dopaminergic innervation in the striatum and in the nucleus accumbens (Martin, Spicer, Lewis, Gluck, & Cork, 1991).
On the behavioral level it remains to be characterized in which way the metabolic, neurochemical, and anatomical changes within the limbic system affect cognitive and emotional behavior of the stressed and/or deprived animals. So far, a consistent behavioral observation in stressed or deprived rats and degus was an increased “hyper-” activity in the open field (Braun, Kremz, Wetzel, Wagner, & Poeggel, 2003; Hall, 1998). In neonatally stressed (by repeatedly separating the pups from their parents and siblings) degus we, in addition, observed a significantly reduced responsiveness of 2-week-old pups towards maternal vocalizations, reflected by their reduced approach behavior and the absence of distress calls (Braun et al.).
Based on the pioneering work of Spitz (1945), Bowlby (1954, 1959), Mary Ainsworth (Ainsworth, Boston, Bowlby, & Rosenbluth, 1956; Ainsworth, 1962), and Skeels (1966) in humans it has been suspected for quite sometime that early traumatic experience, such as the loss or the separation from one or both parents, can be one of the major factors for developing anxiety and depressive disorders in adulthood. Spitz and colleagues have shown that the loss of the mother or parents leads to dramatic emotional disorders in the deprived infants (hospitalism) (Emde, Polak, & Spitz, 1965; Spitz). Recent studies in the biparental species Octodon degus showed that animals which had been raised without a father display significantly reduced densities (−33%) of symmetric shaft synapses layer II of the anterior cingulate cortex compared to biparentally raised controls (Ovtscharoff et al., 2006). The major importance of emotional environment, that is, a stable emotional attachment to the parents or caregivers, in addition to the intellectual input, is illustrated by the studies in Romanian orphans (O’Connor & Rutter, 2000). Clinical studies revealed a significant coincidence between the separation from or the loss of one or both parents and the chance of developing affective disorders (Gilmer & McKinney, 2003; Heim & Nemeroff, 2001), and one of these studies (Canetti et al., 2000) revealed that the separation from the parents due to divorce has a higher impact than the death of one or both parents.
JUVENILE STRESS IMPAIRS ADULT AVOIDANCE LEARNING IN RATS
Research from the laboratory of Gal Richter-Levin at the University of Haifa has shown that stress during adolescence can result in developmental abnormalities, clearly illustrating stress exposure prior to puberty has dramatic effects on later life. Exposure to early life stress is suggested as a significant contributing factor for the emergence of these psychopathologies. Epidemiological studies indicate that early-life stress or childhood emotional trauma is predominantly associated with higher prevalence of both mood and anxiety disorders, particularly depression and PTSD (Agid et al., 1999; Arborelius, Owens, Plotsky, & Nemeroff, 1999; Briere & Elliott, 1994; Draijer & Langeland, 1999; Furukawa et al., 1999; Maughan & McCarthy, 1997; Weiss, Longhurst, & Mazure, 1999). Yet, while some researchers claim that younger children are more vulnerable to trauma due to their impressionability (Browne & Finkelhor, 1986) and show greater symptomology as a result of abuse (Beitchman, Zucker, Hood, daCosta, & Akman, 1992), others claim that young children’s naiveté protects them, especially if children are unaware of the social stigma associated with the type of victimization they have suffered (Browne & Finkelhor).
The significance of the age or developmental stage at the onset of the adversity was also questioned. Heim and colleagues (Heim, Meinlschmidt, & Nemeroff, 2003; Heim, Plotsky, & Nemeroff, 2004) argued that any form of stress—short-term, acute, or chronic may be classified as early life stress as long as it occurs prior to sexual maturity onset—hence constitute a risk factor for the later development of depression and other stress-related psychopathologies. Yet others (Kiser, Heston, Millsap, & Pruitt, 1991) maintain that no systematic relationship exists between the age of the emotional assault and the degree of disturbance.
Studies examining the impact of early life experience in rats usually focus on the first two postnatal weeks (Levine, Huchton, Wiener, & Rosenfeld, 1991; Van Oers, de Kloet, & Levine, 1998). This period of postnatal development (i.e., 3–14 days postnatal) has been suggested to correspond roughly to the 23rd week of gestation in humans (Fitzgerald & Anand, 1993).
We have recently started to study a later developmental period; the Juvenile stage (PND 26–28) (Avital & Richter-Levin, 2005; Tsoory & Richter-Levin, 2005). The Juvenile period, a life period not widely targeted before, is suggested to be of relevance to human childhood (Spear, 2000). At this age the pups become more independent and spend more time in social interactions. Yet, they are still sexually immature (Martin & Berthoz, 2002).
We first examined the ability of adult animals to cope with stress as a function of whether or not they had been exposed to juvenile stress. The exposure to the combination of juvenile and adulthood stress increased anxiety levels as measured in the open field and startle response tests, compared to animals that were exposed to stress only in adulthood (Avital & Richter-Levin, 2005). To verify whether the impact of exposure to juvenile stress was due to the developmental period within which it was applied, or if the effects were due to repeated exposure to the stressor, we compared the juvenile + adulthood stress to a double exposure to stress during adulthood. The exposure to juvenile stress was found to have a significantly stronger impact than a double exposure to stress during adulthood (Avital & Richter-Levin).
The impact of the juvenile + adulthood exposure to stress was found to be long lasting. Examined up to 3 weeks after the adulthood exposure to stress, rats still showed enhanced anxious responses.
In fact, the exposure to stress in juveniles by itself had long-term effects well into adulthood. Adult rats exposed to juvenile stress exhibited reduced exploratory behavior in a novel setting (Tsoory & Richter-Levin, 2005). This was associated with marked alterations in neurosteroid levels in the rat hypothalamus and entorhinal cortex. Juvenile stress increased DHEAS but not DHEA concentrations both in the hypothalamus and the entorhinal cortex, indicating that an exposure to juvenile stress has long-lasting effects on DHEAS levels (Avital, Ram, Maayan, Weizman, & Richter-Levin, 2006).
To examine the impact of an exposure to juvenile stress on the ability to learn under stressful conditions in adulthood, we further examined the ability of adult rats to acquire the two-way shuttle avoidance task, depending on whether or not they were previously exposed to juvenile stress. Juvenile rats exposed to juvenile stress exhibited poor avoidance learning in adulthood, implying that their coping response to the stressful experience might have been compromised by heightened anxiety (Tsoory & Richter-Levin, 2005).
Interestingly, two levels of impairment were evident in the juvenile stress-exposed rats, as indicated by the frequency of no-response trials in the two-way avoidance task. The less severe level of impairment was characterized by poor avoidance learning accompanied by a low frequency of no-response trials; hence it was termed “maladaptive behavior.” The more severe level of impairment was typified by poor avoidance learning and a high frequency of no-response trials, and was termed “learned helplessness-like behavior.” Approximately two-thirds of the juvenile stress rats were characterized by maladaptive behavior in response to the stressful challenge of the avoidance task, while the remaining third was characterized by learned helplessness-like behaviors.
Interestingly, traumatic events often trigger the emergence of a variety of stress-related psychopathologies, including not only acute stress disorder (ASD) and posttraumatic stress disorder (PTSD), but also depressive and anxiety symptoms (Moreau & Zisook, 2002). It is tempting to suggest that the dissociation we observed between maladaptive and learned helplessness-like behaviors is related to that possibility.
The dichotomy among the juvenile stressed rats between two levels of impairment was not found when the young animals were exposed to similar stressors just 1 week later. Adolescent stressed rats exhibited only the less severe level of impairment and were all characterized as maladaptive (Tsoory & Richter-Levin, 2005). Together with finding that juvenile + adulthood stress resulted in more severe impairments than repeated exposures in adulthood (Avital & Richter-Levin, 2005), the results suggest that the juvenile period represents a particularly sensitive developmental period.
DISCUSSION
While each research program reviewed here has taken a unique approach to studying the influences of the environment on emotional development, all show the critical importance of the timing and nature of the environmental conditions to produce the outcome. Indeed, these strikingly different approaches have all contributed a basic research result that expands our understanding of developmental impact of infant experiences on emotional and limbic system development.
The importance of animal models in understanding adult human mental health relies, in part, on homologies between the animal model and humans. Mechanisms underlying the developmental animal models presented here and human adult outcome may not always be identical. Indeed these models clearly illustrate that similar outcomes in adulthood can be achieved through divergent developmental pathways. For example, the detrimental effects of stress on adult emotion may appear similar, yet stress experienced in infancy and the juvenile period may have different pathways to that outcome. Overall, these studies suggest that applying specific mechanisms found in animal models to the myriad adult outcomes and pathologies should be done with caution. Importantly, the research programs outlined here suggest general principles of development that can be readily applied to human populations.
The strength of animal models in research is that they can answer questions concerning specific mechanisms of developmental trajectories through an integrated approach that assesses biological processes throughout development and adulthood. Clinicial studies on human emotional differences and pathologies so often rely on extrapolating backward from the adult disease state to identify developmental time points when genes and environment interact to produce the trajectory to the disease state. Animal research helps us identify developmental time points and types of environmental insults to guide the extrapolating into the clinical populations’ history.
Acknowledgments
The authors thank NICHD for support of the symposium presented at the 2004 International Society for Developmental Psychobiology Annual meeting in Aix-en-Provence, France. Support for research presented in the manuscript was provided for by the German Science Foundation (DFG), the state of Saxony-Anhalt, the VolkswagenStiftung, German-Israeli Foundation (GIF) to K.B.; ETH Zurich, the NCCR: Neural Plasticity and Repair, Swiss National Science Foundation to J.F., U.M., B.K.Y.; NSF, NIH-NICHD, OCAST to R.M.S.; NIH-NIDCD, OCAST to D.A.W.; and The Israel Foundation Trustees, NARSAD Independent Investigator award to G.R.L.
Footnotes
Contract grant sponsor: German Science Foundation (DFG); State of Saxony-Anhalt, VolkswagenStiftung, German-Israeli Foundation (GIF); ETH Zurich, NCCR: Neural Plasticity and Repair; Swiss National Science Foundation; NIH-NICHD; OCAST; The Israel Foundation Trustees; NARSAD Independent Investigator Award
References
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: A case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Molecular Psychiatry. 1999;4(2):163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Ainsworth MDS. The effects of maternal deprivation: A review of findings and controversy in the context of research strategy. Public Health Papers. 1962;14:97–165. [PubMed] [Google Scholar]
- Ainsworth MDS. Object relations, dependency, and attachment: A theoretical review of the infant-mother relationship. Child Development. 1969;40:969–1025. [PubMed] [Google Scholar]
- Ainsworth MDS, Boston M, Bowlby J, Rosenbluth D. The effects of mother-child separation: A follow-up study. British Journal of Medical Psychology. 1956;29:211–247. doi: 10.1111/j.2044-8341.1956.tb00915.x. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. The Journal of Endocrinology. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Avital A, Ram E, Maayan R, Weizman A, Richter-Levin G. Effects of early-life stress on behavior and neurosteroid levels in the rat hypothalamus and entorhinal cortex. Brain Res Bulletin. 2006;68(6):419–424. doi: 10.1016/j.brainresbull.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Avital A, Richter-Levin G. Exposure to juvenile stress exacerbates the behavioral consequences of exposure to stress in the adult rat. International Journal of Neuropsychopharmacology. 2005;8(2):163–173. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Beitchman JH, Zucker KJ, Hood JE, daCosta GA, Akman D. A review of the long-term effects of child sexual abuse. Child Abuse & Neglect. 1992;16(1):101–118. doi: 10.1016/0145-2134(92)90011-f. [DOI] [PubMed] [Google Scholar]
- Best AR, Wilson DA. Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. Journal of Neuroscience. 2004;24:652–660. doi: 10.1523/JNEUROSCI.4220-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best AR, Thompson JV, Fletcher ML, Wilson DA. Cortical metabotropic glutamate receptors contribute to habituation of a simple odor-evoked behavior. Journal of Neuroscience. 2005;25:2513–2517. doi: 10.1523/JNEUROSCI.5298-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof HJ, Rollenhagen A. Behavioural and neurophysiological aspects of sexual imprinting in zebra finches. Behavioral Brain Research. 1999;98:267–276. doi: 10.1016/s0166-4328(98)00093-x. [DOI] [PubMed] [Google Scholar]
- Bock J, Braun K. Differential emotional experience leads to pruning of dendritic spines in the forebrain of domestic chicks. Neural Plasticity. 1998;6:17–27. doi: 10.1155/NP.1998.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Braun K. Blockade of N-methyl-D aspartate receptor activation suppresses learning-induced synaptic elimination. Proceedings of the National Academy of Sciences of the United States of America. 1999a;96:2485–2490. doi: 10.1073/pnas.96.5.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Braun K. Filial imprinting in domestic chicks is associated with spine pruning in the associative area, dorsocaudal neostriatum. European Journal of Neuroscience. 1999b;11:1–5. doi: 10.1046/j.1460-9568.1999.00713.x. [DOI] [PubMed] [Google Scholar]
- Bock J, Gruss M, Becker S, Braun K. Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: Correlation with developmental time windows . Cerebral Cortex. 2005;15:802–808. doi: 10.1093/cercor/bhh181. [DOI] [PubMed] [Google Scholar]
- Bock J, Schnabel R, Braun K. The role of the dorso-caudal neostriatum in filial imprinting of the domestic chick: A pharmacological and autoradigraphical approach focussed on the involvement of NMDA-receptors. European Journal of Neuroscience. 1997;9:1262–1272. doi: 10.1111/j.1460-9568.1997.tb01481.x. [DOI] [PubMed] [Google Scholar]
- Bock J, Wolf A, Braun K. Influence of the N-methyl-D-aspartate receptor antagonist DL-2-amino-5-phosphono valeric acid on auditory filial imprinting in the domestic chick. Neurobiology of Learning and Memory. 1996;65:177–188. doi: 10.1006/nlme.1996.0019. [DOI] [PubMed] [Google Scholar]
- Borish LC, Steinke JW. Cytokines and chemokies. Journal of Allergy and Clinical Immunology. 2003;11:460–475. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arévalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats: Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–221. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- Bowlby J. The effect of separation from the mother in early life. Irish Journal of Medical Science. 1954;6:121–126. doi: 10.1007/BF02952876. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Über das Wesen der Mutter-Kind-Bindung. Psyche. 1959;13:415–456. [Google Scholar]
- Bowlby J. Attachment. New York: Basic Books; 1965. [Google Scholar]
- Bracha HS, Torrey EF, Bigelow LB, Lohr JB, Linington BB. Subtle signs of prenatal maldeve lopment of the hand ectoderm in schizophrenia: A preliminary monozygotic twin study. Biological Psychiatry. 1991;30:719–725. doi: 10.1016/0006-3223(91)90017-g. [DOI] [PubMed] [Google Scholar]
- Bracha HS, Torrey EF, Gottesman II, Bigelow LB, Cunniff C. Second-trimester markers of fetal size in schizophrenia: A study of monozygotic twins. American Journal of Psychiatry. 1992;149:1355–1361. doi: 10.1176/ajp.149.10.1355. [DOI] [PubMed] [Google Scholar]
- Braun K, Bock J. Early traumatic experience alters metabolic brain activity in thalamic, hypothalamic and prefrontal cortical brain areas of Octodon degus. Developmental Psychobiology. 2003;43:248. [Google Scholar]
- Braun K, Kremz P, Wetzel W, Wagner T, Poeggel G. Influence of parental deprivation on the behavioral development in Octodon degus: Modulation by maternal vocalizations. Developmental Psychobiology. 2003;42:237–245. doi: 10.1002/dev.10096. [DOI] [PubMed] [Google Scholar]
- Braun K, Lange E, Metzger M, Poeggel G. Maternal separation followed by early social deprivation affects the development of monoaminergic fiber systems in the medial prefrontal cortex of Octodon degus. Neuroscience. 2000;95:309–318. doi: 10.1016/s0306-4522(99)00420-0. [DOI] [PubMed] [Google Scholar]
- Brayden RM, Altemeier WA, Tucker DD, Dietrich MS, Vietze P. Antecedents of child neglect in the first two years of life. Journal of Pediatrics. 1992;120:426–429. doi: 10.1016/s0022-3476(05)80912-6. [DOI] [PubMed] [Google Scholar]
- Bredenkotter M, Braun K. Changes of neuronal responsiveness in the mediorostral neostriatum/hyperstriatum after auditory filial imprinting in the domestic chick. Neuroscience. 1997;76:355–365. doi: 10.1016/s0306-4522(96)00381-8. [DOI] [PubMed] [Google Scholar]
- Briere JN, Elliott DM. Immediate and long-term impacts of child sexual abuse. The Future of children/Center for the Future of Children, the David and Lucile Packard Foundation. 1994;4(2):54–69. [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Archives of General Psychiatry. 2004a;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 2004b;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Brown MW, Horn G. Learning-related alterations in the visual responsiveness of neurons in a memory system of the chick brain. European Journal of Neuroscience. 1994;6:1479–1490. doi: 10.1111/j.1460-9568.1994.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Brower MC, Price BH. Neuropsychiatry of frontal lobe dysfunction in violent and criminal behaviour: A critical review. Journal of Neurology, Neurosurgery and Psychiatry. 2001;71:720–726. doi: 10.1136/jnnp.71.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne A, Finkelhor D. Impact of child sexual abuse: A review of the research. Psychological Bulletin. 1986;99(1):66–77. [PubMed] [Google Scholar]
- Brunjes PC, Frazier LL. Maturation and plasticity in the olfactory system of vertebrates. Brain Research Reviews. 1986;396:1–45. doi: 10.1016/s0006-8993(86)80188-3. [DOI] [PubMed] [Google Scholar]
- Bryan GK, Riesen AH. Deprived somatosensorymotor experience in stumptailed monkey neocortex: Dendritic spine density and dendritic branching of layer IIIB pyramidal cells. Journal of Comparative Neurology. 1989;286:208–217. doi: 10.1002/cne.902860206. [DOI] [PubMed] [Google Scholar]
- Buck LB. Information coding in the vertebrate olfactory system. Annual Review of Neuroscience. 1996;19:517–544. doi: 10.1146/annurev.ne.19.030196.002505. [DOI] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain, Behavior, and Immunity. 2001;15:411–420. doi: 10.1006/brbi.2001.0644. [DOI] [PubMed] [Google Scholar]
- Canetti L, Bachar E, Bonne O, Agid O, Lerer B, Kaplan De-Nour A, Shalev AY. The impact of parental death versus separation from parents on the mental health of Israeli adolescents. Comprehensive Psychiatry. 2000;41:360–368. doi: 10.1053/comp.2000.9002. [DOI] [PubMed] [Google Scholar]
- Cannon M, Clarke MC. Risk for schizophrenia—Broadening the concepts, pushing back the boundaries. Schizophrenia Research. 2005;79:5–13. doi: 10.1016/j.schres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, Huttunen MO, Keshavan MS, Seidman LJ, Tsuang MT. Early and late neurodevelopmental influences in the prodrome to schizophrenia: Contributions of genes, environment, and their interactions. Schizophrenia Bulletin. 2003;29:653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ, Venturi P, Jones LA, Lewis SW, Sham PC, Gottesman II, Farmer AE, McGuffin P, Reveley AM, Murray RM. Heritability estimates for psychotic disorders: The Maudsley twin psychosis series. Archives of General Psychiatry. 1999;56:162–168. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Done DJ. Prenatal exposure to influenza does not cause schizophrenia. British Journal of Psychiatry. 1992;161:390–393. doi: 10.1192/bjp.161.3.390. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: A study of post institutionalized Romanian orphans. Neuroimage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Curfs JH, Meis JF, Hoogkamp-Korstanje JA. A primer on cytokines: Sources, receptors, effects, and inducers. Clinical Microbiology Reviews. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mother’s voices. Science. 1980;208:1174– 1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Early experience and emotional development. Scientific American. 1963;208:138–146. doi: 10.1038/scientificamerican0663-138. [DOI] [PubMed] [Google Scholar]
- Draijer N, Langeland W. Childhood trauma and perceived parental dysfunction in the etiology of dissociative symptoms in psychiatric inpatients . The American journal of psychiatry. 1999;156(3):379–385. doi: 10.1176/ajp.156.3.379. [DOI] [PubMed] [Google Scholar]
- Emde RN, Polak PR, Spitz RA. Anaclitic depression in an infant raised in an institution. Journal of American Academy of Child Psychiatry. 1965;4:545–553. doi: 10.1016/s0002-7138(09)62157-9. [DOI] [PubMed] [Google Scholar]
- Entrican G. Immune regulation during pregnancy and host-pathogen interactions in infectious abortion. Journal of Comparative Pathology. 2002;126:79–94. doi: 10.1053/jcpa.2001.0539. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: Implications for genesis of autism and schizophrenia. Cellular and Molecular Neurobiology. 2002;22:25–33. doi: 10.1023/a:1015337611258. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, Shier A, Sheikh S, Bailey K. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Molecular Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Anand KJS. The developmental neuroanatomy and neurophysiology of pain. In: Schechter N, Berde C, Yaster M, editors. Pain management in infants, children and adolescents. Baltimore: Williams & Williams; 1993. pp. 11–32. [Google Scholar]
- Fletcher ML, Wilson DA. Ontogeny of odor discrimination: A method to assess novel odor discrimination in neonatal rats. Physiology and Behavior. 2001;74:589–593. doi: 10.1016/s0031-9384(01)00602-3. [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Smith AM, Best AR, Wilson DA. High frequency oscillations are not necessary for simple olfactory discriminations in young rats. Journal of Neuroscience. 2005;25:792–798. doi: 10.1523/JNEUROSCI.4673-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier ME, Joober R, Luheshi GN, Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. Journal of Psychiatric Research. 2004;38:335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Franzek E, Beckmann H. Gene-environment interaction in schizophrenia: Season-of-birth effect reveals etiologically different subgroups. Psychopathology. 1996;29:14–26. doi: 10.1159/000284967. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Neurodynamics: An exploration in mesoscopic brain dynamics. London: Springer; 2000. p. 397. [Google Scholar]
- Fuchs E, Flugge G. Social stress in tree shrews: Effects on physiology, brain function, and behavior of subordinate individuals. Pharmacology, Biochemistry and Behavior. 2002;73:247–258. doi: 10.1016/s0091-3057(02)00795-5. [DOI] [PubMed] [Google Scholar]
- Furukawa TA, Ogura A, Hirai T, Fujihara S, Kitamura T, Takahashi K. Early parental separation experiences among patients with bipolar disorder and major depression: A case-control study. Journal of Affective Disorders. 1999;52(13):85–91. doi: 10.1016/s0165-0327(98)00054-8. [DOI] [PubMed] [Google Scholar]
- Gilmer WS, McKinney WT. Early experience and depressive disorders: Human and non-human primate studies. Journal of Affective Disorders. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF. Exposure to infection and brain development: Cytokines in the pathogenesis of schizophrenia. Schizophrenia Research. 1997;24:365–367. doi: 10.1016/s0920-9964(96)00123-5. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL- 1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology. 2004;29:1221–1229. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Perkins DO, Kliewer MA, Hage ML, Silva SG, Chescheir NC, Hertzberg BS, Sears CA. Fetal brain development of twins assessed in utero by ultrasound: Implications for schizophrenia. Schizophrenia Research. 1996;19:141–149. doi: 10.1016/0920-9964(95)00099-2. [DOI] [PubMed] [Google Scholar]
- Goebel MU, Baase J, Pithan V, Exton M, Saller B, Schedlowski M, Limmroth V. Acute interferon beta-1b administration alters hypothalamic-pituitary-adrenal axis activity, plasma cytokines and leukocyte distribution in healthy subjects. Psychoneuroendocrinology. 2002;27:881–892. doi: 10.1016/s0306-4530(01)00099-3. [DOI] [PubMed] [Google Scholar]
- Golan HM, Lev V, Hallak M, Sorokin Y, Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–917. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72(Suppl):77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Gos T, Becker K, Bock J, Malecki U, Bogerts B, Poeggel G, Braun K. Early neonatal and postweaning social emotional deprivation interferes with the maturation of serotonergic and tyrosine hydroxylase-immunoreactive afferent fiber systems in the rodent nucleus accumbens, hippocampus and amygdala. Neuroscience. 2006;40:811–821. doi: 10.1016/j.neuroscience.2006.02.078. [DOI] [PubMed] [Google Scholar]
- Greenbough WT. Experiential modification of the developing brain. American Scientist. 1975;63(1):37–46. [PubMed] [Google Scholar]
- Gross G. The onset of schizophrenia. Schizophrenia Research. 1997;28:187–198. doi: 10.1016/s0920-9964(97)00118-7. [DOI] [PubMed] [Google Scholar]
- Gruss M, Braun K. Stimulus-evoked increase of glutamate in the mediorostral neostriatum/hyperstriatum ventrale of domestic chick after auditory filial imprinting: An in vivo microdialysis study. Journal of Neurochemistry. 1996;66:1167–1173. doi: 10.1046/j.1471-4159.1996.66031167.x. [DOI] [PubMed] [Google Scholar]
- Gruss M, Westphal S, Luley C, Braun K. Endocrine and behavioural plasticity in response to juvenile stress in the semi-precocial rodent Octodon degus. Psychoneuroendocrinology. 2006;31:361–372. doi: 10.1016/j.psyneuen.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: New insights from morphological and physiological analysis of neuronal circuitry. Chemical Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Saade NE, Safieh-Garabeian B. Cytokines and neuro-immune-endocrine interactions: A role for the hypothalamic-pituitary-adrenal revolving axis. Journal of Neuroimmunology. 2002;133:1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Critical Reviews in Neurobiology. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The affectional systems. In: Schrier A, Harlow HF, Stollnitz F, editors. Behavior of nonhuman primates. Vol. 2. New York: Academic Press; 1965. [Google Scholar]
- Heim C, Meinlschmidt G, Nemeroff CB. Neurobiology of early-life stress. Psychiatric Annals. 2003;33:1–10. [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29(4):641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Ovtscharoff W, Jr, Poeggel G, Braun K. Juvenile emotional experience alters synaptic composition in the anterior cingulate cortex. Cerebral Cortex. 2001a;11:717–727. doi: 10.1093/cercor/11.8.717. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Poeggel G, Braun K. Differential emotional experience induces elevated spine densities on basal dendrites of pyramidal neurons in the anterior cingulate cortex. Neuroscience. 2001b;104:927–931. doi: 10.1016/s0306-4522(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Hess EH. Imprinting. Science. 1959;130:135–141. doi: 10.1126/science.130.3377.733. [DOI] [PubMed] [Google Scholar]
- Holson RR, Ali SF, Scallet AC. The effect of isolation rearing and stress on monoamines in forebrain nigrostriatal, mesolimbic, and mesocortical dopamine systems. Annals of the New York Academy of Sciences. 1988;537:512–514. [Google Scholar]
- Horn G, Bradley P, McCabe BJ. Changes in the structure of synapses associated with learning. Journal of Neuroscience. 1985;5:3161–3168. doi: 10.1523/JNEUROSCI.05-12-03161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn G, Nicols AU, Brown MG. Tracking memory trace. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:55282–55287. doi: 10.1073/pnas.091094798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. Journal of Physiology. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Resarch. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ichikawa M, Mastuoka M, Mori Y. Effect of differential rearing on synaptic density and soma size in rat medial amygdaloid nucleus. Synapse. 1993;13:50–56. doi: 10.1002/syn.890130107. [DOI] [PubMed] [Google Scholar]
- Jakob H, Beckman H. Prenatal development disturbances in the limbic allocortex in schizophrenia. Journal of Neural Transmission. 1986;65:303–326. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA. Olfactory cortical adaptation facilitates detection of odors against background. Journal of Neurophysiology. 2006;95:1888–1896. doi: 10.1152/jn.00812.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Remington G. Atypical antipsychotics: New directions and new challenges in the treatment of schizophrenia. Annual Review of Medicine. 2001;52:503–517. doi: 10.1146/annurev.med.52.1.503. [DOI] [PubMed] [Google Scholar]
- Kay LM, Lancaster LR, Freeman WJ. Reafference and attractors in the olfactory system during odor recognition. International Journal of Neural Systems. 1996;7:489–495. doi: 10.1142/s0129065796000476. [DOI] [PubMed] [Google Scholar]
- Kiser LJ, Heston J, Millsap PA, Pruitt DB. Physical and sexual abuse in childhood: Relationship with post-traumatic stress disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30(5):776–783. [PubMed] [Google Scholar]
- Kleim JA, Swain RA, Armstrong KA, Napper RM, Jones TA, Greenough WT. Selective synaptic plasticity within the cerebellar cortex following complex motor skill learning. Neurobiology of Learning and Memory. 1998;69:274–289. doi: 10.1006/nlme.1998.3827. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: A review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, Abarbanel HD. Odor encoding as an active, dynamical process: Experiments, computation and theory. Annual Review of Neuroscience. 2001;24:263–297. doi: 10.1146/annurev.neuro.24.1.263. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: Consistent or confusing? Reviews of Neuroscience. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Resarch Reviews. 2003;42:23–32. doi: 10.1016/s0165-0173(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma-free corticosterone response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Developmental Psychobiology. 1991;24(8):547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. British Journal of Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- Ling ZD, Potter ED, Lipton JW, Carvey PM. Differentiation of mesencephalic progenitor cells into dopaminergic neurons by cytokines. Experimental Neurology. 1998;149:411–423. doi: 10.1006/exnr.1998.6715. [DOI] [PubMed] [Google Scholar]
- Lorenz K. Der Kumpan in der Umwelt des Vogels. Journal of Ornithology. 1935;83:137–413. [Google Scholar]
- Machón RA, Mednick SA, Huttunen MO. Fetal viral infection and adult schizophrenia: Empirical findings and interpretations. In: Mednick SA, Hollister JM, editors. Neural development and schizophrenia. New York: Plenum Press; 1995. pp. 191–202. [Google Scholar]
- Maguire GA. Comprehensive understanding of schizophrenia and its treatment. American Journal of Health-System Pharmacy. 2002;59:4–11. doi: 10.1093/ajhp/59.suppl_5.S4. [DOI] [PubMed] [Google Scholar]
- Mäki P, Veijola J, Jones PB, Murray GK, Koponen H, Tienari P, Miettunen J, Tanskanen P, Wahlberg KE, Koskinen J, Lauronen E, Isohanni M. Predictors of schizophrenia—A review. British Medical Bulletin. 2005;73:1–15. doi: 10.1093/bmb/ldh046. [DOI] [PubMed] [Google Scholar]
- Maier V, Scheich H. Acoustic imprinting leads to differential 2-deoxy-D-glucose uptake in the chick forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:3860–3864. doi: 10.1073/pnas.80.12.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: Reconciling discrepant findings. Schizophrenia Research. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Spicer DM, Lewis MH, Gluck JP, Cork LC. Social deprivation of rhesus monkeys alters the chemoarchitecture of the brain. I. Subcortical regions. Journal of Neuroscience. 1991;11:3344–3358. doi: 10.1523/JNEUROSCI.11-11-03344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PD, Berthoz A. Development of spatial firing in the hippocampus of young rats. Hippocampus. 2002;12:465–480. doi: 10.1002/hipo.10021. [DOI] [PubMed] [Google Scholar]
- Marx CE, Jarskog LF, Lauder JM, Lieberman JA, Gilmore JH. Cytokine effects on cortical neuron MAP-2 immunoreactivity: Implications for schizophrenia. Biological Psychiatry. 2001;50:743–749. doi: 10.1016/s0006-3223(01)01209-4. [DOI] [PubMed] [Google Scholar]
- Maughan B, McCarthy G. Childhood adversities and psychosocial disorders. British Medical Bulletin. 1997;53(1):156–169. doi: 10.1093/oxfordjournals.bmb.a011597. [DOI] [PubMed] [Google Scholar]
- McDonald C, Murray RM. Early and late environmental factors for schizophrenia. Brain Resarch, Reviews. 2000;31:130–137. doi: 10.1016/s0165-0173(99)00030-2. [DOI] [PubMed] [Google Scholar]
- McGrath J, Castle D, Murray R. How can we judge whether or not prenatal exposure to influenza causes schizophrenia? In: Mednick SA, Hollister JM, editors. Neural development and schizophrenia. New York: Plenum Press; 1995. pp. 203–214. [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Archives of General Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Meek LR, Dittel PL, Sheehan MC, Chan JY, Kjolhaug SR. Effects of stress during pregnancy on maternal behavior in mice. Physiological Behavior. 2001;72:473–479. doi: 10.1016/s0031-9384(00)00431-5. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neuroscience and Biobehavioral Reviews. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: A link between neurodevelopment and adult psychopathology. Brain, Behavior, and Immunity. 2006a;20:378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. Journal of Neuroscience. 2006b;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Schwendener S, Feldon J, Yee BK. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Experimental Brain Research. 2006c doi: 10.1007/s00221-006-0419-5. in press. [DOI] [PubMed] [Google Scholar]
- Miller AL. Combination treatments for schizophrenia. CNS Spectrum. 2004;9:19–23. doi: 10.1017/s1092852900004363. [DOI] [PubMed] [Google Scholar]
- Miller MT, Stromland K, Ventura L, Johansson M, Bandim JM, Gillberg C. Autism associated with conditions characterized by developmental errors in early embryogenesis: A mini review. International Journal of Developmental Neuroscience. 2005;23:201–219. doi: 10.1016/j.ijdevneu.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Mino Y, Oshima I, Tsuda T, Okagami K. No relationship between schizophrenic birth and influenza epidemics in Japan. Journal of Psychiatric Research. 2000;34:133–138. doi: 10.1016/s0022-3956(00)00003-0. [DOI] [PubMed] [Google Scholar]
- Miura H, Qiao H, Ohta T. Influence of aging and social isolation on changes in brain monoamine turnover and biosynthesis of rats elicited by novelty stress. Synapse. 2002;46:116–124. doi: 10.1002/syn.10133. [DOI] [PubMed] [Google Scholar]
- Moreau C, Zisook S. Rationale for a posttraumatic stress spectrum disorder. Psychiatric Clinics of North America. 2002;25(4):775–790. doi: 10.1016/s0193-953x(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Morgan V, Castle D, Page A, Fazio S, Gurrin L, Burton P, Montgomery P, Jablensky A. Influenza epidemics and incidence of schizophrenia, affective disorders and mental retardation in Western Australia: No evidence of a major effect. Schizophrenia Research. 1997;26:25–39. doi: 10.1016/S0920-9964(97)00033-9. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. European Journal of Neuroscience. 2006 doi: 10.1111/j.1460-9568.2006.05024.x. in press. [DOI] [PubMed] [Google Scholar]
- Murray RM, Jones P, O’Callaghan E. Fetal brain development and later schizophrenia. Ciba Foundation Symposium. 1991;156:155–163. doi: 10.1002/9780470514047.ch10. [DOI] [PubMed] [Google Scholar]
- Murray RM, O’Callaghan E, Castle DJ, Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophrenia Bulletin. 1992;18:319–332. doi: 10.1093/schbul/18.2.319. [DOI] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler BE, Wallhausser-Franke E, DeVoogd TJ. Regressive development in neuronal structure during song learning in birds. Journal of Neurobiology. 1995;27:204–215. doi: 10.1002/neu.480270207. [DOI] [PubMed] [Google Scholar]
- O’Callaghan E, Sham PC, Takei N, Murray G, Glover G, Hare EH, Murray RM. The relationship of schizophrenic births to 16 infectious diseases. British Journal of Psychiatry. 1994;165:353–356. doi: 10.1192/bjp.165.3.353. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: A neurodevelopmental animal model of schizophrenia. Biological Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Rutter M. Attachment disorder behavior following early severe deprivation: Extension and longitudinal follow-up. English and Romanian Adoptees Study Team. Journal of American Academy of Child Adolescence Psychiatry. 2000;39:703–712. doi: 10.1097/00004583-200006000-00008. [DOI] [PubMed] [Google Scholar]
- O’Malley A, O’Connell C, Murphy KJ, Regan CM. Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience. 2000;99:229–232. doi: 10.1016/s0306-4522(00)00182-2. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Jr, Helmeke C*, Braun K. Lack of paternal care affects synaptic development in the anterior cingulate cortex. Brain Res. 2006 doi: 10.1016/j.brainres.2006.07.106. in press. [DOI] [PubMed] [Google Scholar]
- Patin V, Lordi B, Vincent A, Thoumas JL, Vaudry H, Caston J. Effects of prenatal stress on maternal behavior in the rat. Brain Research Developmental Brain Research. 2002;139:1–8. doi: 10.1016/s0165-3806(02)00491-1. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection: Window on neuroimmune interactions in fetal brain development and mental illness. Current Opinion in Neurobiology. 2002;12:115–118. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- Pearce BD. Schizophrenia and viral infection during neurodevelopment: A focus on mechanisms. Molecular Psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- Petronis A. The origin of schizophrenia: Genetic thesis, epigenetic antithesis, and resolving synthesis. Biological Psychiatry. 2004;55:965–970. doi: 10.1016/j.biopsych.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Poeggel G, Helmeke C, Abraham A, Schwabe T, Friedrich P, Braun K. Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2003a;100:16137–16142. doi: 10.1073/pnas.2434663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeggel G, Nowicki L, Braun K. Early social deprivation alters monoaminergic afferents in the orbital prefrontal cortex of Octodon degus. Neuroscience. 2003b;116:617–620. doi: 10.1016/s0306-4522(02)00751-0. [DOI] [PubMed] [Google Scholar]
- Potter ED, Ling ZD, Carvey PM. Cytokineinduced conversion of mesencephalic-derived progenitor cells into dopamine neurons. Cell Tissue Research. 1999;296:235–246. doi: 10.1007/s004410051285. [DOI] [PubMed] [Google Scholar]
- Raine A, Buchsbaum M, LaCasse L. Brain abnormalities in murderers indicated by positron emission tomography. Biological Psychiatry. 1997;42:495–508. doi: 10.1016/S0006-3223(96)00362-9. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: Implications for learning, memory, and mental illness. Progress in Brain Research. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Rantakallio P, von Wendt L. Risk factors for mental retardation. Archives of Disease in Childhood. 1985;60:946–952. doi: 10.1136/adc.60.10.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch G, Scheich H. Dendritic spine loss and enlargement during maturation of the speech control system in the mynah bird (Gracula religiosa) Neuroscience Letters. 1982;29:129–133. doi: 10.1016/0304-3940(82)90341-x. [DOI] [PubMed] [Google Scholar]
- Remington G, Shammi C. The use of pharmacotherapy in the prodrome of schizophrenia. CNS Spectrum. 2004;9:579–586. doi: 10.1017/s1092852900002741. [DOI] [PubMed] [Google Scholar]
- Revello MG, Gerna G. Pathogenesis and prenatal diagnosis of human cytomegalovirus infection. Journal of Clinical Virology. 2004;29:71–83. doi: 10.1016/j.jcv.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Hyman SL. Early environmental factors in autism. Mental Retardation and Developmental Disabilities Research Reviews. 1998;4:121–128. [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: Effects of training and experience on brain and behavior. Behavioral Brain Research. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: A study with functional MRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Sanches-Tosccano F, Sanchez M, Garzon J. Changes in the number of spines in the medial preoptic nucleus during a premature long-term social isolation in rats. Neuroscience Letters. 1991;122:1–3. doi: 10.1016/0304-3940(91)90178-v. [DOI] [PubMed] [Google Scholar]
- Sargent IL. Maternal and fetal immune responses during pregnancy. Experimental and Clinical Immunogenetics. 1993;10:85–102. [PubMed] [Google Scholar]
- Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Experimental Neurology. 1975;47:392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in the aging human limbic system. Experimental Neurology. 1976;53:420–430. doi: 10.1016/0014-4886(76)90082-0. [DOI] [PubMed] [Google Scholar]
- Schneirla TC. Behavioral development and comparative psychology. Quarterly Review of Biology. 1966;41(3):283–302. doi: 10.1086/405056. [DOI] [PubMed] [Google Scholar]
- Selten JP, Slaets JP. Evidence against maternal influenza as a risk factor for schizophrenia. British Journal of Psychiatry. 1994;164:674–676. doi: 10.1192/bjp.164.5.674. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. Journal of Neuroscience. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- Skeels HM. Adult status of children with contrasting early life experiences: A follow-up study. Monographs of the Society for Research in Child Development. 1966;31:1–65. [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spitz RA. Hospitalism: An inquiry into the genesis of psychiatric conditions in early childhood. Psychoanal Study Child. 1945;1:53–74. [PubMed] [Google Scholar]
- Sporn AL, Addington AM, Gogtay N, Ordonez AE, Gornick M, Clasen L, Greenstein D, Tossell JW, Gochman P, Lenane M, Sharp WS, Straub RE, Rapoport JL. Pervasive developmental disorder and childhood-onset schizophrenia: Comorbid disorder or a phenotypic variant of a very early onset illness? Biological Psychiatry. 2004;55:989–994. doi: 10.1016/j.biopsych.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Stöber G, Franzek E, Beckmann H, Schmidtke A. Exposure to prenatal infections, genetics and the risk of systematic and periodic catatonia. Journal of Neural Transmission. 2002;109:921–929. doi: 10.1007/s007020200075. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy: Ontogeny of conditioned fear and the amygdala. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ, Harlow HF, Domek CJ. Effect of repetitive infant-infant separation of young monkeys . Journal of Abnormal Psychology. 1970;76(2):161–172. doi: 10.1037/h0029809. [DOI] [PubMed] [Google Scholar]
- Thompson JV, Best AR, Wilson DA. Ontogeny of cortical synaptic depression underlying olfactory sensory gating in the rat. Developments in Brain Research. 2005;158:107–110. doi: 10.1016/j.devbrainres.2005.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tienari P, Wynne LC, Sorri A, Lahti I, Laksy K, Moring J, Naarala M, Nieminen P, Wahlberg KE. Genotype-environment interaction in schizophreniaspectrum disorder. Long-term follow-up study of Finnish adoptees. British Journal of Psychiatry. 2004;184:216–222. doi: 10.1192/bjp.184.3.216. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Richter-Levin G. Learning under stress in the adult rat is differentially affected by ‘juvenile’ or ‘adolescent’ stress. International Journal of Neuropsychopharmacology. 2005 Dec 2;:1–16. doi: 10.1017/S1461145705006255. [DOI] [PubMed] [Google Scholar]
- Tsuang M. Schizophrenia: Genes and environment. Biological Psychiatry. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Turkewitz G, Gordon EW, Birch HG. Head turning in the human neonate: Effect of prandial condition and lateral preference. Journal of Comparative and Physiological Psychology. 1965;59:189–192. doi: 10.1037/h0021859. [DOI] [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Research. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Van Oers HJ, de Kloet ER, Levine S. Early vs. late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Brain research Developmental Brain Research. 1998;111(2):245–252. doi: 10.1016/s0165-3806(98)00143-6. [DOI] [PubMed] [Google Scholar]
- Wahlberg KE, Wynne LC, Oja H, Keskitalo P, Pykalainen L, Lahti I, Moring J, Naarala M, Sorri A, Seitamaa M, Laksy K, Kolassa J, Tienari P. Gene-environment interaction in vulnerability to schizophrenia: Findings from the Finnish Adoptive Family Study of Schizophrenia. American Journal of Psychiatry. 1997;154:355–362. doi: 10.1176/ajp.154.3.355. [DOI] [PubMed] [Google Scholar]
- Wallhausser E, Scheich H. Auditory imprinting leads to differential 2-deoxyglucose uptake and dendritic spine loss in the chick rostral forebrain. Brain Research. 1987;428:29–44. doi: 10.1016/0165-3806(87)90080-0. [DOI] [PubMed] [Google Scholar]
- Webster JI, Sternberg EM. Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. Journal of Endocrinology. 2004;181:207–221. doi: 10.1677/joe.0.1810207. [DOI] [PubMed] [Google Scholar]
- Weiss EL, Longhurst JG, Mazure CM. Childhood sexual abuse as a risk factor for depression in women: Psychosocial and neurobiological correlates. The American Journal of Psychiatry. 1999;156(6):816–828. doi: 10.1176/ajp.156.6.816. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ. The fundamental role of memory in olfactory perception. Trends in Neurosciences. 2003;26:243–247. doi: 10.1016/S0166-2236(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. Journal of Neurophysiology. 1998;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Winterfeld KT, Teuchert-Noodt G, Dawirs RR. Social environment alters both ontogeny of dopamine innervation of the medial prefrontal cortex and maturation of working memory in gerbils (Meriones unguiculatus) Journal of Neuroscience Research. 1998;52:201–209. doi: 10.1002/(SICI)1097-4547(19980415)52:2<201::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Yadon CA, Wilson DA. The role of metabotropic glutamate receptors and cortical adaptation in habituation of odor-guided behavior. Learn Memory. 2005;12:601–605. doi: 10.1101/lm.41405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: A novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology. 2003;169:308–313. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. Journal of Psychiatric Research. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]


