Abstract
Supplementation of maternal diet with the essential nutrient, choline, during the second half of pregnancy in rats causes long-lasting improvements in spatial memory in the offspring and protects them from the memory decline characteristic of old age. In contrast, prenatal choline deficiency is associated with poor performance in certain cognitive tasks. The mechanism by which choline influences learning and memory remains unclear; however, it may involve changes to the hippocampal cholinergic system. Previously, we showed that the hippocampi of prenatally [embryonic days (E) 11–17] choline-deficient animals have increased synthesis of acetylcholine (ACh) from choline transported by the high-affinity choline transporter (CHT) and reduced ACh content relative to the control and to the E11–17 choline-supplemented rats. In the current study, we found that, during postnatal period [postnatal days (P) 18-P480)], prenatal choline deficiency increased the expression of CHT mRNA in the septum and CHT mRNA and protein levels in the hippocampus and altered the pattern of CHT immunoreactivity in the dentate gyrus. CHT immunoreactivity was more prominent in the inner molecular layer in prenatally choline-deficient rats compared to controls and prenatally choline-supplemented animals. In addition, in all groups, we observed a population of hilar interneurons that were CHT immunoreactive. These neurons are the likely source of the hippocampal CHT mRNA as their number correlated with the levels of this mRNA. The abundance of hippocampal CHT mRNA rose between P1 and P24 and then declined reaching 60% of the P1 value by P90. These data show that prenatal availability of choline alters its own metabolism (i.e. CHT expression). While the upregulated CHT expression during the period of prenatal choline deficiency may be considered as a compensatory mechanism that could enhance ACh synthesis when choline supply is low, the persistent upregulation of CHT expression subsequent to the brief period of prenatal deprivation of choline in utero might be beneficial during choline deficiency in adulthood.
Keywords: choline transporter, acetylcholine, hippocampus, septum, nutrition, pregnancy
1. Introduction
The availability of the essential nutrient, choline, during gestation in rodents causes changes in brain organization that are apparent in the fetus, in the young, in the adult, and in the aged animal (Blusztajn, 1998; McCann et al., 2006; Meck and Williams, 2003; Zeisel, 2004). In the most studied model, that employs pregnant rats consuming diets of varying choline content during the seven day period of the second half of gestation (embryonic days E11–17), animals that were supplemented with choline are characterized by improved spatial and temporal memory and improved attention at a young age and in adulthood (Meck et al., 1988; Meck et al., 1989; Meck and Williams, 1997a; Meck and Williams, 1997b; Meck and Williams, 1997c; Meck and Williams, 1999). These animals do not exhibit the normally occurring age-related decline in memory performance, i.e. they are protected from the cognitive deficits of old age (Blusztajn, 1998; Meck and Williams, 2003). Taken together these data suggest that cognitive decline is not an inevitable outcome of old age, but rather can be prevented by increasing the supply of choline during a critical period of prenatal development. In contrast, rats deprived of choline during the E11–17 period have deficits in certain memory tasks (Meck and Williams, 1997c). One of the physiological functions of choline is to serve as the precursor of the neurotransmitter, acetylcholine (ACh) in cholinergic neurons. The rate limiting factor in the synthesis of ACh is the supply of choline (Blusztajn and Wurtman, 1983), and our previous studies provided evidence that, in young rats, this process is profoundly affected by the availability of choline in utero in the septohippocampal cholinergic neurons that are thought to participate in the mechanisms underlying learning, memory, and attention (Gold, 2003; Sarter et al., 2003). Specifically, the prenatally choline-supplemented rats derived a high proportion of choline for ACh synthesis from the membrane-bound choline pool in the form of phosphatidylcholine (Cermak et al., 1998; Holler et al., 1996), whereas prenatally choline-deficient rats were characterized by upregulated transport of choline from the extracellular space catalyzed by the high-affinity choline transporter, CHT (Cermak et al., 1998). The latter result could be due to either increased CHT expression or its activation (Sarter and Parikh, 2005) as choline transport catalyzed by CHT can be rapidly stimulated by increased neuronal firing (Collier and Ilson, 1977; Murrin and Kuhar, 1976; Polak et al., 1977; Sherman et al., 1978) that appears to be mediated by a recruitment of CHT molecules from a synaptic vesicular pool to the plasma membrane resulting in an increased Vmax of choline transport without a change in Kt (Ferguson et al., 2003). In the current study we found that prenatal choline deficiency is associated with increased CHT mRNA expression in the septum (where the cell bodies of the cholinergic septohippocampal neurons reside) and increased CHT mRNA and protein levels in the hippocampus. The CHT immunoreactive septohippocampal projection, which terminates in the inner molecular layer of the dentate gyrus, is augmented in the choline deficient animals and CHT immunoreactive local circuit neurons in the hilus of the hippocampus are in many cases more prominent than in controls and choline supplemented animals.
2. Results
Initially, we examined CHT expression in the medial septum on P18, P90, and P480 (16 months old) by RTPCR (Fig. 1). At each of the three ages, prenatally choline-deficient animals had the highest level of CHT mRNA expression with the most significant difference on P480. The medial septal cholinergic neurons project to the hippocampus and, consistent with CHT mRNA expression in the septum, the levels of CHT protein in the hippocampus were at least 2-fold higher in prenatally choline deficient rats as compared to the other two groups on both P18 and P480 (Fig. 2). Previous studies have noted that a small number CHT-expressing neurons can also be found in the hippocampus, specifically the hilus of the dentate gyrus (Misawa et al., 2001). Moreover, we previously reported the presence of CHT mRNA in the hippocampus (Berse et al., 2005). We therefore performed a developmental study of CHT mRNA expression in the hippocampus (Fig. 3). During the first postnatal week CHT mRNA expression increased and then plateaued from P8 to P24. Subsequently there was a dramatic drop in CHT mRNA levels that reached a nadir by P34, followed by a new, apparently stable, plateau until P90. The latter level of CHT was approximately 50% of that seen at the peak.
Figure 1. CHT mRNA expression in the medial septum.
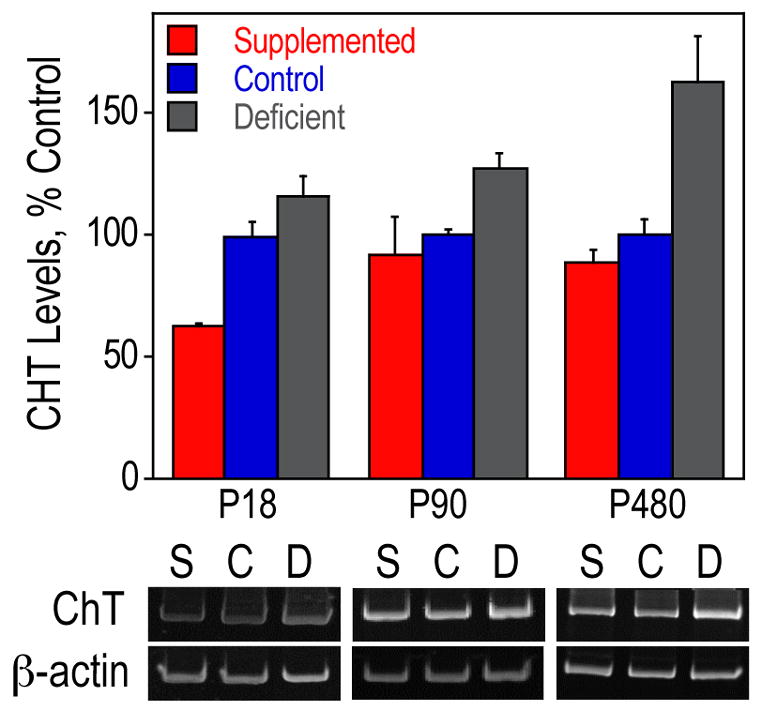
Septal RNA from P18, P90 and P480 rats was used for RT-PCR of CHT and β-actin. CHT levels were normalized using β-actin levels and are presented as means ± SEM (n=4 per group on P18 and P90, n=3 per group on P480). The levels of CHT were significantly different among the three groups of animals on each day as determined by ANOVA (p<0.005, p<0.05, and p<0.01, respectively). The ANOVA was followed up with Tukey tests at a 5% procedure wise error rate. Prenatally choline-deficient animals had a significantly higher amount of CHT mRNA compared to prenatally choline-supplemented animals on all days and to control animals at P480. On P18, the CHT mRNA levels were significantly higher in the control animals than prenatally choline-supplemented animals. S, prenatally choline-supplemented; C, control; D, prenatally choline-deficient.
Figure 2. CHT protein levels in the hippocampus.
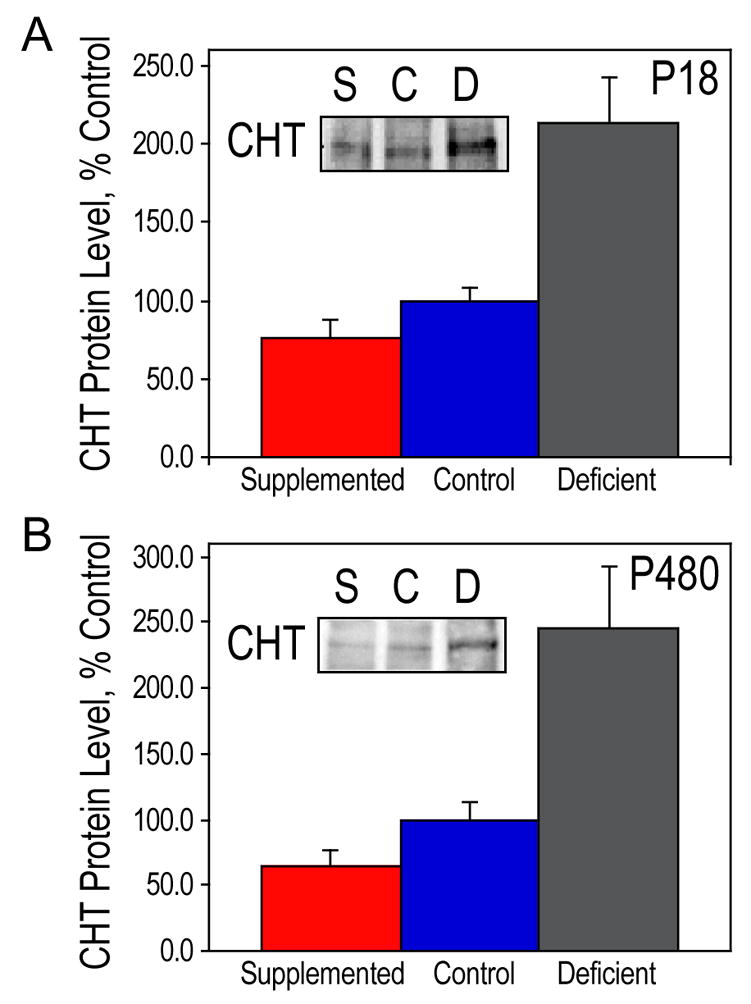
Hippocampal lysates from P18 (A) and P480 (B) rats were used for Western blot analysis of CHT. CHT protein levels are presented as means ± SEM, n=3 per group. The levels of CHT were significantly different among the three groups of animals on both days as determined by ANOVA (p<0.005, and p<0.01, respectively). The ANOVA was followed up with Tukey tests at a 5% procedure wise error rate. On both P18 and P480, prenatally choline-deficient animals had a significantly higher amount of CHT protein compared to control animals and to prenatally choline-supplemented animals. S, prenatally choline-supplemented; C, control; D, prenatally choline-deficient.
Figure 3. Developmental changes in CHT expression in the hippocampus of control rats.
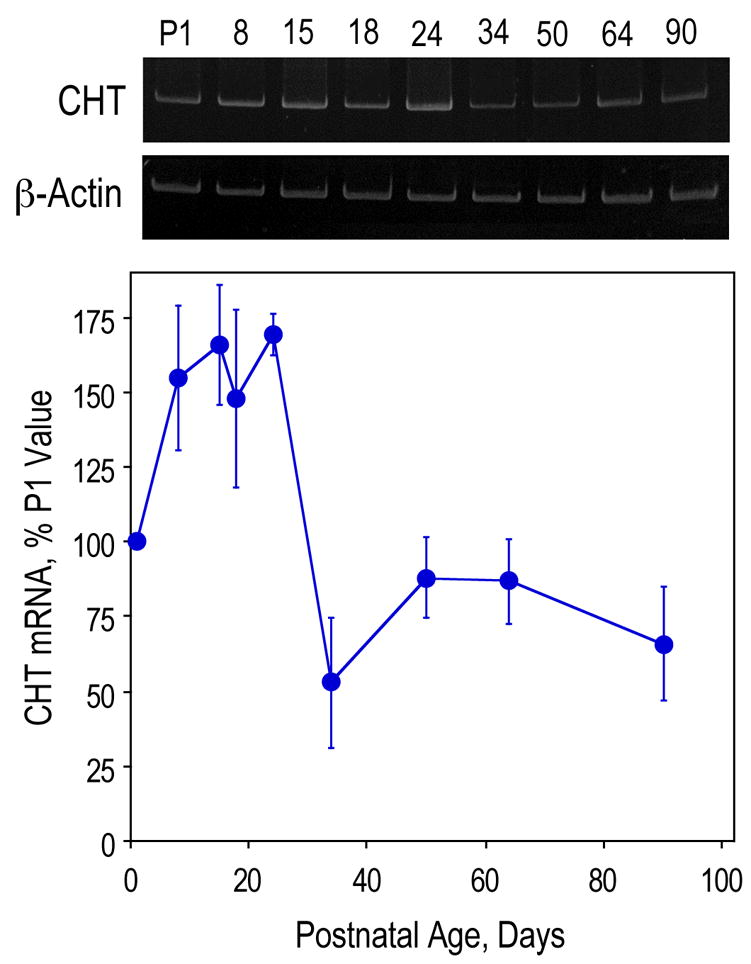
Hippocampal RNA from P1, 8, 15, 18, 34, 50, 64 and 90 rats was used for RT-PCR of CHT and β-actin. CHT levels were normalized using β-actin levels and are presented as means ± SEM, n=6 per group.
In the hippocampal formation, CHT-immunoreactivity was primarily located in non-pyramidal neurons in CA4 and in fibers and terminals in the supragranular plexus and inner molecular layer of the dentate gyrus (Fig. 4). Less prominent fiber staining was seen in the stratum pyramidale and stratum oriens of CA1–3. In P18 animals that experienced reduced dietary choline availability in utero, CHT-immunoreactivity was more prominent in the inner molecular layer of the dentate gyrus and CHT-immunoreactivity neurons were more prominent in CA4 compared to control and supplemented groups despite similar staining intensity patterns among the three groups in other brain regions (Fig. 4). Quantitation of CHT-immunoreactive neurons in CA4 showed a significant difference among the three groups of P18 animals with supplemented animals having a lower density than either deficient or controls (Fig. 5A). The CHT mRNA expression in the hippocampus of the same P18 animals was determined. Prenatally choline-supplemented animals had a significantly lower amount of CHT expression as compared to that of the other two groups of animals (Fig. 5B). Regression analysis showed a significant correlation between the hilar cell counts and CHT mRNA in the hippocampus (Fig. 5C).
Figure 4. CHT immunocytochemistry in the hippocampus and medial septum of P18 rats.
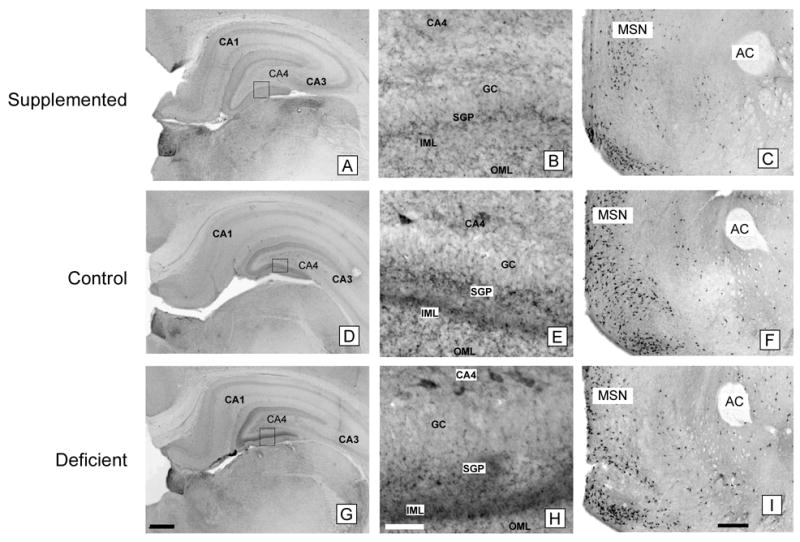
Prenatally choline-supplemented (A, B, C), control (D, E, F), deficient (G, H, I) animals. GC, granular cell layer; SGP, supragranular plexus; IML, inner molecular layer; OML, outer molecular layer; MSN, medial septal nucleus; AC, anterior commissure. The pattern of CHT-immunoreactivity in the molecular layer of the dentate gyrus differed among the three groups and CHT-immunoreactive neurons were generally more prominent in CA4 in the deficient group (G, H) and less prominent in the supplemented group (A, B) compared to controls (D, E) despite similar staining intensities in other brain regions such as the medial septal nucleus (C, F, I). Magnification bars: A, D, G - 500 microns; B, E, H - 50 microns; C, F, I - 500 microns.
Figure 5. Correlation between the number of CHT-positive cells in the hilus and the level of CHT mRNA in the hippocampus at P18.
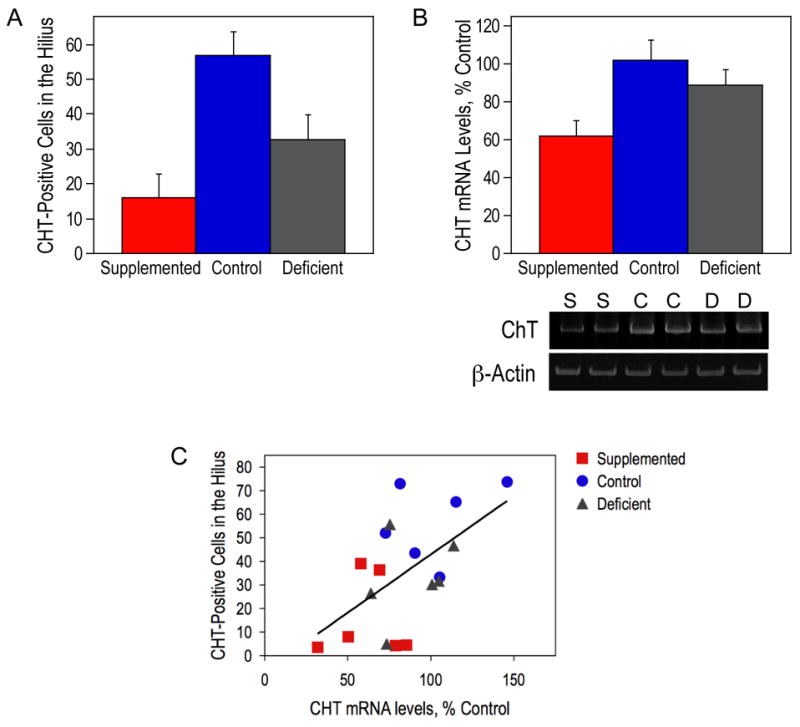
(A) CHT-positive neuronal perikarya in the CA4 regions of the hippocampal formation of P18 animals were quantitated and analyzed using a semi-automated computerized morphometry system. The number of CHT-positive cells was significantly different among the three groups of animals as determined by ANOVA (p<0.005). The ANOVA was followed up with Tukey tests at a 5% procedure wise error rate. Control animals had a significantly higher number of CHT-positive cells compared to prenatally choline-supplemented animals. (B) Hippocampal RNA from P18 rats was used for RT-PCR of CHT and β-actin. CHT levels were normalized using β-actin levels and are presented as means ± SEM (n=6 per group). The levels of CHT were significantly different among the three groups of animals as determined by ANOVA (p<0.05). The ANOVA was followed up with Tukey tests at a 5% procedure wise error rate. Prenatally choline-deficient and control animals had a significantly higher amount of CHT mRNA compared to prenatally choline-supplemented animals. (C) A significant correlation between the number of CHT-positive cells in the hilus and the level of CHT mRNA in the hippocampus was observed (R=0.567 and p<0.05). S, prenatally choline-supplemented; C, control; D, prenatally choline-deficient.
At P72, the differences in CHT-immunoreactivity among the three groups were less consistent but a similar pattern of altered CHT-immunoreactivity was seen in deficient animals in some cases (Fig. 6). There were no distinguishable differences in CHT-immunoreactivity basal forebrain neurons among the three groups at P18 or P72 (Fig. 4C, F, I and 6C, F, I). Analysis of P72 animals did not show a significant difference in cell density among the three groups (Fig. 7A). Because the P72 animals were perfused, the tissue could not be used for RNA extraction and parallel analysis of CHT mRNA as performed on the P18 animals. As an alternative, hippocampal tissue from rats that were similar in age (P64) was used to measure the expression level of CHT. Prenatally choline-deficient animals had 2–3 fold higher amounts of CHT mRNA than animals from each of the other two groups (Fig. 7B). These results suggest that the prenatally choline-deficient animals maintain a higher level of CHT in the hippocampus, possibly by increased CHT expression per cell rather than a higher number CHT-positive cells.
Figure 6. CHT immunocytochemistry in the hippocampus and medial septum of P72 rats.
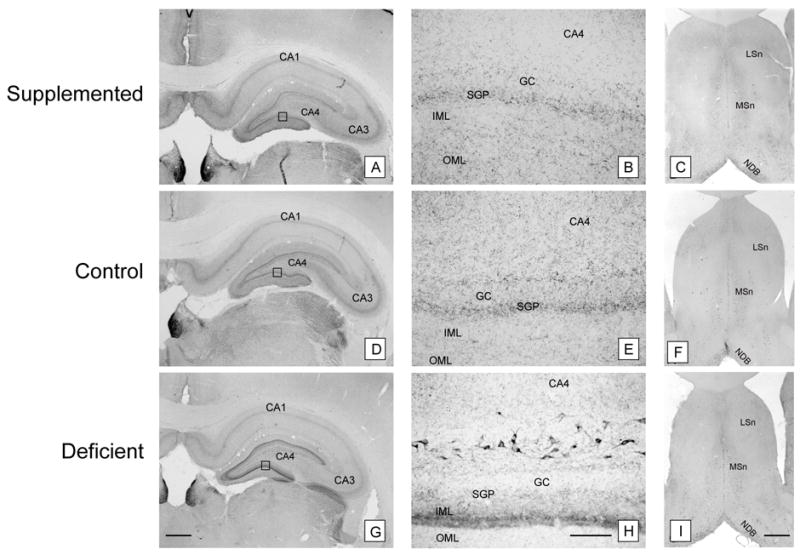
Prenatally choline-supplemented (A, B, C), control (D, E, F), and deficient (G, H, I) animals. GC, granular cell layer; SGP, supragranular plexus; IML, inner molecular layer; OML, outer molecular layer; MSn, medial septal nucleus; LSn, Lateral septal nucleus; NDB, nucleus of the digonal band. The pattern of CHT-immunoreactivity seen at P18 persisted at P72. CHT-immunoreactivity was more prominent in the inner molecular layer of the dentate gyrus and CHT-immunoreactive neurons were generally more prominent in CA4 in the deficient group (G, H) compared to the supplemented (A, B) and control (D, E) groups despite similar staining intensities in other brain regions such as the medial septal nucleus (C, F, I). The prominence of inner molecular layer staining generally paralleled the number CHT-immunoreactive hilar neurons even in the face of case to case variability (B, E, H). Magnification bars: A, D, G - 500 microns; B, E, H - 50 microns; C, F, I - 500 microns.
Figure 7. CHT-positive cells in the hilus at P72.
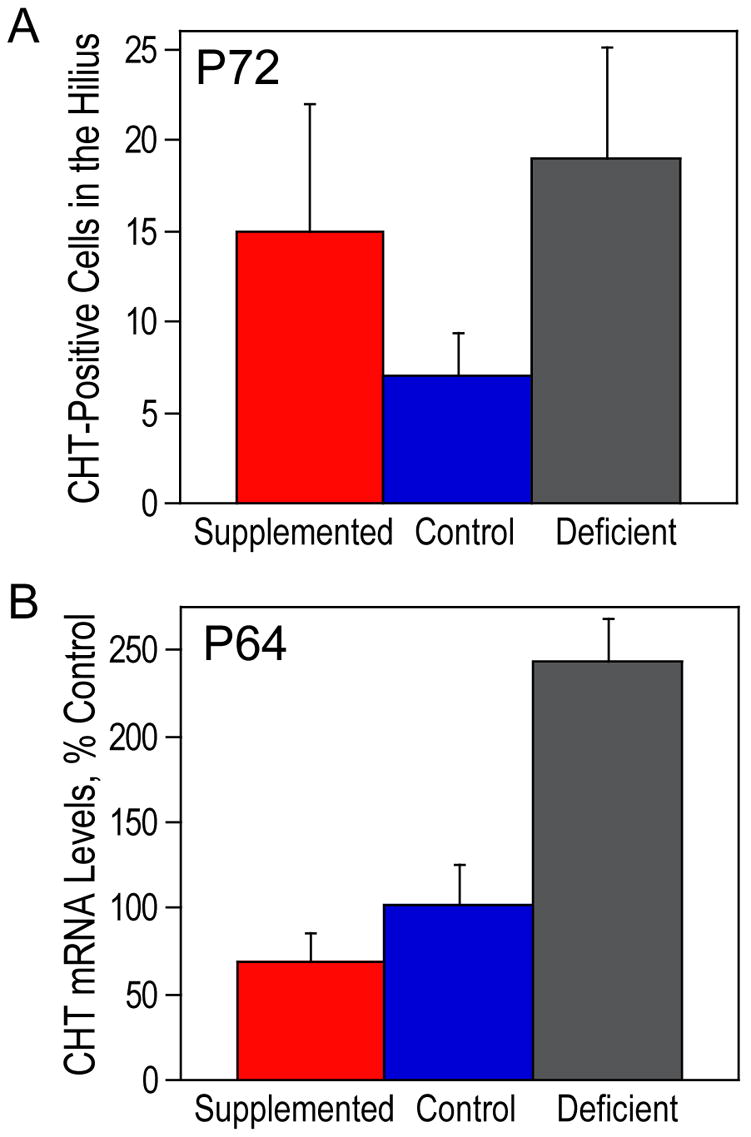
(A) CHT-positive neuronal perikarya in the CA4 regions of the hippocampal formation of P18 animals were quantitated and analyzed using a semi-automated computerized morphometry system. There were no significant differences between the groups. (B) Hippocampal RNA from P64 rats was used for RT-PCR of CHT and β-actin. CHT levels were normalized using β-actin levels and are presented as means ± SEM (n=6 per group). The levels of CHT were significantly different among the three groups of animals as determined by ANOVA (p<0.0005). The ANOVA was followed up with Tukey tests at a 5% procedure wise error rate. Prenatally choline-deficient animals had a significantly higher amount of CHT mRNA compared to prenatally choline-supplemented and control animals.
3. Discussion
The data presented here indicate that the previously-observed upregulation of CHT activity in hippocampal slices from the prenatally choline-deficient rats (Cermak et al., 1998) can be accounted for by the increased expression of CHT in these animals. Prenatal choline deficiency caused a long-term upregulation of the abundance of CHT mRNA in the septum and hippocampus. CHT protein levels were also dramatically increased in the hippocampi from the prenatally choline-deficient rats as compared to the control and prenatally choline-supplemented animals. These biochemical findings were further corroborated by the increased prominence of CHT immunoreactivity in the inner molecular layer of the dentate gyrus in prenatally choline-deficient animals. However, the number of CHT-immunoreactive neurons in the medial septal nucleus were similar among the three groups of rats, suggesting that the increased hippocampal fiber immunoreactivity in the prenatally choline-defcient animals is likely due to plasticity and enhancement of the axonal projections, synaptic terminations, and/or CHT protein levels in the nerve terminals of neurons originating from the medial septum (Mesulam et al., 1983). Interestingly, previous studies showed that prenatal choline deficiency reduced (McKeon-O’Malley et al., 2003), while supplementation caused an increase (Williams et al., 1998) in the average size of the medial septal cholinergic neurons as assessed by immunostaining with antibodies against the p75 neurotrophin receptor, NGFR. Given that both CHT and NGFR are markers for the same population of medial septal cholinergic neurons, it appears that prenatal choline availability has a profound impact on the development of these cells with low choline consumption resulting in upregulated CHT expression and reduced size of these neurons, while high choline intake causing an increase in the neuronal size, possibly mediated by the previously-reported elevated expression of hippocampal nerve growth factor (Sandstrom et al., 2002) – a trophic protein for these neurons (Hefti et al., 1984; Koliatsos et al., 1991; Mobley et al., 1986). In contrast to the profound impact of prenatal choline deficiency on CHT expression, prenatal choline supplementation had a minor effect on CHT with significantly reduced levels of its mRNA levels relative to the control animals observed only at P18 in the septum and hippocampus.
In behavioral studies, prenatally choline-supplemented adult rats are characterized by improved performance relative to prenatally-deficient and control animals in tasks measuring spatial memory, temporal processing, and attention (Brandner, 2002; Meck et al., 1989; Meck and Williams, 1997a; Meck and Williams, 1997b; Meck and Williams, 1997c; Meck and Williams, 1999; Schenk and Brandner, 1995). Prenatally choline-deficient animals are normal in many behavioral tests, however they exhibit impairments in attentional- and demanding memory tasks (Meck and Williams, 1997c; Meck and Williams, 1999), and, unlike the prenatally choline-supplemented rats that are characterized by enhanced hippocampal long-term potentiation (LTP) studied in vitro in slice preparations, these animals have LTP deficits (Jones et al., 1999; Pyapali et al., 1998), possibly as a result of diminished slice viability. NMDA receptor-mediated neurotransmission was also reduced in hippocampal slices from prenatally choline-deficient rats as compared to controls (Montoya and Swartzwelder, 2000). Moreover, phosphorylation of hippocampal mitogen-activated protein kinase (MAPK) and cAMP response element binding protein (CREB) evoked by NMDA, glutamate, or depolarizing concentrations of potassium was reduced by prenatal choline deficiency and increased by prenatal choline supplementation (Mellott et al., 2004). These data suggested that prenatal availability of choline has long-term effects on multiple neuronal systems. We tested this hypothesis using gene expression profiling with oligonucleotide microarray techniques (Mellott et al., in press) and found alterations in expression of multiple genes that were, in most cases, transient occurring during the P15–P34 period. Prenatally choline deficient rats had the highest expression of calcium/calmodulin-dependent protein kinase (CaMK) IIβ, protein kinase Cβ2, and GABAB receptor 1 isoforms c and d in the hippocampus. Prenatally choline-supplemented rats had the highest expression of CaMKI and insulin-like growth factor II in the cortex and of the transcription factor Zif268/EGR1 in the cortex and hippocampus.
Upregulation of CHT expression also occurs under conditions that can be collectively described as “hypocholinergic.” Mice heterozygous for a mutation in the gene encoding the ACh-synthesizing enzyme, choline acetyltransferase (ChAT), have 1.5–2 fold increase in CHT mRNA and protein expression, as well as activity, as compared to the wild type animals (Brandon et al., 2004). This upregulation of CHT contributes to the apparently normal behavioral phenotype and ACh turnover in the ChAT heterozygotes. Similarly, overexpression of the ACh-hydrolyzing enzyme, acetylcholinesterase (AChE), in mice, doubled the striatal binding of a CHT-specific ligand, hemicholinium-3 (Beeri et al., 1997). Perhaps surprisingly, AChE knockout mice had increased CHT protein expression in the striatum (Volpicelli-Daley et al., 2003). It is possible that in the latter case ACh levels are high at the expense of free choline and that this relative choline deficiency results in high CHT expression. CHT-null mice die shortly after birth due to apnea caused by the deficit in the cholinergic neurotransmission at the neuromuscular junctions of the respiratory muscles (Ferguson et al., 2004). CHT heterozygous mice are normal in multiple behavioral tasks (Bazalakova et al., 2006). However, these animals have a spontaneous phenotype, i.e. they show more rearing behavior in the open field than the wild type littermates (Bazalakova et al., 2006). They also display early fatigue on a treadmill (Bazalakova et al., 2006) suggesting that a single CHT allele is insufficient to support normal physical performance. In addition, CHT heterozygotes have reduced levels of hippocampal, striatal, and cerebral cortical ACh concomitant with elevations in the free choline concentrations (Bazalakova et al., 2006). The latter observations are consistent with the model of Farber et al. who estimated that even though cholinergic neurons in the striatum constitute only 1% of all cells, it is the activity of CHT expressed by these neurons that accounts for 60% of the total choline uptake in this tissue (Farber et al., 1996). Similar conclusions have been reached by Parikh and Sarter based on in vivo studies (Parikh and Sarter, 2006). Thus, when CHT activity is reduced, such as that occurring in the CHT heterozygotes, ACh levels drop and, the presumably extracellular, brain free choline levels are allowed to rise (Bazalakova et al., 2006).
The current public databases list 71 single nucleotide polymorphisms (SNPs) within the human CHT gene (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=60482&chooseRs=all). Only one of those CHT alleles, occurring with an approximately 6% frequency among Ashkenazi Jews and North American Whites (Bazalakova et al., 2006; Okuda et al., 2002), produces an amino acid substitution in the CHT protein (I89V). This results in a 40–50% reduced velocity of choline transport as compared to the wild type, when measured in a cell culture expression system, although no phenotype has been reported to date. Multiple additional SNPs are found near the promoter regions and in the 3′ untranslated region of the CHT mRNA. Given the highly plastic regulation of CHT expression, its subcellular distribution (Ferguson and Blakely, 2004), and posttranslational regulation (Gates et al., 2004) it is possible that these polymorphisms may be associated with subtle phenotypes in the human population.
In addition to describing modulation of brain CHT expression by prenatal nutrition, our data provide new information on the intrinsic hippocampal CHT-immunoreactive neurons. Consistent with the studies of Misawa et al. (Misawa et al., 2001), we find CHT-positive hilar neurons in the rat. However, when we counted these neurons at the level of the habenular nucleus, their number varied greatly among the animals, from very few to over 100 cells (Fig. 5). Given a remarkably high correlation between the number of these neurons observed with immunohistochemical techniques and the levels of CHT mRNA measured in the contralateral hippocampus, the data suggest that these CHT-immunoreactive neurons are the most likely source of the hippocampal CHT mRNA. Moreover, the current data confirm our previous studies in mice showing a decline in the hippocampal abundance of CHT mRNA during the early postnatal period (Berse et al., 2005). It is not clear at present if these hilar CHT-positive cells also express ChAT, and are thus bona fide, cholinergic. Several (Kanaya-Ida and Ben Ari, 1989; Levey et al., 1984; Matthews et al., 1987; Wainer et al., 1985), but not all (Blaker et al., 1988; Ichikawa et al., 1997), investigators have previously observed ChAT-positive neurons in the rat hilus. These, possibly cholinergic (Kanaya-Ida and Ben Ari, 1989; Levey et al., 1984; Matthews et al., 1987; Wainer et al., 1985), and CHT-immunoreactive hilar neurons (polymorphic layer of the dentate gyrus) could also contribute local projections in the dentate gyrus and thus be the source of the increased CHT immunoreactivity observed in the inner molecular layer of the prenatally choline-deficient rats. The overall lower number of the hilar CHT positive cells identified at P72 (Fig. 7) as compared to P18 (Fig. 5) roughly parallels the reduction in CHT mRNA that normally occurs during development as shown in figure 3. The lower cell counts at P72 could either reflect cell death or reduced CHT gene expression in cells that do not die. It is possible that choline supplementation interferes with this normal developmental pattern and results in persistent expression of CHT or survival of CHT positive neurons.
The mechanism of the long-term regulation of CHT expression by prenatal supply of choline remains to be elucidated. One possibility is that it is related to the function of choline as a donor of methyl groups. The availability of the metabolic methyl groups is known to affect DNA methylation in a cell (see below) and, because the DNA methylation patterns are passed on to cell progeny following mitosis, the patterns established in embryogenesis may persist throughout lifetime. Choline is a source of methyl groups for enzymatic methylations (Blusztajn, 1998; Zeisel, 2006) because its metabolite betaine is used to synthesize methionine and subsequently S-adenosylmethionine – a methyl group donor for most biological methylation reactions including the methylation of cytidines in CpG dinucleotide sequences of DNA (Waterland, 2006). This cytidine methylation of CpG-rich sequences near promoters of multiple genes can modulate transcription. In general hypermethylated DNA binds transcriptional repressors whereas hypomethylation stimulates transcription by preventing this binding (Li, 2002; Paulsen and Ferguson-Smith, 2001; Reinhart et al., 2002; Robertson and Wolffe, 2000; Tucker, 2001). Thus, low availability of choline during the time of neurogenesis of the basal forebrain cholinergic neurons (E13–E19 in the rat, (Koh and Loy, 1989)) could lead to hypomethylation of regulatory regions within the CHT gene causing its long-term overexpression. Analysis of the rat CHT gene reveals the presence of a CpG island (chr9:133805–134277; UCSC Genome Browser http://genome.ucsc.edu/) near its promoter, consistent with this model. Indeed, animals fed diets deficient in methyl donors (choline and methionine) had hypomethylated DNA (Locker et al., 1986; Niculescu et al., 2006; Tsujiuchi et al., 1999; Wainfan et al., 1989) and in choline deficient mouse fetuses, global and gene-specific DNA methylation was decreased in the ventricular and subventricular zones of hippocampal Ammon’s horn and this correlated with the induction of expression of Cdkn3, a gene known to be silenced by DNA methylation (Niculescu et al., 2006). Together with previous findings, the current data show that the availability of an essential nutrient, choline, in utero causes changes in its own turnover in the hippocampus later in life. In prenatally choline-deficient animals this results in efficient recycling of choline for ACh synthesis catalyzed by upregulated expression of CHT (Cermak et al., 1998). Prenatally choline-supplemented rats, instead, exhibit greater reliance on phosphatidylcholine-stored choline for ACh synthesis (Cermak et al., 1998). These adaptations seem appropriate for the periods when choline availability is altered (i.e. prenatally), however they are long-lasting, i.e. they are observed at a time when all animals consume the control diet, months after the termination of treatment. Therefore, elevated dietary choline supply in adult animals that were prenatally choline-deficient could result in increases of ACh synthesis, whereas it might have a lesser effect in prenatally choline-supplemented animals. Conversely, choline deficiency in adults that were prenatally choline-deficient might be well tolerated due to upregulation of CHT. Thus, cholinergic neurotransmission may be differentially sensitive to the availability of choline in adulthood, based on its supply in utero.
4. Experimental Procedure
Animal Subjects
Pregnant Sprague-Dawley rats (Charles River Laboratories) were divided into three groups: choline-deficient, control, and choline-supplemented. During days 11–17 of pregnancy, the rats were fed an AIN76A diet (Bieri, 1980; Bieri et al., 1977) (Dyets Inc., Bethlehem, PA) that contained no choline (deficient), 7.9 mmol/kg of choline (control), or 35.6 mmol/kg of choline (supplemented). Following embryonic day 17, all rats were returned to a control AIN76A diet. Offspring also consumed a control diet once weaned at postnatal day (P) 25.
For immunohistochemical studies male subjects were used. For all other studies equal number of males and females per dietary group was used. Four to six individual offspring (each from a different litter) were used per group. At various postnatal ages, offspring were anesthetized and the septum and hippocampus were removed rapidly on ice. For protein, the tissue was immediately frozen on dry ice and stored until later use. For RNA, the brain tissues were immediately homogenized in cold guanidine isothiocynate solution and placed on dry ice. First, the RNA was extracted using phenol/chloroform method and then precipitated. The RNA pellet was resuspended in RNase-free water. The quantity of RNA was determined using QuantiT™ RiboGreen® RNA assay kit (Molecular Probes) and the Victor3 multi-label plate reader (PerkinElmer Life Sciences).
Reverse Transcriptase PCR
RNAs were used for reverse transcriptase PCR using Superscript™ One-Step RT-PCR with Platinum® Taq (Invitrogen Life Technologies). First strand cDNA synthesis was performed with 25 ng of total RNA (except 50 ng of hippocampal RNA for CHT), oligo dT primer and reverse transcriptase at 48 °C (45 min). Primers used for PCR include β-actin (Forward: CACAGCTGAGAGGGAAATC, Reverse: TCAGCAATGCCTGGGTAC), and CHT (Forward: CGGGGAACCATTGAATTCGTTGAAGTCTAC, Reverse: GGGGCAAGCTTCCACTTTCAA ATAGATACT). PCR was performed using Platinum Taq DNA polymerase with a denaturing step for 2 min at 94 °C, followed by 32 cycles for β-actin or 36 cycles for CHT of 1 min at 94 °C, 1 min at 55 °C and 2 min at 72 °C, and terminated by an elongation step at 72 °C for 7 min. PCR products were displayed on a 10% polyacrylamide gel and stained with ethidium bromide. PCR products were visualized with Kodak Image Station 440 and product intensities were quantified using Kodak software.
Western Blot Analysis
For Western blot analysis, whole tissue extracts were prepared by adding lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% Nonidet NP-40, 10% glycerol, 2 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 1 mg/ml leupeptin, 2 mg/ml aprotinin, 2 mg/ml pepstatin) to a frozen sample of a CNS region or hippocampal slice, gently sonicating, incubating for 15 min on ice, and briefly centrifuging to clear. The extracts were normalized for total protein and subjected to SDS-PAGE. After transfer of protein to an Immobilon P membrane (Millipore), the membrane was blocked with 5% nonfat dry milk in 1X Tris-buffered saline (TBS) containing 0.1% Tween 20 for 1 hour and then probed with affinity-purified rabbit anti-CHT polyclonal antibody AB5966 (1:1000) (Chemicon) overnight. The antibody/antigen complexes on the membranes were detected using a peroxidase-conjugated anti-rabbit IgG (1:5000) and visualized using the enhanced chemiluminescence method (Western Lightning, Perkin Elmer) and a Kodak Image Station 440. Digitized images of immunoblots were quantified using Kodak ID software.
Immunocytochemistry
For 18-day-old male rats, six animals per dietary group were anesthetized. The brain was rapidly removed and bisected through the midsagittal plane. The left hemibrain was dissected for neurochemistry and the right was submerged in phosphate buffered PLP fixative (4% paraformaldehyde, 75 mM lysine, 10 mM sodium periodate; pH 7.4) for immunocytochemistry. Brains were post-fixed for 24 hours in PLP at 4°C, then cryoprotected in a graded series of 10% and 20% glycerol/2% dimethylsulfoxide, in 0.1 M PBS, pH 7.3. For 72-day-old male rats, groups of four from each of the three dietary groups were deeply anesthetized with pentobarbital and euthanized by transcardial perfusion with 150 ml of normal saline, followed by 300 ml of PLP. Brains were removed, post-fixed for four hours in PLP at 4°C, and then cryoprotected as described above.
Brains were serially sectioned at 50 μm, using a freezing microtome. Every fifth section was stained with affinity-purified rabbit anti-CHT polyclonal antibody AB5966 (1:1000) (Chemicon) for P72 rats or anti-CHT polyclonal antibody (1:5000) (generous gift from Dr. Blakely) (Ferguson et al., 2003) for P18 rats. Standard immunohistochemical procedures were used as previously reported (McKeon-O’Malley et al., 2003).
CHT positive neuronal perikarya in the CA4 regions of the hippocampal formation of P18 and P72 animals were quantitated and analyzed using a semi-automated computerized morphometry system (MicroBrightField, Colchester, VT, Neurolucida™ and StereoInvestigator™, Burlington, VT). All CHT-immunoreactive profiles in the CA4 were mapped in 3–4 step sections spaced at 250 micron intervals at the level of the habenular nucleus and confirmed by visual inspection.
Statistical Analyses
Data were analyzed by Analysis of Variance (ANOVA). If significant effects were found data were further analyzed by Tukey’s multiple comparison test to determine individual group differences. Analyses were performed with the statistical program SYSTAT (SYSTAT Software Inc., San Jose, CA).
Acknowledgments
These studies were supported by a grant from the National Institute on Aging AG009525 and the Department of Veterans Affairs (NWK).
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- CaMK
calcium/calmodulin-dependent protein kinase
- ChAT
choline acetyltransferase
- CHT
choline transporter
- CREB
cAMP response element binding protein
- E
embryonic day
- GABA
gamma-aminobutyric acid
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinase
- NMDA
N-methyl-D-aspartate
- P
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bazalakova MH, Wright J, Schneble EJ, McDonald MP, Heilman CJ, Levey AI, Blakely RD. Deficits in acetylcholine homeostasis, receptors and behaviors in choline transporter heterozygous mice. Genes Brain Behav. 2006 doi: 10.1111/j.1601-183X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- Beeri R, Le Novère N, Mervis R, Huberman T, Grauer E, Changeux JP, Soreq H. Enhanced hemicholinium binding and attenuated dendrite branching in cognitively impaired acetylcholinesterase-transgenic mice. J Neurochem. 1997;69:2441–2451. doi: 10.1046/j.1471-4159.1997.69062441.x. [DOI] [PubMed] [Google Scholar]
- Berse B, Szczecinska W, Lopez-Coviella I, Madziar B, Zemelko V, Kaminski R, Kozar K, Lips KS, Pfeil U, Blusztajn JK. Expression of high affinity choline transporter during mouse development in vivo and its upregulation by NGF and BMP-4 in vitro. Brain Res Dev Brain Res. 2005;157:132–40. doi: 10.1016/j.devbrainres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Bieri JG. Second report of the ad hoc committee on standards for nutritional studies. J Nutr. 1980;110:1726. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- Bieri JG, Stoewsand GS, Briggs GM, Phillips RW, Woodard JC, Kanapka JJ. Report of the American Institute of Nutrition ad hoc committee on standards for nutritional studies. J Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- Blaker SN, Armstrong DM, Gage FH. Cholinergic neurons within the rat hippocampus: response to fimbria-fornix transection. J Comp Neurol. 1988;272:127–38. doi: 10.1002/cne.902720109. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983;221:614–620. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- Brandner C. Perinatal choline treatment modifies the effects of a visuo-spatial attractive cue upon spatial memory in naive adult rats. Brain Res. 2002;928:85–95. doi: 10.1016/s0006-8993(01)03363-7. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Mellott T, Pizzo DP, Coufal N, D’Amour KA, Gobeske K, Lortie M, Lopez-Coviella I, Berse B, Thal LJ, Gage FH, Blusztajn JK. Choline transporter 1 maintains cholinergic function in choline acetyltransferase haploinsufficiency. J Neurosci. 2004;24:5459–66. doi: 10.1523/JNEUROSCI.1106-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak JM, Holler T, Jackson DA, Blusztajn JK. Prenatal availability of choline modifies development of the hippocampal cholinergic system. FASEB J. 1998;12:349–357. doi: 10.1096/fasebj.12.3.349. [DOI] [PubMed] [Google Scholar]
- Collier B, Ilson D. The effect of preganglionic nerve stimulation on the accumulation of certain analogues of choline by a sympathetic ganglion. J Physiol. 1977;264:489–509. doi: 10.1113/jphysiol.1977.sp011679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber SA, Savci V, Wei A, Slack BE, Wurtman RJ. Choline’s phosphorylation in rat striatal slices is regulated by the activity cholinergic neurons. Brain Res. 1996;723:90–99. doi: 10.1016/0006-8993(96)00221-1. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Bazalakova M, Savchenko V, Tapia JC, Wright J, Blakely RD. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc Natl Acad Sci U S A. 2004;101:8762–7. doi: 10.1073/pnas.0401667101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Blakely RD. The choline transporter resurfaces: new roles for synaptic vesicles? Mol Intervent. 2004;4:22–37. doi: 10.1124/mi.4.1.22. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J Neurosci. 2003;23:9697–9709. doi: 10.1523/JNEUROSCI.23-30-09697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates J, Jr, Ferguson SM, Blakely RD, Apparsundaram S. Regulation of choline transporter surface expression and phosphorylation by protein kinase C and protein phosphatase 1/2A. J Pharmacol Exp Ther. 2004;310:536–45. doi: 10.1124/jpet.104.066795. [DOI] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem. 2003;80:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Hefti F, David A, Hartikka J. Chronic intraventricular injections of nerve growth factor elevate hippocampal choline acetyltransferase activity in adult rats with partial septo-hippocampal lesions. Brain Res. 1984;293:305–311. doi: 10.1016/0006-8993(84)91237-x. [DOI] [PubMed] [Google Scholar]
- Holler T, Cermak JM, Blusztajn JK. Dietary choline supplementation in pregnant rats increases hippocampal phospholipase D activity of the offspring. FASEB J. 1996;10:1653–1659. doi: 10.1096/fasebj.10.14.9002559. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Ajiki K, Matsuura J, Misawa H. Localization of two cholinergic markers, choline acetyltransferase and vesicular acetylcholine transporter in the central nervous system of the rat: in situ hybridization histochemistry and immunohistochemistry. J Chem Neuroanat. 1997;13:23–39. doi: 10.1016/s0891-0618(97)00021-5. [DOI] [PubMed] [Google Scholar]
- Jones JPH, Meck W, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Dev Brain Res. 1999;118:159–67. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- Kanaya-Ida S, Ben Ari Y. Transient increase in the number of cholinergic neurons in the developing rat dentate gyrus. Neurosci Lett. 1989;101:23–8. doi: 10.1016/0304-3940(89)90434-5. [DOI] [PubMed] [Google Scholar]
- Koh S, Loy R. Localization and development of nerve growth factor-sensitive rat basal forebrain neurons and their afferent projections to hippocampus and neocortex. J Neurosci. 1989;9:2999–0318. doi: 10.1523/JNEUROSCI.09-09-02999.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos VE, Applegate MD, Knüsel B, Junard EO, Burton LE, Mobley WC, Hefti FF, Price DL. Recombinant human nerve growth factor prevents retrograde degeneration of axotomized basal forebrain cholinergic neurons in the rat. Exp Neurol. 1991;112:161–173. doi: 10.1016/0014-4886(91)90066-l. [DOI] [PubMed] [Google Scholar]
- Levey AI, Wainer BH, Rye DB, Mufson EJ, Mesulam MM. Choline acetyltransferase-immunoreactive neurons intrinsic to rodent cortex and distinction from acetylcholinesterase-positive neurons. Neuroscience. 1984;13:341–53. doi: 10.1016/0306-4522(84)90234-3. [DOI] [PubMed] [Google Scholar]
- Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–73. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- Locker J, Reddy TV, Lombardi B. DNA methylation and hepatocarcinogenesis in rats fed a choline-devoid diet. Carcinogenesis. 1986;7:1309–1312. doi: 10.1093/carcin/7.8.1309. [DOI] [PubMed] [Google Scholar]
- Matthews DA, Salvaterra PM, Crawford GD, Houser CR, Vaughn JE. An immunocytochemical study of choline acetyltransferase-containing neurons and axon terminals in normal and partially deafferented hippocampal formation. Brain Res. 1987;402:30–43. doi: 10.1016/0006-8993(87)91044-4. [DOI] [PubMed] [Google Scholar]
- McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biobehav Rev. 2006;30:696–712. doi: 10.1016/j.neubiorev.2005.12.003. [DOI] [PubMed] [Google Scholar]
- McKeon-O’Malley C, Siwek D, Lamoureux JA, Williams CL, Kowall NW. Prenatal choline deficiency decreases the cross-sectional area of cholinergic neurons in the medial septal nucleus. Brain Res. 2003;977:278–283. doi: 10.1016/s0006-8993(03)02599-x. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport. 1997a;8:2831–2835. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997b;8:3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport. 1997c;8:3045–3051. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Dev Brain Res. 1999;118:51–9. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Mellott TJ, Follettie MT, Diesl V, Hill AA, Lopez-Coviella I, Blusztajn JK. Prenatal choline availability modulates hippocampal and cerebral cortical gene expression. FASEB J. doi: 10.1096/fj.06-6597com. in press. [DOI] [PubMed] [Google Scholar]
- Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 2004;18:NIL412–NIL427. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10:1185–201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Misawa H, Nakata K, Matsuura J, Nagao M, Okuda T, Haga T. Distribution of the high-affinity choline transporter in the central nervous system of the rat. Neuroscience. 2001;105:87–98. doi: 10.1016/s0306-4522(01)00147-6. [DOI] [PubMed] [Google Scholar]
- Mobley WC, Rutkowski JL, Tennekoon GI, Gemski J, Buchanan K, Johnston MV. Nerve growth factor increases choline acetyltransferase activity in developing basal forebrain neurons. Brain Res. 1986;387:53–62. doi: 10.1016/0169-328x(86)90020-3. [DOI] [PubMed] [Google Scholar]
- Montoya D, Swartzwelder HS. Prenatal choline supplementation alters hippocampal N-methyl-D-aspartate receptor-mediated neurotransmission in adult rats. Neurosci Lett. 2000;296:85–88. doi: 10.1016/s0304-3940(00)01660-8. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Kuhar MJ. Activation of high-affinity choline uptake in vitro by depolarizing agents. Mol Pharmacol. 1976;12:1082–1090. [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. Faseb J. 2006;20:43–9. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Okamura M, Kaitsuka C, Haga T, Gurwitz D. Single nucleotide polymorphism of the human high-affinity choline transporter alters transport rate. J Biol Chem. 2002;277:45315–45322. doi: 10.1074/jbc.M207742200. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cortical choline transporter function measured in vivo using choline-sensitive microelectrodes: clearance of endogenous and exogenous choline and effects of removal of cholinergic terminals. J Neurochem. 2006;97:488–503. doi: 10.1111/j.1471-4159.2006.03766.x. [DOI] [PubMed] [Google Scholar]
- Paulsen M, Ferguson-Smith AC. DNA methylation in genomic imprinting, development, and disease. J Pathol. 2001;195:97–110. doi: 10.1002/path.890. [DOI] [PubMed] [Google Scholar]
- Polak RL, Molenaar PC, van Gelder M. Acetylcholine metabolism and choline uptake in cortical slices. J Neurochem. 1977;29:477–485. doi: 10.1111/j.1471-4159.1977.tb10696.x. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Williams CL, Meck WH, Swartzwelder HS. Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J Neurophysiol. 1998;79:1790–1796. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- Reinhart B, Eljanne M, Chaillet JR. Shared role for differentially methylated domains of imprinted genes. Mol Cell Biol. 2002;22:2089–2098. doi: 10.1128/MCB.22.7.2089-2098.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat Rev Genet. 2000;1:11–19. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Loy R, Williams CL. Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Res. 2002;947:9–16. doi: 10.1016/s0006-8993(02)02900-1. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem. 2003;80:245–56. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- Schenk F, Brandner C. Indirect effect of peri- and postnatal choline treatment on place-learning abilities in rat. Psychobiology. 1995;23:302–313. [Google Scholar]
- Sherman KA, Zigmond MJ, Hanin I. High affinity choline uptake in striatum and hippocampus: Differential effects of treatments which release acetylcholine. Life Sci. 1978;23:1863–1870. doi: 10.1016/0024-3205(78)90119-4. [DOI] [PubMed] [Google Scholar]
- Tsujiuchi T, Tsutsumi M, Sasaki Y, Takahama M, Konishi Y. Hypomethylation of CpG sites and c-myc gene overexpression in hepatocellular carcinomas, but not hyperplastic nodules, induced by a choline-deficient L-amino acid-defined diet in rats. Jpn J Cancer Res. 1999;90:909–913. doi: 10.1111/j.1349-7006.1999.tb00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL. Methylated cytosine and the brain: a new base for neuroscience. Neuron. 2001;30:649–652. doi: 10.1016/s0896-6273(01)00325-7. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Hrabovska A, Duysen EG, Ferguson SM, Blakely RD, Lockridge O, Levey AI. Altered striatal function and muscarinic cholinergic receptors in acetylcholinesterase knockout mice. Mol Pharmacol. 2003;64:1309–1316. doi: 10.1124/mol.64.6.1309. [DOI] [PubMed] [Google Scholar]
- Wainer BH, Levey AI, Rye DB, Mesulam MM, Mufson EJ. Cholinergic and non-cholinergic septohippocampal pathways. Neurosci Lett. 1985;54:45–52. doi: 10.1016/s0304-3940(85)80116-6. [DOI] [PubMed] [Google Scholar]
- Wainfan E, Dizik M, Stender M, Christman JK. Rapid appearance of hypomethylated DNA in livers of rats fed cancer- promoting, methyl-deficient diets. Cancer Res. 1989;49:4094–4097. [PubMed] [Google Scholar]
- Waterland RA. Assessing the effects of high methionine intake on DNA methylation. J Nutr. 2006;136:1706S–1710S. doi: 10.1093/jn/136.6.1706S. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH, Heyer D, Loy R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794:225–238. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Nutritional importance of choline for brain development. J Am Coll Nutr. 2004;23:621S–626S. doi: 10.1080/07315724.2004.10719433. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Choline: Critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]


