Abstract
Zinc is an essential cofactor for the activity and folding of up to ten percent of mammalian proteins and can modulate the function of many others. Because of the pleiotropic effects of zinc on every aspect of cell physiology, deficits of cellular zinc content, resulting from zinc deficiency or excessive rise in its cellular concentration, can have catastrophic consequences and are linked to major patho-physiologies including diabetes and stroke. Thus, the concentration of cellular zinc requires establishment of discrete, active cellular gradients. The cellular distribution of zinc into organelles is precisely managed to provide the zinc concentration required by each cell compartment. The complexity of zinc homeostasis is reflected by the surprisingly large variety and number of zinc homeostatic proteins found in virtually every cell compartment. Given their ubiquity and importance, it is surprising that many aspects of the function, regulation, and crosstalk by which zinc transporters operate are poorly understood. In this mini-review, we will focus on the mechanisms and players required for generating physiologically appropriate zinc gradients across the plasma membrane and vesicular compartments. We will also highlight some of the unsolved issues regarding their role in cellular zinc homeostasis.
INTRODUCTION
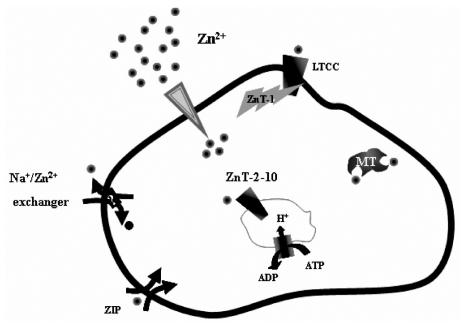
The uniqueness of zinc, in contrast to other abundant transition metals in the body, e.g., Fe3+ and Cu2+, is that it lacks redox activity. It has been suggested that early in evolution, Fe3+ fulfilled the roles of Zn2+ as a cofactor in domains that were predecessors to Zn-fingers (1). The emergence of oxygen and oxidative phosphorylation led to the rapid extinction of this form of heavy metal biochemistry. Importantly, between three and ten percent of all proteins in mammalian genomes are considered to bind zinc (2). In these proteins, zinc is essential for folding, conformational change, or activity. It is, therefore, not surprising that zinc deficiency has catastrophic consequences, reflected in, among other syndromes, erosion of the gastrointestinal tract, skin lesions, cardiac failure, and malformations of brain and the male reproductive system (3,4). Zinc, particularly the “free” or loosely-bound form of the ion, is nevertheless profoundly toxic to mammalian cells (5). Numerous studies have shown that a rise in cellular free Zn2+ resulting from permeation of synaptic zinc or from release of this ion from intracellular stores in neurons (6–9), is a key factor in neuronal death following seizure or an ischemic episode (10,11). Dysregulation of free zinc has also been implicated in the formation of β-amyloid plaques associated with Alzheimer’s disease (12). Permeation of zinc that is co-released with insulin from pancreatic β-cells is emerging as an important factor in the degeneration of these cells (13–15). These paradoxical aspects of cellular zinc dictate that it be distributed in highly regulated gradients with respect to the plasma membrane and intracellular compartments. It also demands that an adequate supply of this ion be available to the numerous zinc-binding proteins, while preventing its accumulation and the potentially devastating effects it can readily initiate. The magnitude of the zinc gradient present at the plasma membrane rivals even that of calcium (16). Zinc concentrations in the synaptic cleft, for example, are estimated to rise to micomolar concentrations, while the intracellular free-Zn2+ concentration is estimated to be in the picomolar range (17). Similarly steep zinc gradients are also formed within the cells, with millimolar concentrations of Zn2+ existing in the synaptic and secretory vesicles (18–20). The complexity and importance of zinc homeostasis is reflected by the large number of proteins that are potentially dedicated to Zn2+ transport and buffering (Figure 1), among them, at least ten members of the ZnT (Zn2+ Transporter) family (21), 15 members of the ZIP (i.e., Zn2+-regulated metal transporter, Iron-regulated metal transporter-like protein) family (22), and 3 distinct isoforms of metallothionein (23).
Figure 1.
A schematic representation on the mechanisms of zinc homeostasis in mammalian cells. Zinc is distributed at large transmembrane and vesicular gradients. These are generated by the orchestrated activity of multiple zinc transporters and regulators of zinc transport. For the sake of simplicity, the ZnT2-10 have been illustrated on a single compartment although they may be found on multiple organelles.
ACTIVE ZINC TRANSPORT ACROSS THE CELL MEMBRANE
Ion gradients are generated by two main mechanisms: 1) A primary pump, utilizing the energy of ATP-hydrolysis; or 2) a secondary active mechanism that uses an ion gradient, such as Na+, for generating Zn2+ gradients. A Zn2+ pump has been demonstrated in bacteria, where several forms of p-type ATPases have been shown to catalyze active Zn2+ transport (24). Recently, a similar ATPase, which transports Zn2+ and Cd2+ and to a lesser extent other heavy metals, has been discovered in Arabidopsis (25,26). Surprisingly, there is still no evidence for a Zn2+ pump in either yeast or mammalian cells, though a Cu pump has been identified that is linked to heavy metal ion transport (27).
A Na+-dependent secondary active mechanism has, however, been suggested to facilitate formation of the transmembrane Zn2+ gradient in neurons. Early studies suggested that the neuronal Na+/Ca2+ exchanger mediates Zn2+ extrusion (28), but more recent findings seem to support the existence of a distinct Na+/Zn2+ exchanger. These studies have indicated that a putative Na+/Zn2+ exchanger, probably a member of the Na+/Ca2+ exchanger superfamily, operates with a stoichiometry of 3Na+/1Zn2+, promoting Zn2+ efflux against a 500-fold transmembrane gradient (29). This mechanism is pharmacologically and molecularly distinct from the classical Na+/Ca2+ exchangers. Whether this exchanger is the principle plasma membrane extruder of Zn2+ or is accompanied by an as yet unidentified Zn2+ pump, is an open and intriguing question.
THE ROLE OF ZnT PROTEINS IN CELLULAR ZINC HOMEOSTASIS
To date, human CDF, also known as SLC30, genes, code for ten zinc transporters (ZnTs), i.e., ZnT-1-10 (30). The expression and cellular distribution of the ZnTs is highly regulated by changes in zinc (31–33). The rapid changes occurring in extracellular Zn2+, and the existence of numerous pathways for permeation of this ion, suggest an important role for the ZnT proteins in physiology and pathology (Table 1), though relatively little is known about their mechanism of activity or their regulation. Studies of yeast CDF proteins and the bacterial ZitB protein, which is a distantly related homologue of the ZnT proteins, have suggested mechanisms by which these proteins control Zn2+ transport (34,35). Functional analyses of their bacterial and yeast homologues have suggested that these proteins might catalyze H+/Zn2+ exchange (35). If a similar mechanism underlies the activity of mammalian Zn2+ transporters, it may have important physiological implications. For instance, metabolic acidosis or a change in pH triggered by inflammation, might attenuate ZnT-dependent Zn2+ transport. Glial cells, normally more resistant to zinc toxicity than neurons, are indeed rendered more susceptible by reduction in pHi (36), possibly as a result of lower ZnT-dependent transport.
Table 1.
ZnT proteins-expression and physiological roles.
| Expression pattern | Cellular distribution | Disease model | Altered expression phenotype | Ref | |
|---|---|---|---|---|---|
| ZnT-1 | Ubiquitous | Plasma membrane |
Overexpression: reduced [Zn2+]i and enhanced resistance against Zn2+ toxicity.
KO: Lethal at embryonic stage. |
(37,42,43,71) | |
| ZnT-2 | small intestine, kidney, placenta, pancreas, testis, seminal vesicles, and mammary gland | Vesicles, lysosomes | Overexpression: enhanced lysosomal and vesicular Zn2+ accumulation | (72,73) | |
| ZnT-3 | brain | Synaptic vesicles(Glutamatergic and GABAergic) | KO: synaptic Zn2+ deficiency, enhanced susceptibility to seizure, loss of gender specific Alzheimer’s disease plaque formation in a mouse model, decreased susceptibility to amyloid angiopathy | (49,74) | |
| ZnT-4 | mammary gland, brain, small intestine and mast cells | Intracellular compartments | Lethal milk syndrome in mice, Asthma (mice), Alzheimer’s disease | (75–79) | |
| ZnT-5 | Pancreatic β-cells, intestine, heart brain, liver, kidney | Insulin secretory vesicles, Golgi
Spliced isoform: plasma membrane Complexed with ZnT-6 |
KO: poor growth, osteopenia, male specific fatal arrhythmias. Essential for folding and secretion of Zn2+-binding enzymes. | (53–55,59,80) | |
| ZnT-6 | liver, brain, and small intestine | Complexed with ZnT-5 | Alzheimer’s disease (mice) | (53,57,79) | |
| ZnT-7 | small intestine, liver, retina, spleen, kidney, and lung | Golgi | Essential for folding and secretion of Zn2+-binding enzymes. | (54,81,82) | |
| ZnT-8 | Pancreatic β-cells | Insulin secretory vesicles | Polymorphism marker in diabetes type II | Overexpression: enhances glucose dependent insulin secretion | (60–62) |
Although the ZnTs are increasingly recognized as critical players in cellular zinc homeostasis, for the sake of brevity, only a few examples will be discussed.
ZnT-1
ZnT-1, a ubiquitously expressed member of the SLC30 zinc transporter family, is found on the plasma membrane of neurons (37) and glia cells (38,39). In the mouse brain, ZnT-1 is, in general, localized in regions rich in synaptic Zn2+ (though cerebellar Purkinje cells are also intensely ZnT-1-immunoreactive) and its expression is developmentally regulated in correlation with the appearance of the synaptic Zn2+ (39,40). ZnT-1 has been shown to reduce Zn2+ toxicity in neurons and glial cells (37,38,41). Expression of this protein is highly regulated by Zn2+, via the transcription factor MTF-1 (32), and priming of glial cells with non-toxic Zn2+ exposures promotes ZnT-1 expression (38). Thus, sublethal exposure to Zn2+ might induce ZnT-1 (and perhaps MT) expression to counteract subsequent (toxic) rises in [Zn2+]i. However, it must be emphasized that the mechanism by which ZnT-1 maintains low [Zn2+]i is more complex than previously thought.
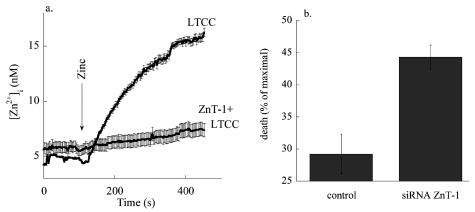
Initially, it was widely accepted that ZnT-1 reduces [Zn2+]i by acting as a Zn extruder (37,41). However, changes in Zn2+ effected by ZnT-1 appear to occur without concomitant changes in the concentration of counter ions, such as K+, Na+, Ca2+, or protons. Nor are changes in cytoplasmic Zn2+ affected by depletion of intracellular ATP. This indicates that as a proposed mechanism of ion transport, zinc extrusion fits poorly with the well-described modus operandi of traditional transporters. Recent data support the idea that ZnT-1 can affect Zn2+ homeostasis through regulating L-type calcium channels (LTCC) (38,42,43). We have found that heterologous expression of ZnT-1 in HEK-293 cells was followed by a decrease in Zn2+ accumulation but not by an apparent increase in zinc efflux (43). Notably, the expression of ZnT-1 reduced the Zn2+ influx mediated through the L-type Ca2+ channels (Figure 2A). Moreover, expression of ZnT-1 in cells endogenously expressing LTCC, as well as heterologous co-expression of ZnT-1 and LTCC, resulted in reduction of [Zn2+]i following opening of the LTCC (43). We have determined the physiological implication of this mode of activation in astroglia (38). Induction of ZnT-1 expression mediated by preincubation with zinc or by heterologous expression of ZnT-1 was followed by attenuated influx of zinc via the L-type Ca2+ channels. Importantly, heterologous expression of ZnT-1 dramatically reduced astroglia cell death mediated by zinc influx through the latter channels. Our results also indicate that by regulating the activity of the L-type Ca2+ channel ZnT-1 confers resistance against toxic rise in Zn2+i in neurons (Figure 2B). Because ZnT-1 regulates L-type calcium channels, it is conceivable that it also will regulate permeation of other cations that permeate through this pathway, e.g., calcium and cadmium. Recently, we have demonstrated that silencing of endogenous ZnT-1 by siRNA results in enhanced calcium and cadmium influx into neurons (42). The enhanced Cd2+ influx was followed by an increase in neuronal Cd2+ toxicity. The enhanced Ca2+ influx, on the other hand, was linked to an increase in synaptic transmission. Our results therefore indicate that regulation of the L-type Ca2+ channel, mediated by ZnT-1, has a more diverse role in cation homeostasis than previously thought. An open and intriguing question is how ZnT-1 affects the activity of the L-type Ca2+ channel (42). We have found that ZnT-1 does not modulate the expression of the L-type Ca2+ channel. Furthermore, direct interaction between ZnT-1 and the L-type calcium channel has not been established. It may be that interaction of ZnT-1 with the LTCC, or one of its accessory subunits, is too labile to be monitored using co-immunoprecipitation analysis. Alternatively, this may indicate that ZnT-1 indirectly modulates the activity of the L-type Ca2+ channel. The demonstration that ZnT-1 is regulated by the activity of Raf-1 suggests a possible pathway for such interaction. Therefore, further studies will be required to elucidate this intriguing and open question.
Figure 2.
Expression of ZnT-1 is followed by attenuation of Zn2+ influx and toxicity. A. HEK-293 cells were co-transfected with ZnT-1 and LTCC plasmids, or with LTCC only. Cells were loaded with Fura-2 (5 μM), which is also a highly sensitive Zn2+ dye, and imaged while superfusing with a high K+ Ringer’s solution (50mM) thereby opening the LTCC in the presence of Zn2+ (200 μM). B. Neurons were transfected with a ZnT-1 siRNA construct or control siRNA. Zn2+ (200 μM) was applied to a neuronal culture in the presence of high K + Ringer’s (ten minutes). Cell death was determined 24 h later using LDH.
ZNT-3
Possibly the most extensively-studied ZnT, ZnT-3 is localized to the membranes of Zn2+-containing vesicles in glutamatergic synaptic boutons (44). Support for the idea that this transporter is essential for uploading Zn2+ into synaptic vesicles comes from ZnT-3 knockout mice, which lack chelatable Zn2+ throughout the brain. While early studies on this mutant failed to discern a distinct phenotype (45,46), later studies, focusing on brain disorders, such as stroke, epileptic seizure, and Alzheimer’s disease, have demonstrated a role for synaptic Zn2+, e.g., increasing susceptibility to seizures (47–49). An intriguing question emerging from this work is whether synaptic Zn2+ participates directly in fundamental processes of synaptic transmission such as LTP. Two recent studies (50,51) show that LTP in the cortico-amygdala pathway and hippocampus, respectively, does require Zn2+ release, while others (e.g.16,52) have indicated that LTP is not required. Although this issue is still unresolved, the ZnT-3 KO model continues to provide an excellent tool to examine these questions.
ZnT-5 and ZnT-6
ZnT-5 and ZnT-6, vesicular ZnTs found on Golgi and ER, are thought to functionally interact (53,54). Deletion of the ZnT-5 gene leads to abnormal bone development, weight loss, and lethal, male-specific, cardiac arrhythmias (55). Interestingly, one of the hallmarks of zinc deficiency is cardiac dysfunction (56). Several studies have indicated that ectopically expressed ZnT-5 or ZnT-6 are capable of independently transporting Zn2+, while others suggest that the activity of the Zn2+-dependent protein, TNAP (tissue-nonspecific alkaline phosphatase), requires their hetero-oligomerization (53–55,57,58). While this links ZnT5/6 and vesicular Zn2+, the precise mechanism involved and their regulation remain poorly understood. Studies on yeast and bacterial ZnT homologues, considered together with the distribution of mammalian ZnTs in acidic cellular compartments, suggest that intracellular ZnTs are linked to both, Zn2 and H+ transport. Results from our group indicate that ZnT-5 is capable of independently mediating Zn2+ transport, but coexpression of ZnT-5 and ZnT-6 accelerates its rate. We have further demonstrated that Zn2+ transport mediated by ZnT-5 is linked to changes in Golgi pH (Ohana et al. in preparation).
Localization of splice variants of ZnT-5 at various stations in cells (59) suggests a more versatile role in intracellular zinc homeostasis then was previously envisioned.
ZnT-8
ZnT-8 is exclusively expressed in pancreatic β-cells (60). Zinc is essential for the proper processing and packaging of insulin into secretory vesicles. Indeed, recent studies indicate that heterologous expression of ZnT-8 results in enhanced insulin accumulation and secretion (61). Furthermore, it has been shown recently that a polymorphism of ZnT-8 in humans is linked genetically to susceptibility to Type II diabetes (62). It should be noted that other ZnT family members, among them ZnT-5 and ZnT-6, are also found on secretory vesicles, influencing insulin production and secretion. Thus, the question is whether a functional interaction occurs between these Zn2+ transporters, and what their specific role in insulin production and secretion is.
ADDITIONAL QUESTIONS AND CHALLENGES FOR FUTURE RESEARCH
As seen by the brief discussion above, the characterization of multiple ZnT proteins has not yet led to elucidation of a mechanism of action for most of them. Many fundamental questions remain about their activity and regulation, as well as their interaction with other ion transporters. Of particular interest is the question of how these proteins are capable of transporting zinc against the vanishingly low intracellular concentration existing in mammalian cells. One possible mechanism is direct buffering of Zn2+ by the ZnTs. Alternatively, the transporters may interact with metallothioneins (MT) which will transfer zinc to the ZnTs. A similar mechanism has been shown for the shuttling of Fe3+ and Cu2+ (63). Finally, release of zinc from metallothioneins, in response to specific intracellular signals (e.g., NO) or ischemia, may also provide free Zn2+ to the ZnTs.
Another question regards the existence of functional crosstalk between the Zn2+ transporters and other ion transport mechanisms, particularly proteins involved in maintaining cellular pH homeostasis, such as the Na+/H+ exchangers. This is especially relevant considering the putative mechanism of H+/Zn2+ exchange, which may underlie the activity of the intracellular ZnTs.
The structural organization of the ZnTs also is poorly understood at this time. Hetero-oligomerization, has been suggested to underlie the activity of at least some of the transporters. Is this phenomenon exclusive to ZnT5 and ZnT6, or is it shared by other ZnT members? Such interactions would increase significantly the functional repertoire of zinc transport proteins. The functional interaction between ZnT-3 and the vesicular glutamate transporter, Vglut1, would support such a mechanism (64).
Finally, at the dawn of the era of proteomics and cellular networking, is it possible to begin drawing the network map of the zinc transport proteins? Powerful tools to address these issues, e.g., fluorescent zinc sensors targeted to specific organelles, have been developed and are being constantly refined (65–69). The advantage of such an approach has been demonstrated previously for H+ and Ca2+ using GFP-based chameleon and pHluo-rin constructs. Based on seminal studies performed on yeast and mammalian cells, the feasibility of studying zinc in organelles using such an approach is promising, (65,70). The results will have an impact far beyond cellular zinc homeostasis.
ACKNOWLEDGMENTS
Thanks to the many members in the zinc community for their invaluable insight and discussions. This work was supported in part by Binational Science Foundation Grant 2001101 (to M. H.), Israel Science Foundation Grant 456/02.1 (to I. S.), German Israel Foundation (GIF, project nr. I-588-99.1/1998 to I.S.), and Israel Science Foundation equipment Grant 456/02.2 (to I. S.).
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–5. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 2.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol-Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 3.Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000;130:1500S–8S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 5.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–62. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 6.Frazzini V, Rockabrand E, Mocchegiani E, Sensi SL. Oxidative stress and brain aging: is zinc the link? Biogerontology. 2006;7:307–14. doi: 10.1007/s10522-006-9045-7. [DOI] [PubMed] [Google Scholar]
- 7.Sensi SL, Jeng JM. Rethinking the excitotoxic ionic milieu: the emerging role of Zn(2+) in ischemic neuronal injury. Curr Mol Med. 2004;4:87–111. doi: 10.2174/1566524043479211. [DOI] [PubMed] [Google Scholar]
- 8.Pal S, He K, Aizenman E. Nitrosative stress and potassium channel-mediated neuronal apoptosis: is zinc the link? Pflugers Arch. 2004;448:296–303. doi: 10.1007/s00424-004-1256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Wang H, Li J, et al. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J Neurosci. 2004;24:10616–27. doi: 10.1523/JNEUROSCI.2469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JM, Zipfel GJ, Park KH, He YY, Hsu CY, Choi DW. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience. 2002;115:871–8. doi: 10.1016/s0306-4522(02)00513-4. [DOI] [PubMed] [Google Scholar]
- 11.Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci. 1998;21:347–75. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 12.Cherny RA, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–76. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim BJ, et al. Zinc as a paracrine effector in pancreatic islet cell death. Diabetes. 2000;49:367–72. doi: 10.2337/diabetes.49.3.367. [DOI] [PubMed] [Google Scholar]
- 14.Chang I, et al. Role of calcium in pancreatic islet cell death by IFN-gamma/TNF-alpha. J Immunol. 2004;172:7008–14. doi: 10.4049/jimmunol.172.11.7008. [DOI] [PubMed] [Google Scholar]
- 15.Priel T, Hershfinkel M. Zinc influx and physiological consequences in the beta-insulinoma cell line, Min6. Biochem Biophys Res Commun. 2006;346:205–12. doi: 10.1016/j.bbrc.2006.05.104. [DOI] [PubMed] [Google Scholar]
- 16.Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–96. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 17.Bozym RA, Thompson RB, Stoddard AK, Fierke CA. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem Biol. 2006;1:103–11. doi: 10.1021/cb500043a. [DOI] [PubMed] [Google Scholar]
- 18.Gee KR, Zhou ZL, Qian WJ, Kennedy R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J Am Chem Soc. 2002;124:776–8. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- 19.Dunn MF. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer — a review. Biometals. 2005;18:295–303. doi: 10.1007/s10534-005-3685-y. [DOI] [PubMed] [Google Scholar]
- 20.Frederickson CJ, Moncrieff DW. Zinc-containing neurons. Biol Signals. 1994;3:127–39. doi: 10.1159/000109536. [DOI] [PubMed] [Google Scholar]
- 21.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–72. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 22.Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 23.Maret W. Cellular zinc and redox states converge in the metallothionein/thionein pair. J Nutr. 2003;133:1460S–2S. doi: 10.1093/jn/133.5.1460S. [DOI] [PubMed] [Google Scholar]
- 24.Banci L, Bertini I, Ciofi-Baffoni S, Finney LA, Outten CE, O’Halloran TV. A new zinc-protein coordination site in intracellular metal trafficking: solution structure of the Apo and Zn(II) forms of ZntA(46–118) J Mol Biol. 2002;323:883–97. doi: 10.1016/s0022-2836(02)01007-0. [DOI] [PubMed] [Google Scholar]
- 25.Eren E, Kennedy DC, Maroney MJ, Arguello JM, Eren E, Arguello JM. A novel regulatory metal binding domain is present in the C terminus of Arabidopsis Zn2+-ATPase HMA2. J Biol Chem. 2006;281:33881–91. doi: 10.1074/jbc.M605218200. [DOI] [PubMed] [Google Scholar]
- 26.Eren E, Arguello JM. Arabidopsis HMA2, a divalent heavy metal-transporting P(IB)-type ATPase, is involved in cytoplasmic Zn2+ homeostasis. Plant Physiol. 2004;136:3712–23. doi: 10.1104/pp.104.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrukhin K, Lutsenko S, Chernov I, Ross BM, Kaplan JH, Gilliam TC. Characterization of the Wilson disease gene encoding a P-type copper transporting ATPase: genomic organization, alternative splicing, and structure/function predictions. Hum Mol Genet. 1994;3:1647–56. doi: 10.1093/hmg/3.9.1647. [DOI] [PubMed] [Google Scholar]
- 28.Sensi SL, et al. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci. 1997;17:9554–64. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohana E, et al. A sodium zinc exchange mechanism is mediating extrusion of zinc in Mammalian cells. J Biol Chem. 2004;279:4278–84. doi: 10.1074/jbc.M309229200. [DOI] [PubMed] [Google Scholar]
- 30.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–9. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 31.Dufner-Beattie J, Langmade SJ, Wang F, Eide D, Andrews GK. Structure, function, and regulation of a subfamily of mouse zinc transporter genes. J Biol Chem. 2003;278:50142–50. doi: 10.1074/jbc.M304163200. [DOI] [PubMed] [Google Scholar]
- 32.Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem. 2000;275:34803–9. doi: 10.1074/jbc.M007339200. [DOI] [PubMed] [Google Scholar]
- 33.Devergnas S, et al. Differential regulation of zinc efflux transporters ZnT-1, ZnT-5 and ZnT-7 gene expression by zinc levels: a real-time RT-PCR study. Biochem Pharmacol. 2004;68:699–709. doi: 10.1016/j.bcp.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 34.MacDiarmid CW, Milanick MA, Eide DJ. Biochemical properties of vacuolar zinc transport systems of Saccharomyces cerevisiae. J Biol Chem. 2002;277:39187–194. doi: 10.1074/jbc.M205052200. [DOI] [PubMed] [Google Scholar]
- 35.Chao Y, Fu D. Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter, ZitB. J Biol Chem. 2004;279:12043–50. doi: 10.1074/jbc.M313510200. [DOI] [PubMed] [Google Scholar]
- 36.Sensi SL, Rockabrand E, Canzoniero LM. Acidosis enhances toxicity induced by kainate and zinc exposure in aged cultured astrocytes. Biogerontology. 2006;7:367–74. doi: 10.1007/s10522-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 37.Palmiter RD. Protection against zinc toxicity by metallothionein and zinc transporter 1. Proc Natl Acad Sci U S A. 2004;101:4918–23. doi: 10.1073/pnas.0401022101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nolte C, et al. ZnT-1 expression in astroglial cells protects against zinc toxicity and slows the accumulation of intracellular zinc. Glia. 2004;48:145–55. doi: 10.1002/glia.20065. [DOI] [PubMed] [Google Scholar]
- 39.Sekler I, et al. Distribution of the zinc transporter ZnT-1 in comparison with chelatable zinc in the mouse brain. J Comp Neurol. 2002;447:201–9. doi: 10.1002/cne.10224. [DOI] [PubMed] [Google Scholar]
- 40.Nitzan YB, Sekler I, Hershfinkel M, Moran A, Silverman WF. Postnatal regulation of ZnT-1 expression in the mouse brain. Brain Res Dev Brain Res. 2002;137:149–57. doi: 10.1016/s0165-3806(02)00437-6. [DOI] [PubMed] [Google Scholar]
- 41.Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO-J. 1995;14:639–49. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohana E, et al. Silencing of ZnT-1 expression enhances heavy metal influx and toxicity. J Mol Med. 2006;84:753–63. doi: 10.1007/s00109-006-0062-4. [DOI] [PubMed] [Google Scholar]
- 43.Segal D, Ohana E, Besser L, Hershfinkel M, Moran A, Sekler I. A role for ZnT-1 in regulating cellular cation influx. Biochem Biophys Res Commun. 2004;323:1145–50. doi: 10.1016/j.bbrc.2004.08.211. [DOI] [PubMed] [Google Scholar]
- 44.Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93:14934–9. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc Natl Acad Sci U S A. 1999;96:1716–21. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole TB, Martyanova A, Palmiter RD. Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensorimotor functions in the mouse. Brain Res. 2001;891:253–65. doi: 10.1016/s0006-8993(00)03220-0. [DOI] [PubMed] [Google Scholar]
- 47.Lopantsev V, Wenzel HJ, Cole TB, Palmiter RD, Schwartzkroin PA. Lack of vesicular zinc in mossy fibers does not affect synaptic excitability of CA3 pyramidal cells in zinc transporter 3 knockout mice. Neuroscience. 2003;116:237–48. doi: 10.1016/s0306-4522(02)00570-5. [DOI] [PubMed] [Google Scholar]
- 48.Lee JY, Kim JH, Palmiter RD, Koh JY. Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol. 2003;184:337–47. doi: 10.1016/s0014-4886(03)00382-0. [DOI] [PubMed] [Google Scholar]
- 49.Friedlich AL, et al. Neuronal zinc exchange with the blood vessel wall promotes cerebral amyloid angiopathy in an animal model of Alzheimer’s disease. J Neurosci. 2004;24:3453–9. doi: 10.1523/JNEUROSCI.0297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kodirov SA, Takizawa S, Joseph J, Kandel ER, Shumyatsky GP, Bolshakov VY. Synaptically released zinc gates long-term potentiation in fear conditioning pathways. Proc Natl Acad Sci U S A. 2006;103:15218–23. doi: 10.1073/pnas.0607131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J Neurosci. 2006;26:7181–8. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mellor J, Nicoll RA. Hippocampal mossy fiber LTP is independent of postsynaptic calcium. Nature Neuroscience. 2001;4:125–6. doi: 10.1038/83941. [DOI] [PubMed] [Google Scholar]
- 53.Ishihara K, et al. Zinc transport complexes contribute to the homeostatic maintenance of secretory pathway function in vertebrate cells. J Biol Chem. 2006;281:17743–50. doi: 10.1074/jbc.M602470200. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki T, et al. Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J Biol Chem. 2005;280:637–43. doi: 10.1074/jbc.M411247200. [DOI] [PubMed] [Google Scholar]
- 55.Inoue K, et al. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Hum Mol Genet. 2002;11:1775–84. doi: 10.1093/hmg/11.15.1775. [DOI] [PubMed] [Google Scholar]
- 56.Afridi HI, Kazi TG, Kazi GH, Jamali MK, Shar GQ. Essential trace and toxic element distribution in the scalp hair of Pakistani myocardial infarction patients and controls. Biol Trace Elem Res. 2006;113:19–34. doi: 10.1385/BTER:113:3. [DOI] [PubMed] [Google Scholar]
- 57.Huang L, Kirschke CP, Gitschier J. Functional characterization of a novel mammalian zinc transporter, ZnT6. J Biol Chem. 2002;277:26389–95. doi: 10.1074/jbc.M200462200. [DOI] [PubMed] [Google Scholar]
- 58.Ellis CD, Macdiarmid CW, Eide DJ. Heteromeric protein complexes mediate zinc transport into the secretory pathway of eukaryotic cells. J Biol Chem. 2005;280:28811–8. doi: 10.1074/jbc.M505500200. [DOI] [PubMed] [Google Scholar]
- 59.Jackson KA, Helston RM, McKay JA, O’Neill ED, Mathers JC, Ford D. Splice variants of the human zinc transporter ZnT5 (SLC30A5) are differentially localized and regulated by zinc through transcription and mRNA stability. J Biol Chem. 2007;282:10423–31. doi: 10.1074/jbc.M610535200. [DOI] [PubMed] [Google Scholar]
- 60.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–7. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 61.Chimienti F, et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci. 2006;119:4199–206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- 62.Sladek R, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–5. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 63.Prohaska JR, Gybina AA. Intracellular copper transport in mammals. J Nutr. 2004;134:1003–6. doi: 10.1093/jn/134.5.1003. [DOI] [PubMed] [Google Scholar]
- 64.Salazar G, Craige B, Love R, Kalman D, Faundez V. Vglut1 and ZnT3 co-targeting mechanisms regulate vesicular zinc stores in PC12 cells. J Cell Sci. 2005;118:1911–21. doi: 10.1242/jcs.02319. [DOI] [PubMed] [Google Scholar]
- 65.Hara H, Aizenman E. A molecular technique for detecting the liberation of intracellular zinc in cultured neurons. J Neurosci Methods. 2004;137:175–80. doi: 10.1016/j.jneumeth.2004.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang CJ, Nolan EM, Jaworski J, Burdette SC, Sheng M, Lippard SJ. Bright fluorescent chemosensor platforms for imaging endogenous pools of neuronal zinc. Chem Biol. 2004;11:203–10. doi: 10.1016/j.chembiol.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 67.Burdette SC, Frederickson CJ, Bu W, Lippard SJ. ZP4, an improved neuronal Zn2+ sensor of the Zinpyr family. J Am Chem Soc. 2003;125:1778–87. doi: 10.1021/ja0287377. [DOI] [PubMed] [Google Scholar]
- 68.Crivat G, et al. Fluorescence-based zinc ion sensor for zinc ion release from pancreatic cells. Anal Chem. 2006;78:5799–804. doi: 10.1021/ac060764i. [DOI] [PubMed] [Google Scholar]
- 69.Komatsu K, Kikuchi K, Kojima H, Urano Y, Nagano T. Selective zinc sensor molecules with various affinities for Zn2+, revealing dynamics and regional distribution of synaptically released Zn2+ in hippocampal slices. J Am Chem Soc. 2005;127:10197–204. doi: 10.1021/ja050301e. [DOI] [PubMed] [Google Scholar]
- 70.Qiao W, Mooney M, Bird AJ, Winge DR, Eide DJ. Zinc binding to a regulatory zinc-sensing domain monitored in vivo by using FRET. Proc Natl Acad Sci U S A. 2006;103:8674–9. doi: 10.1073/pnas.0600928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andrews GK, Wang H, Dey SK, Palmiter RD. Mouse zinc transporter 1 gene provides an essential function during early embryonic development. Genesis. 2004;40:74–81. doi: 10.1002/gene.20067. [DOI] [PubMed] [Google Scholar]
- 72.Falcon-Perez JM, Dell’angelica EC. Zinc transporter 2 (SLC30A2) can suppress the vesicular zinc defect of adaptor protein 3-depleted fibroblasts by promoting zinc accumulation in lysosomes. Exp Cell Res. 2007;313:1473–83. doi: 10.1016/j.yexcr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palmiter RD, Cole TB, Findley SD. ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO-J. 1996;15:1784–91. [PMC free article] [PubMed] [Google Scholar]
- 74.Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci U S A. 2002;99:7705–10. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat Genet. 1997;17:292–7. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- 76.Michalczyk A, Varigos G, Catto-Smith A, Blomeley RC, Ackland ML. Analysis of zinc transporter, hZnT4 (Slc30A4), gene expression in a mammary gland disorder leading to reduced zinc secretion into milk. Hum Genet. 2003;113:202–10. doi: 10.1007/s00439-003-0952-2. [DOI] [PubMed] [Google Scholar]
- 77.Murgia C, Vespignani I, Cerase J, Nobili F, Perozzi G. Cloning, expression, and vesicular localization of zinc transporter Dri 27/ZnT4 in intestinal tissue and cells. Am J Physiol. 1999;277:G1231–9. doi: 10.1152/ajpgi.1999.277.6.G1231. [DOI] [PubMed] [Google Scholar]
- 78.Lang C, et al. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am J Physiol Lung Cell Mol Physiol. 2007;292:L577–84. doi: 10.1152/ajplung.00280.2006. [DOI] [PubMed] [Google Scholar]
- 79.Smith JL, Xiong S, Markesbery WR, Lovell MA. Altered expression of zinc transporters-4 and –6 in mild cognitive impairment, early and late Alzheimer’s disease brain. Neuroscience. 2006;140:879–88. doi: 10.1016/j.neuroscience.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 80.Kambe T, et al. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem. 2002;277:19049–55. doi: 10.1074/jbc.M200910200. [DOI] [PubMed] [Google Scholar]
- 81.Kirschke CP, Huang L. ZnT7, a novel mammalian zinc transporter, accumulates zinc in the Golgi apparatus. J Biol Chem. 2003;278:4096–102. doi: 10.1074/jbc.M207644200. [DOI] [PubMed] [Google Scholar]
- 82.Wang X, et al. Localization of ZnT7 and zinc ions in mouse retina—immunohistochemistry and selenium autometallography. Brain Res Bull. 2006;71:91–6. doi: 10.1016/j.brainresbull.2006.08.002. [DOI] [PubMed] [Google Scholar]