Abstract
One-day-old, awake infants underwent an olfactory classical conditioning procedure to assess associative learning within the olfactory system of newborns. Experimental infants received ten 30-second pairings of a novel olfactory conditioned stimulus (a citrus odor of neutral value) and tactile stimulation provided by stroking as the reinforcing unconditioned stimulus (a stimulus with positive properties). Control babies received only the odor, only the stroking, or the stroking followed by the odor presentation. The next day, all infants, in either the awake or sleep state, were given five 30-second presentations of the odor. Results were analyzed from video tapes scored by an observer unaware of the infants’ training condition. The results indicate that only those infants who received the forward pairings of the odor and stroking exhibited conditioned responding (head turning toward the odor) to the citrus odor. The performance of the conditioned response was not affected by the state of the baby during testing, because both awake and sleeping infants exhibited conditioned responses. Furthermore, the expression of the conditioned response was odor specific; a novel floral odor presented during testing did not elicit conditioned responses in the experimental babies. These results suggest that complex associative olfactory learning is seen in newborns within the first 48 hours of life. These baseline findings may serve as normative data against which observation from neonates at risk for neurological sequelae may be compared.
Keywords: mother-infant interaction, neonate, olfaction, learning
Olfactory modulation of mother-infant interactions has been studied in a variety of mammalian species, including rats,1-6 mice,7 sheep,8 cats,9,10 and nonhuman primates.11-15 Indeed, according to recent evidence, olfactory information is important in human parent-infant interactions (for extensive reviews see.7,16,17 For instance, there is a mutual olfactory recognition between parents and their offspring. A breast-fed infant is attracted to its mother’s odor,18-20 and both mothers20-22 and fathers21 are capable of identifying their infant’s odor. It is unclear whether these olfactory attractions are genetically determined19,21 or learned through experience.20
Human infants are capable of detecting a myriad of different odors soon after birth,23,24 with certain odors associated with a particular hedonic value.25-30 Infants also appear capable of retaining some memory of these odors, as indicated by olfactory discrimination and nonassociative learning paradigms such as habituation.30-36
In the present experiment, we evaluated associative learning in the olfactory system of newborns using a classical conditioning experimental paradigm. Specifically, we sought to determine whether a newborn infant could learn to associate two stimuli, an odor and a reinforcer. According to extensive recent evidence in nonprimates, olfactory associative conditioning appears to rely on normal temporal lobe and limbic system function.37,38 For example, severing olfactory inputs to the hippocampus retards olfactory associative conditioning, at least with complex odors. Thus, development of an olfactory associative conditioning task applied to human newborns could potentially provide a screening test for temporal lobe dysfunction soon after birth. We provide the first evidence that infants are capable of olfactory associative learning during the first day after birth.
MATERIALS AND METHODS
Subjects
The subjects were 66 male and female infants born at the University of California, Irvine Medical Center. All infants had Apgar scores of at least 8 and 9 at 1 and 5 minutes, respectively, following birth40 and were assessed as normal during routine postnatal examinations (In the Table, we show additional subject variables (n = 48) and their distribution across training conditions for the conditioning experiment. An additional 18 infants participated in the odor specificity experiment).
TABLE.
Subject Population Variables as a Function of Training Condition*
| Variables | Conditioning Group |
|||
|---|---|---|---|---|
| Forward Odor & Stroke | Backward Stroke/Odor | Odor Only | Stroke Only | |
| Activity level | ||||
| Preodor | 21.0 ± 4.74 | 15.4 ± 5.8 | 16.9 ± 3.2 | 21.6 ± 4.4 |
| Odor | 31.0 ± 3.57 | 12.0 ± 5.84 | 15.5 ± 3.6 | 21.6 ± 4.3 |
| Head turns toward odor | ||||
| Preodor | 6.7 ± 1.6 | 3.33 ± 1.8 | 3.7 ± 1.0 | 5.86 ± 2.1 |
| Odor | 9.4 ± 2.1 | 3.16 ± 2.1 | 4.1 ± 1.7 | 4.85 ± 1.8 |
| % of breast-fed babies | 69 | 50 | 58 | 33 |
| % of vaginally delivered babies | 58 | 69 | 50 | 83 |
| % of female babies | 42 | 33 | 50 | 42 |
Values are given as means ± standard errors of the mean.
Conditioning
Infants were conditioned on the first day of life during quiet, alert behavioral states III and IV.41 Infants in sleep-wake transition42 during training were not included in the study. All infants remained in their bassinets during conditioning. Immediately prior to conditioning, infants were taken into the odor exposure room, which was a small, well-ventilated, dimly lit, quiet room. Infants were randomly assigned to the experimental Forward Odor and Stroke Group or one of the three control groups. The infants received 10 training trials with an intertrial interval of at least 1 minute. The four conditioning groups were as follows: (1) Forward Odor and Stroke—the infant received a 30-second odor presentation with stroking given concurrently during the last 25 seconds (2) Backwards Odor and Stroke—the infant received 25 seconds of stroking followed by a 30-second odor presentation; (3) Odor Only—only the 30-second odor presentation was given; and (4) Stroke Only—only the 25-second stroking was given. The 30-second odor presentation was delivered on a cotton swab held laterally 10 cm from the infant’s nose. The cotton swab was held by hand so that it could continuously be repositioned to maintain the lateral 10-cm distance when the infant moved. The swab held 0.05 mL of a citrus odor (conditioned stimulus odor) provided by International Flavors and Fragrances (Calabasas, CA). This odor and concentration were chosen because of the seemingly neutral response of infants to the odor presentation during pilot work (ie, respiration increased, but little head turning occurred). Stroking action consisted of gentle but firm rubbing of all accessible areas of the infant’s body. The stroking resembled massage. The experimenter attempted to equally stroke all areas of the infant’s body without changing the overall position of the infant; thus, only the frontside of the babies was stroked. Furthermore, the direction and site of the stroking were varied throughout each 30-second trial. Such stimulation invariably produced an increase in general activity in babies. The infant was unable to see the person stroking or holding the cotton swab, because the experimenter stood at the head of the bassinet. On the two occasions when an infant turned his or her head to see the experimenter, the experimenter moved out of the infant’s view. The cotton swab containing the odor was visible to the infants.
The tactile stimulation provided by stroking was chosen as the reinforcing unconditioned stimulus to mimic normal mother-infant interactions.43 Although all infants received an amount of stroking sufficient to produce an increase in activity, the level of stimulation each infant received varied among infants. This was necessary because some infants responded with distress to what may be called “overstimulation.”44 This “overstimulation” does not appear to be limited to tactile stimulation; it also occurs in other sensory modalities.43 In these cases, the level was decreased. Thus, the level of stroking was not standardized; rather, the infant’s response that was elicited by the stroking was standardized. The infants were randomly assigned to the different treatment groups, and infants in the different treatment groups did not respond differently to stroking. Following training, infants were placed in clean bassinets with clean clothes to minimize lingering odors.
Testing
On day 2, 48 infants (12 per group) were tested for the acquisition of conditional responding. The test consisted of five 30-second conditioned stimulus citrus odor presentations with an intertrial interval of two min. A total of 18 infants (9 per group) were used to assess whether the olfactory learning was specific to the learned odor or was generalized to other odors. Of these infants, 9 were tested with the conditioned stimulus citrus odor and the remainder were tested with a novel floral odor (0.01 mL from International Flavors and Fragrances). Testing was conducted in the same room in which conditioning took place with the infant remaining in his or her bassinet. The odor presentation procedure was identical with that used during training. All testing sessions were conducted by persons unfamiliar with the infant’s training experiences. The test was videotaped for later analysis.
Scoring Video Tapes
The video tapes were scored by someone who was not familiar with the training condition of the baby. Babies were identified only by subject number. Thus, those scoring the tape could view the entire tape without knowing the previous training condition of the infant. The infant behaviors assessed were divided into three broad categories: general behavioral activity, turning of head toward and away from the odor, and infant behavioral state. General activity was assessed by noting the number of appendages (ie, arms, legs, and head) that the infants moved. The scoring scale was as follows: 0, the baby was not active; 1, the baby was slightly active (ie, mouth movements); 2, the baby was moving one appendage; 3, the baby was moving two appendages; 4, the baby was moving three appendages; and 5, the baby was moving four appendages.
Head turns were scored by first assigning a stable baseline head position to each infant. A deviation from this baseline either toward or away from the odor source was considered a head turn. During both training and testing, the positioning of the cotton swab containing the odor was repositioned with infant head movement so that the cotton swab was always the same distance from the infant’s nose. This was done to ensure consistent odor exposure between all treatment and test conditions. The state of the baby was assessed based on Prechtl’s41 state categories, as well as by those described by Thoman et al.42
Data Analysis
The data were analyzed using a ratio of the 30-second behavior during the odor divided by the 30-second behavior during the odor presentation plus the 30-second behavior prior to odor onset. Thus a score of .5 indicates no change in behavior in response to odor onset. A score greater than .5 indicates excitation and a score less than .5 indicates inhibition. The data were subsequently analyzed using a two-way analysis of variance (state X training condition) followed by a post hoc test. The odor specificity study was analyzed with a t test.
RESULTS AND DISCUSSION
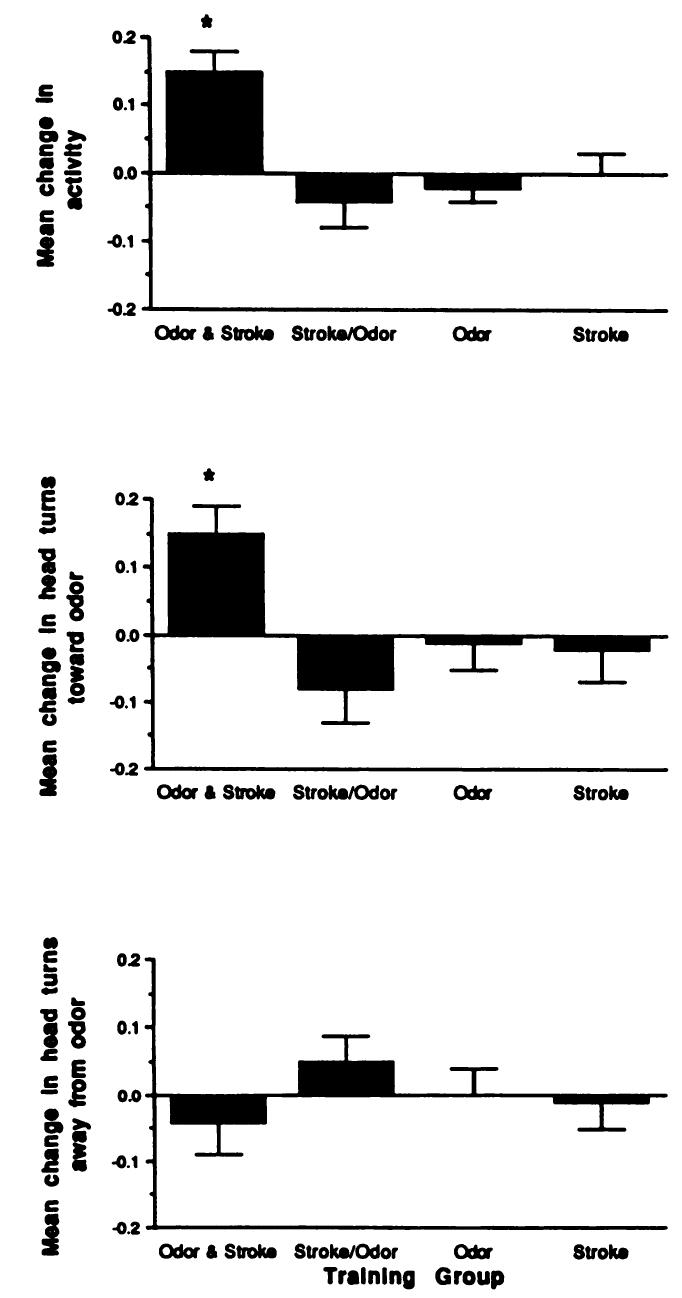
Infants are capable of complex olfactory learning as assessed by a classical conditioning paradigm (Fig. 1). Only infants who were trained in the Forward Odor and Stroke group exhibited conditioned responses and head turns toward the conditioned stimulus odor during the test presentations of odor only. These experimental (Forward Odor and Stroke) infants exhibited both a conditioned behavioral activation, which is the unconditioned response to stroking, and conditioned head turning toward the odor. The head turning response does not seem to be a function of the conditioned activity because no conditioning of head turning away from the odor was seen (2 × 4 analysis of variance for conditioned activity, F = 6.64, P < .01 and head turning toward the odor F = 4.24, P < .05; post hoc test results showed that the Forward Odor and Stroke group was significantly different from each of the other treatment groups in terms of both activity and head turning toward the odor). Control infants (Backward Odor and Stroke Group, Stroke Only Group, and Odor Only group) did not exhibit these behaviors in response to test conditioned stimulus odor presentations. Furthermore, only the experimental Forward Odor and Stroke group babies exhibited more activity and head turns toward the odor source during the odor presentation as compared with the time preceding the odor presentation (t = 7.47, P < .01). The baseline activity and head turning levels did not differ between groups (Table 1).
Fig 1.
Mean change (± standard error) in (top panel) general activity, (middle panel) head turning toward odor, and (bottom panel) head turning away from odor during odor only test as function of training condition. Infants were tested in either the awake or the asleep state.
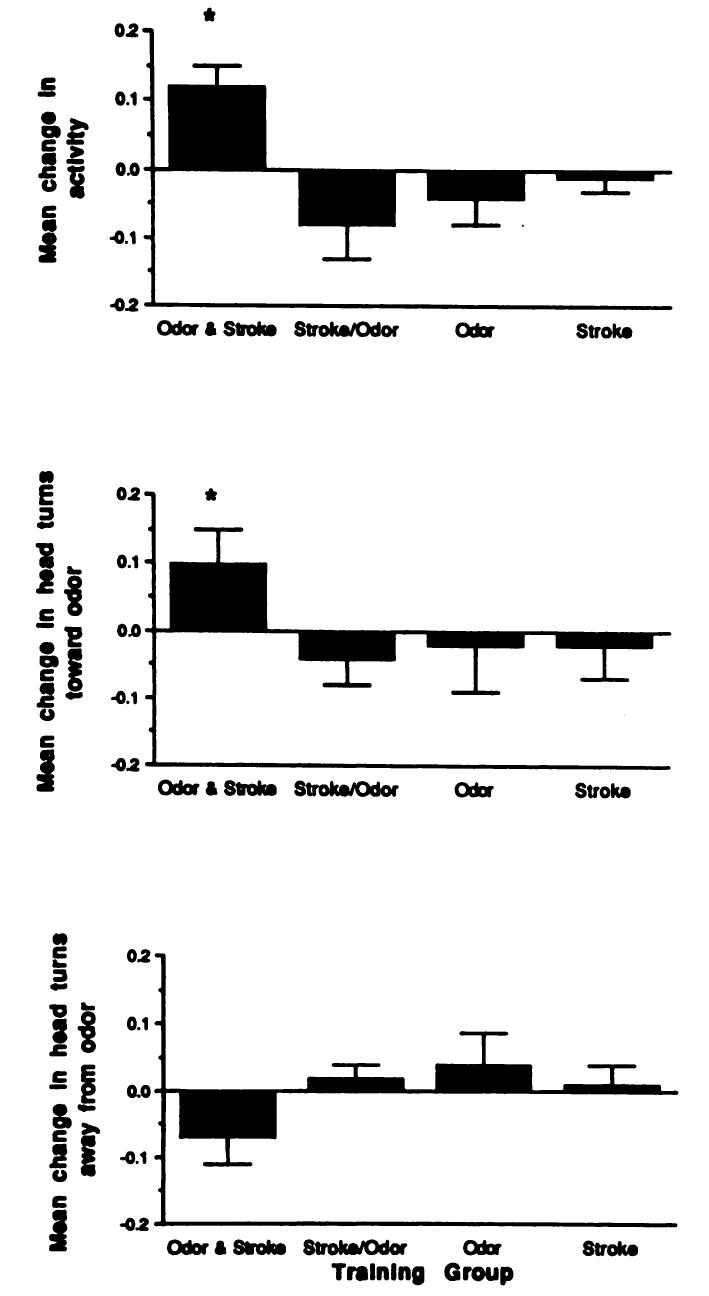
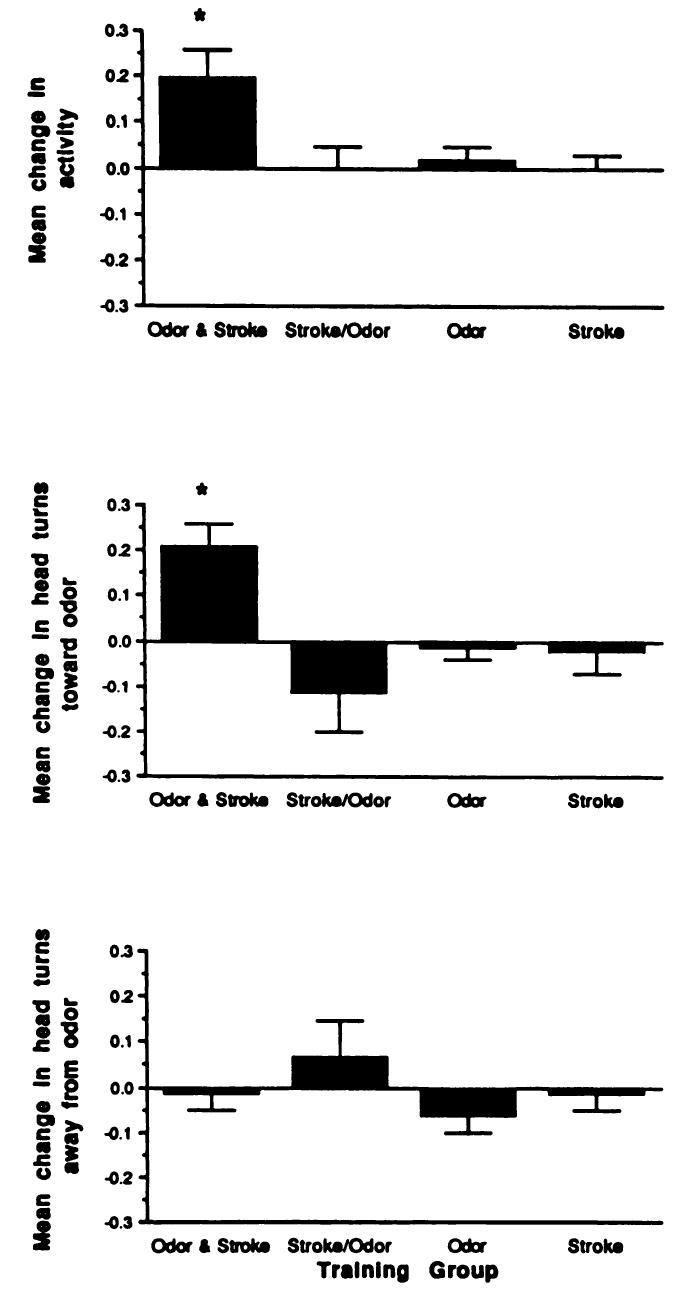
Although all infants were awake during training, infants were tested in both awake and sleep states. The state of the infant during testing did not appear to influence test results; both sleeping and awake babies in the Forward Odor and Stroke group exhibited conditioned responses (Figs 2 and 3; 2 × 4 analysis of the variance, no significant effect for state at testing).
Fig 2.
Mean change (± standard error) in (top panel) general activity, (middle panel) head turning toward odor, and (bottom panel) head turning away from odor during odor only test as function of training condition. All infants were in the awake state during testing.
Fig 3.
Mean change (± standard error) in (top panel) general activity, (middle panel) head turning toward odor, and (bottom panel) head turning away from odor during odor only test as function of training condition. All infants were in the sleep state during testing.
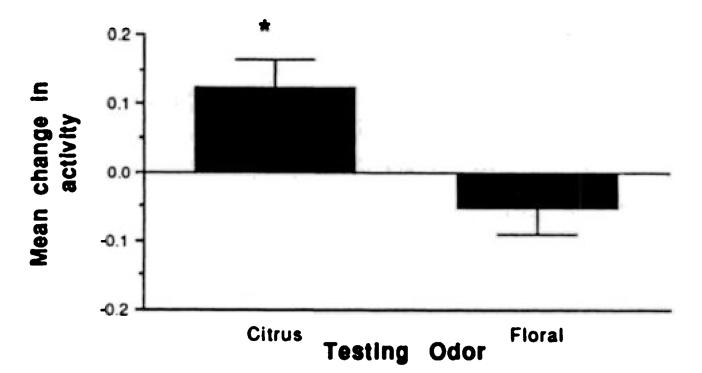
This learning appears to be odor specific—ie, presentation of a novel floral odor during testing did not result in the expression of conditioned responses. (Fig. 4; t = 5.877, P <.05). Furthermore, it is suggested by these results that the visual stimulus of the cotton swab, which occurred concurrently with both the conditioned citrus odor and the novel floral odor, was not salient to the infant.
Fig 4.
Mean change (± standard error) in general activity during odor only test for forward odor and stroke group infants tested with learned conditioned stimulus citrus odor and novel floral odor.
The ability to classically condition an odor preference in human infants appears quite robust; since, infants differing in a number of unique characteristics were equally as likely to learn (Table 1). Within the Forward Odor and Stroke group, the following variables had no detectable effect on infant learning (nonsignificant t tests): sex of infant, method of delivery (cesarean vs vaginal), and method of feeding (bottle vs breast). The inability of these variables to influence olfactory functioning to novel odors is consistent with previous research.23-36
COMPLEX PROCESSING OF OLFACTORY INFORMATION
Although this is the first demonstration of complex olfactory learning in human neonates, remarkably complex learning abilities in neonatal infants during the first few days of life have been shown in previous studies within other sensory modalities (ie, proprioceptive, gustatory, auditory).45-48
Furthermore, there has been some indication that infants are capable of complex processing of odors. Infants fed bottle milk with a taste/smell of a mint nipple on their bottle later show heightened responsiveness to mint odor in a learning experiment (Irzhanskaia and Felberbaum, 1954; cited in Ref 47). Thus, infants may have been associating feeding with the mint taste or odor. However, there was no odor-only control used in the experiment; it is possible that experience with the odor without the feeding would also have resulted in the behavioral change. In the later case, simple nonassociative learning, such as habituation, could account for the results. Furthermore, these infants were much older (1.5 to 2.5 months of age) than those in the present study.
In a recent study, Balogh and Porter49 assessed the neonate’s ability to learn a preference for a novel odor as indicated by head turns toward the odor. In that study, neonates were exposed to a novel odor placed in their bassinet for 23 hours. These infants subsequently exhibited a relative preference for this odor as compared with those experiencing the odor for the first time. It was hypothesized that this neonatal odor preference was due to odor familiarization as a result of simple exposure to the odor. The present results suggest, however, that associative learning may be involved in the infant’s response to the odor. During periods of simple odor exposure in the Balogh and Porter49 study, infants received customary care. Thus, infants received a number of types of stimulation during the odor exposure, many of which have reinforcing properties; such as milk,50 tactile stimulation provided by stroking (present study), and mother’s voice.45 Furthermore, a sex difference in learning was found in the Balogh and Porter49 study. Females but not males exhibited a change in responsiveness to the odor after a 23-hour exposure.
There is also some suggestion of infant complex olfactory processing in more naturalistic experiments. Breast-fed infants exhibit a relative preference for the odor of a breast-feeding mother.16-19 This odor preference is not limited to breast odors,17-20 preference responses in newborns are also elicited by axillary,21 and neck17 odor. It is unclear whether the infant’s response to the maternal odor is due to the mother excreting a pheromone19 with infants genetically predisposed to like maternal odor7 or whether the infant is learning about the mother’s odor through nonassociative or associative learning.16,17,48
TACTILE STIMULATION AS REINFORCEMENT
Tactile stimulation of an infant increases his or her attention to a stimulus.50-52 Therefore, one may argue that infants who received the Forward Odor and Stroke training were simply attending to the odor more than control infants did during training. Thus, experimental infants may exhibit subsequent enhanced responding to the odor because of habituation rather than classical conditioning. In this rationale, it is assumed that infants initially exhibit an aversive response to an odor that inhibits responsiveness to the odor. Following habituation to the odor, the inhibition of response is removed and infants exhibit what appears to be enhancement of response. However, there is evidence indicating that infants are exhibiting classical conditioning rather than habituation. First, infants in control groups who did and did not experience the odor during training perform similarly during testing. Second, infants in the Backward Odor and Stroke group, who presumably experienced the attentional effects of stroking stimulation, exhibited behavioral responsiveness to the odor similar to that of the Odor Only and Stroke Only training groups. Third, in previous studies30-36 of olfactory habituation it was shown that infants decrease their responsiveness to an odor with experience, even to what would appear to be an aversive odor. Thus, it appears as though stroking has reinforcing value to human infants.
The ability of stroking to function as a reward in an olfactory classical conditioning paradigm does not appear to be limited to human infants. During the neonatal period, stroking similar to that used in the present study was shown to have reinforcing value to infant rats.54 Indeed, this type of early learning produces neural, metabolic, and structural changes within the rat olfactory bulb.55-58 These neurobehavioral changes are dependent on classical conditioning.59
SUMMARY AND IMPLICATIONS
In summary, infants appear capable of complex processing of olfactory information as early as the first hours of life, as indicated by classical conditioning. Additionally, it appears as though the tactile stimulation provided by stroking has reinforcing value to human newborns. It is possible that this type of olfactory learning plays a role in the neonate’s recognition of its mother’s odor.
These baseline data may serve as normative data against which observations from neonates at risk for neurological sequelae may be compared. Accordingly, this relatively simple conditioning paradigm may be used as one of a battery of tests to assess cognitive functioning in newborn infants and thus expedite the diagnosis of neurological pathologies associated with impaired cognitive functioning. Thus, treatment for these neurological pathologies may begin during the neonatal period when the brain is still highly plastic.
An additional clinical application of these results may be to assist premature infants in developing an attraction to their mothers’ odors. Premature infants must be separated from their mothers to provide optimal medical care. However, this separation may not be ideal for the mother-infant relationship. It is possible that the olfactory conditioning procedure described here may be used to assist infants in developing an attraction to an odor. If this odor is then applied to the mother (in some cases, to the father or significant caretaker), it is possible that the infant’s responsiveness to the mother or caretaker will be enhanced and perhaps strengthen the mother-infant relationship.
ACKNOWLEDGMENTS
Funding for this research was provided by NIDCD grant DC00480 to Dr Sullivan and the PEW Charitable Trust.
We thank Adrian Correa and Rebecca Wong for assistance in data analysis, Susan Shull for help in typing, and Don Wilson for his comments on an earlier draft of this manuscript. We also thank the nursing staff of the University of California, Irvine Medical Center Full-Term Nursery, for their assistance and patience.
REFERENCES
- 1.Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Dev Brain Res. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- 2.Hofer MA, Shair H, Singh P. Evidence that maternal ventral skin substances promote suckling in infant rats. Physiol Behav. 1976;17:131–136. doi: 10.1016/0031-9384(76)90279-1. [DOI] [PubMed] [Google Scholar]
- 3.Leon M. Chemical communication in mother-young interactions. In: Vandenbergh JG, editor. Pheromones and Reproduction in Mammals. Academic Press; New York, NY: 1983. pp. 39–77. [Google Scholar]
- 4.Johanson IB, Polefrone JM, Hall WG. Appetitive conditioning in neonatal rats: conditioned ingestive responding to stimuli paired with oral infusions of milk. Dev Psychobiol. 1984;17:357–381. doi: 10.1002/dev.420170404. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan RM, Brake SC, Hofer MA, et al. Huddling and independent feeding of neonatal rats is enhanced by a conditioned change in behavioral state. Dev Psychobiol. 1986;19:625–635. doi: 10.1002/dev.420190613. [DOI] [PubMed] [Google Scholar]
- 6.Singh PJ, Tobach E. Olfactory bulbectomy and nursing behavior in rat pups. Dev Psychobiol. 1975;8:151–164. doi: 10.1002/dev.420080207. [DOI] [PubMed] [Google Scholar]
- 7.Porter RH, Balogh RD, Makin JW. Olfactory influences on mother-infant interactions. In: Rovee-Collier C, Lipsitt LP, editors. Advances in Infancy Research. L. P. ABLEX Publication Corporation; City of Publication, NJ: 1988. pp. 39–68. [Google Scholar]
- 8.Vince MA, Lynch JJ, Mottershead B, et al. Sensory factors involved in the immediately postnatal ewe/lamb bonding. Behav. 1986;93:60–83. [Google Scholar]
- 9.Larson MA, Stein BE. The use of tactile and olfactory cues in neonatal orientation and localization of the nipple. Dev Psychobiol. 1984;17:423–436. doi: 10.1002/dev.420170408. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt JS, Turkewitz G, Schneirla TC. Development of home orientation in newly born kittens. NY Acad Sci. 1969;31:231–250. doi: 10.1111/j.2164-0947.1969.tb02903.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan JN. Perceptual properties of attachment in surrogate-reared and mother-reared squirrel monkeys. In: Chevalier-Skolnikoff S, Poirier FE, editors. Garland, NY: 1977. pp. 225–234. [Google Scholar]
- 12.Kaplan JN, Cubiciotti DD., III . Early perceptual experience and social preferences in squirrel monkey. In: Smotherman WP, Bell RW, editors. Maternal Influences and Early Behavior. Spectrum Publication Corporation; NY: 1981. pp. 253–270. [Google Scholar]
- 13.Kaplan JN, Cubiciotti DD, III, Redican WK. Olfactory discrimination of squirrel monkey mothers by their infants. Dev Psychobiol. 1977;12:1–10. doi: 10.1002/dev.420100505. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan JN, Russell M. Olfactory recognition in the infant squirrel monkey. Dev Psychobiol. 1974;7:15–19. doi: 10.1002/dev.420070104. [DOI] [PubMed] [Google Scholar]
- 15.Redican WK, Kaplan JN. Effects of synthetic odors on filial attachment in infant squirrel monkeys. Physiol Behav. 1978;320:79–85. doi: 10.1016/0031-9384(78)90206-8. [DOI] [PubMed] [Google Scholar]
- 16.Meisami E. The uses of olfaction and its neural development in the neonate. In: Jones CT, editor. Fetal and Neonatal Development. I. Perinatology Press; Ithaca, NY: 1988. pp. 195–202. [Google Scholar]
- 17.Schaal B. Review: Olfaction in infants and children: developmental and functional perspectives. Chem Senses. 1988;13:145–190. [Google Scholar]
- 18.Macfarlane AJ. Olfaction in the development of social preferences in the human neonate. Ciba Found Symp. 1975;33:103–117. doi: 10.1002/9780470720158.ch7. [DOI] [PubMed] [Google Scholar]
- 19.Russell MJ. Human olfactory communication. Nature. 1976;260:520–522. doi: 10.1038/260520a0. [DOI] [PubMed] [Google Scholar]
- 20.Schaal B, Montagner H, Hertling E, et al. Les stimulations olfactives dans les relations entr l’enfant et la mere. Reprod Nutr Dev. 1980;20:843–858. [PubMed] [Google Scholar]
- 21.Porter RH, Balogh RD, Cernoch JM, et al. Recognition of kin through characteristic body odors. Chem Senses. 1986;11:389–395. [Google Scholar]
- 22.Porter RH, Cernoch JM, McLaughlin FJ. Maternal recognition of neonates through olfactory cues. Physiol Behav. 1983;30:151–154. doi: 10.1016/0031-9384(83)90051-3. [DOI] [PubMed] [Google Scholar]
- 23.Lipsitt LP, Engen T, Kaye H. Developmental changes in the olfactory threshold of the neonate. Child Dev. 1963;34:371–376. [Google Scholar]
- 24.Shimada M, Takahashi S, Imur S, et al. Olfaction in neonates. Chem Senses. 1987;12:518. [Google Scholar]
- 25.Peterson F, Rayey LH. The beginnings of mind in the newborn. Bull Lying-in Hosp. (NYC) 1911 [Google Scholar]
- 26.Ciulo L. Sulla funzione olfattoria nel neonato. Valsalva. 1934;10:22–34. [Google Scholar]
- 27.Steiner JE. Facial expressions of the neonate infant indicating the hedonics of food-related stimuli. In: Weiffenbach JM, editor. Taste and Development: The Genesis of Sweet Preference. National Institutes of Health, Dept of Health, Education, and Welfare; Bethesda, MD: 1977. pp. 173–188. [Google Scholar]
- 28.Steiner JE. Human facial expressions in response to taste and smell stimulation. In: Lipsitt LP, Reese HW, editors. Advances in Child Development. Vol. 13. Academic Press; New York, NY: 1979. pp. 257–295. [DOI] [PubMed] [Google Scholar]
- 29.Stirnimann F. Le gou et l’ordorant du noveau-ne. Rev Fr Pediatr. 1936;12:453–485. [Google Scholar]
- 30.Engen T. Psychophysical analysis of the odor intensity of homologous alcohols. J Exp Psychol. 1965;70:611–616. doi: 10.1037/h0022685. [DOI] [PubMed] [Google Scholar]
- 31.Engen T, Lipsitt LP. Decrement and recovery of responses to olfactory stimuli. J Comp Physiol Psychol. 1965;59:312–316. doi: 10.1037/h0021820. [DOI] [PubMed] [Google Scholar]
- 32.Engen T, Lipsitt LP, Kaye H. Olfactory responses and adaptation in the human neonate. J Physiol Psychol. 1963;56:73–77. [Google Scholar]
- 33.Engen T, Cain WS, Rovee CK. Direct scaling of olfaction in the newborn infant and the adult observer. In: Tanyolac N, editor. Theories of Odor Measurement. Academic Press; New York, NY: 1968. pp. 271–294. [Google Scholar]
- 34.Rovee CK. Psychophysical scaling of olfactory response to the aliphatic alcohols in human neonates. J Exp Psychol. 1969;7:245–254. doi: 10.1016/0022-0965(69)90047-2. [DOI] [PubMed] [Google Scholar]
- 35.Sarnat HB. Olfactory reflexes in the newborn infant. J Pediatr. 1978;92:624–626. doi: 10.1016/s0022-3476(78)80307-2. [DOI] [PubMed] [Google Scholar]
- 36.Self PA, Horowitz RD, Paden LY. Olfaction in newborn infants. Dev Psychol. 1972;7:349–363. [Google Scholar]
- 37.Kucharski D, Hall WG. New routes to early memories. Science. 1987;238:786–788. doi: 10.1126/science.3672125. [DOI] [PubMed] [Google Scholar]
- 38.Kucharski D, Hall WG. Developmental change in the access to olfactory memories. Behav Neurosci. 1988;102:340–348. doi: 10.1037//0735-7044.102.3.340. [DOI] [PubMed] [Google Scholar]
- 39.Staubli U, Ivy G, Lynch G. Hippocampal denervation causes rapid forgetting of olfactory information in rats. Proc Nat Acad Sci (USA) 1984;81:5885–5887. doi: 10.1073/pnas.81.18.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Apgar V. Proposal of a new method of evaluating newborn infants. Anesth Analg. 1953;32:260–267. [PubMed] [Google Scholar]
- 41.Prechtl HFR. The behavioral states of the newborn infant. Brain Res. 1974;76:185–212. doi: 10.1016/0006-8993(74)90454-5. [DOI] [PubMed] [Google Scholar]
- 42.Thomas EB, Davis DH, Denenberg V. The sleeping and waking states of infants: correlations across time and person. Physiol Behav. 1987;41:53–537. doi: 10.1016/0031-9384(87)90307-6. [DOI] [PubMed] [Google Scholar]
- 43.Korner AF, Thoman EB. The relative efficacy of contact and vestibular-proprioceptive stimulation in soothing neonates. Child Dev. 1972;43:443–453. [PubMed] [Google Scholar]
- 44.Gardner JM, Karmel BZ. Attention and arousal in preterm and full-term neonates. In: Field T, Sostek A, editors. Infants born at risk: Physiological, Perceptual, and Cognitive Processes. Grune & Stratton; New York, NY: 1983. pp. 69–98. [Google Scholar]
- 45.DeCasper AJ, Fifer WP. Of human bonding: newborns prefer their mothers’ voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- 46.Lipsitt LP, Kaye H, Bosack TN. Enhancement of neonatal sucking through reinforcement. J Exp Child Psychol. 1966;4:163–168. doi: 10.1016/0022-0965(66)90016-6. [DOI] [PubMed] [Google Scholar]
- 47.Marquis DP. Can conditioned reflexes be established in the newborn infant? J Genet Psychol. 1931;39:479–492. [Google Scholar]
- 48.Spelt D. The conditioning of the human fetus in utero. J Exp Psychol. 1948;38:338–346. doi: 10.1037/h0059632. [DOI] [PubMed] [Google Scholar]
- 49.Balogh RD, Porter RH. Olfactory preference resulting from mere exposure in human neonates. Infant Behav Dev. 1986;9:395–401. [Google Scholar]
- 50.Papousek H. Conditioned head rotation reflexes in infants in the first months of life. Acta Paediatr. 1961;50:565–576. doi: 10.1111/j.1651-2227.1961.tb08047.x. [DOI] [PubMed] [Google Scholar]
- 51.Gregg CL, Haffner ME, Korner AF. The relative efficacy of vertibular-proprioceptive stimulation and the upright position in enhancing visual pursuit in neonates. Child Dev. 1976;47:309–314. [PubMed] [Google Scholar]
- 52.Korner AF, Thoman EB. Visual alertness in neonates as evoked by maternal care. J Exp Child Psychol. 1970;10:67–78. doi: 10.1016/0022-0965(70)90045-7. [DOI] [PubMed] [Google Scholar]
- 53.Muir D, Field J. Newborn infants orient to sound. Child Dev. 1979;50:431–436. [PubMed] [Google Scholar]
- 54.Sullivan RM, Hall WG. Reinforcers in infancy: classical conditioning using stroking or intra-oral infusions of milk as a UCS. Dev Psychobiol. 1986;20:215–223. doi: 10.1002/dev.420210303. [DOI] [PubMed] [Google Scholar]
- 55.Coopersmith R, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;225:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Dev Brain Res. 1986;27:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- 57.Woo CC, Coopersmith R, Leon M. Localized changes in olfactory bulb morphology associated with early olfactory learning. J Comp Neurol. 1987;263:113–125. doi: 10.1002/cne.902630110. [DOI] [PubMed] [Google Scholar]
- 58.Wilson DA, Sullivan RM, Leon M. Single-unit analysis of postnatal olfactory learning: modified olfactory bulb output response patterns to learned attractive odors. J Neurosci. 1987;7:3154–3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sullivan RM, Wilson DA, Leon M. Associative processes in early olfactory preference acquisition: neural and behavioral consequences. Psychobiol. 1988;17:29–33. doi: 10.3758/bf03337814. [DOI] [PMC free article] [PubMed] [Google Scholar]