Abstract
Zinc translocation from presynaptic nerve terminals to postsynaptic neurons has generally been considered the critical step leading to the accumulation of intracellular free zinc and subsequent neuronal injury. Recent evidence, however, strongly suggests that the liberation of zinc from intracellular stores upon oxidative and nitrative stimulation contributes significantly to the toxicity of this metal not only to neurons, but also to oligodendrocytes. The exact cell death signaling pathways triggered by zinc are beginning to be deciphered. In this review, we describe how the activation of 12-lipoxygenase and mitogen-activated protein kinase (MAPK) contribute to the toxicity of liberated zinc to neurons and oligodendrocytes.
INTRODUCTION
Zinc is the second most abundant trace element in the body and is present in particularly high concentrations in the mammalian brain (1). The presence of zinc in synaptic vesicles in certain populations of glutamatergic neurons results in the co-release of this metal together with glutamate (2,3). The synaptically released zinc, in turn, has been suggested to modulate the activity of postsynaptic glutamate receptors (4). Under pathological conditions, zinc can enter postsynaptic neurons via NMDA receptors, calcium permeable AMPA/kainate receptors, and voltage sensitive calcium channels (1). Once the intracellular free zinc concentrations rise, neuronal cell death will quickly follow (5). Trans-synaptic zinc movement has been regarded previously as the necessary step leading to intracellular zinc accumulation and the ensuing neuronal death that is observed in several neurological conditions, such as ischemia, epilepsy, and head trauma (6,7). However, the hypothesis that trans-synaptic zinc movement fully accounts for the accumulation of this metal in postsynaptic cells has to be reconsidered given the recent demonstration that neurons destined to die in mice lacking presynaptic vesicular zinc still undergo zinc accumulation (8). Although the sources of zinc in these instances have yet to be determined, it is most likely cytoplasmic metal binding proteins, such as metallothioneins, or the mitochondria (4,9).
OXIDATIVE STRESS AND INTRACELLULAR ZINC LIBERATION
Zinc is an important structural and functional component in many cellular proteins and enzymes, such as zinc finger transcription factors, metallothioneins and the antioxidant enzyme copper, zinc superoxide dismutase. Zinc is normally tightly bound to these proteins, limiting the extent of intracellular free zinc concentrations [Zn2+]i in eukaryotic cells. These concentrations are generally believed to be in the low picomolar to low nanomolar range (6,10,11). The increase in [Zn2+]i beyond these levels is generally believed to contribute to rapid cellular demise (4).
Zinc and Metallothionein
Metallothioneins (MTs) are low molecular weight metal binding proteins encoding 61–68 amino acids, 20 of which are cysteines. Four isoforms of mammalian MT have been identified: MT-1 and MT-2 are expressed ubiquitously, and largely expressed in astrocytes in the central nervous system (12), MT-3 is found primarily in neurons (13), and MT-4 is expressed in differentiated squamous epithelial cells (14). These cysteine-rich proteins can bind up to seven zinc ions, and thus provide strong buffering capacity for intracellular zinc. On the other hand, these proteins also provide a reservoir from which zinc can be released, typically in response to thiol oxidization (5,15). For example, studies with cell-free assays demonstrated that redox agents, such as 2,2′-dithiodipyridine (DTDP) can oxidize the cysteine residues within MTs to readily release all zinc ions (16). In primary neuronal cultures, DTDP has also been found to release zinc from metallothioneins and trigger apoptotic cell death (17). DTDP also is able to induce zinc release from astrocytes that overexpress MT-2 and cause toxicity in this system (18). In vivo studies using zinc transporter 3 (ZnT-3) knockout mice suggest that zinc released from MT in the CA1 region following ischemia leads to toxicity within CA1 neurons (19). Recently we also have demonstrated that cytosolic zinc levels increase in rat thalamic neurons, specifically in lateral geniculate nucleus (LGN) neurons after target loss, which normally lack presynaptic zinc input (20). We speculate that zinc in this system originates from MT-3, as zinc-associated thalamic cell death is nearly abolished in MT-3 gene knockout mice (19).
Zinc and Mitochondria
Mitochondria are important cellular organelles that have been shown to sequester intracellular zinc and also release it in response to specific stimuli or injury (5). Zinc ions may alter mitochondrial function as well, leading to modulation of the activity of several enzymes involved in ATP synthesis under both physiological and pathophysiological conditions (21–23). Zinc may enter mitochondria via the calcium uniporter (24). Through the use of zinc fluorescent dyes and manipulation of mitochondrial membrane potential, a pool of labile zinc in mitochondria was revealed (16). Specifically, zinc can be readily released from this pool once the mitochondrial membrane is depolarized (25). Therefore, the integrity of the mitochondrial membrane and the stability of its membrane potential can strongly influence the status of mitochondrial sequestration or release of zinc. Upon exposure of cells to oxidative or nitrative stressors, the decreased energy production and increased generation of reactive oxygen species (ROS) is likely to impair mitochondrial membrane potential, resulting in increased cytosolic zinc levels and subsequent cell injury. It has been shown that the pro-apoptotic protein ΔN-BCL-xL, a cleavage product of the outer mitochondrial membrane protein BCL-xL (26), induces large conductance (multi-conductance) channel activity and neuronal death following injurious stimuli (27). The increased large conductance channel activity has recently been found to be associated with the elevated levels of zinc in mitochondria, and chelation of zinc blocks the activation of the large conductance channel activity and, importantly, the delayed neuronal death observed in transient global ischemia (28).
MECHANISMS OF ZINC TOXICITY TO NEURONS
Using primary neuronal cultures from rat brain, we have found that intracellular zinc release triggered by oxidative and nitrative stressors is the necessary 1st step to initiate cell death signaling pathways (17,29–32).
p38 MAPK
DTDP, a cell permeant thiol oxidant, has been found to liberate zinc from intracellular stores (17) and induce neuronal apoptosis via activation of p38 MAPK (29). The activation of p38 MAPK is followed by potassium efflux and the subsequent activation of caspases (29). The efflux of potassium ions is mediated by the direct phosphorylation of Kv2.1-encoded K+ channels by p38, leading to a SNARE-dependent insertion of the channel and a dramatic increase in K+ currents (33–35). This cell death pathway also appears to contribute to the toxicity of nitric oxide to neurons (36,37). In this paradigm, the toxicity is mediated by the intracellular formation of peroxynitrite and the subsequent release of zinc from intracellular stores. Indeed, we have found that administration of peroxynitrite generator, SIN-1, or authentic peroxynitrite can liberate zinc from intracellular stores and cause apoptosis in neurons (31). Thus, p38 MAPK activation is downstream of mitochondria dysfunction and ROS generation following zinc liberation. In addition, studies using peroxynitrite as an oxidizing agent also indicate that 12-lipoxygenase (12-LOX) activation contributes to mitochondria independent ROS generation, also occurring upstream of p38 MAPK activation (31).
Extracellular Signal Regulated Protein Kinase 1/2 (ERK1/2)
ERK1/2 activation is typically associated with cell survival, proliferation, and differentiation (38). However, a growing number of in vitro and in vivo studies also implicate a detrimental role for ERK1/2 signaling during oxidative neuronal injury (39). Methylisothiazolinone (MIT), a commonly used industrial and household biocide, has been found to trigger intracellular zinc release likely via the oxidization of cellular thiols (30). Interestingly, unlike the other oxidizing and nitrating agents mentioned above, neuronal cell death induced by MIT is mediated by activation of ERK1/2, and not p38 MAPK. Oxidative neuronal cell death in this model also involves activation of NADPH oxidase, generation of ROS, DNA damage, and the activation of poly (ADP-ribose) polymerase, all occurring downstream of zinc release and ERK1/2 activation (30). The sequence of this signaling cascade is in harmony with those observed when exogenous zinc is applied to neurons in culture (40–43), supporting the notion that zinc released from intracellular stores by MIT triggers the activation of ERK1/2 and the subsequent cell death signaling pathways. Notably, persistent ERK1/2 activation also is observed in glutamate-induced oxidative toxicity in the HT22 neuroblastoma cell line and immature primary cortical neurons (44,45). Similar to what has been observed in mature neurons (17,30,31), we have recently found that ERK1/2 activation caused by glutathione depletion is also mediated by intracellular zinc liberation in HT22 cells and in immature neurons (unpublished data). It is noteworthy that in these model systems, ERK1/2 activation is associated with a caspase-independent cell death pathway, in direct contrast with the zinc-mediated p38 cell death pathway described earlier (29,31).
Activation of 12-LOX
Mitochondria are generally believed to be the major source of ROS generation induced by loss of cellular zinc homeostasis. However, recent studies also provide evidence of mitochondria-independent ROS production, such as those generated from the activation of NADPH oxidase and 12-LOX (30,31,41, 42). 12-LOX is the primary LOX expressed in the brain (46), and belongs to a family of arachidonic acid metabolizing enzymes, which also include 5-LOX, cyclooxygenase, and epoxygenase (47). In primary cultures of immature neurons, glutamate-induced glutathione depletion causes 12-LOX activation, ROS generation, and cell death (48). Activation of 12-LOX also has been found in MIT- and peroxynitrite-induced toxicity in mature neurons in culture (30,31). N, N, N′, N′-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN), a zinc chelator, blocks the membrane translocation and the activity of 12-LOX, strongly suggesting that in-tracellular zinc release causes the activation of this enzyme. In a mouse model of transient cerebral ischemia, the expression of 12-LOX is upregulated in the area surrounding the primary lesion, primarily in neurons (49). Significantly, 12-LOX inhibitors are protective against the neuronal injury observed in the ischemic brain (49,50). It is therefore reasonable to speculate that rising levels of zinc during ischemic injury (51,52) contributes to 12-LOX activation and neuronal death (31). The cell death pathways triggered by intracellular free zinc following oxidative or nitrative stress are illustrated in Figure 1.
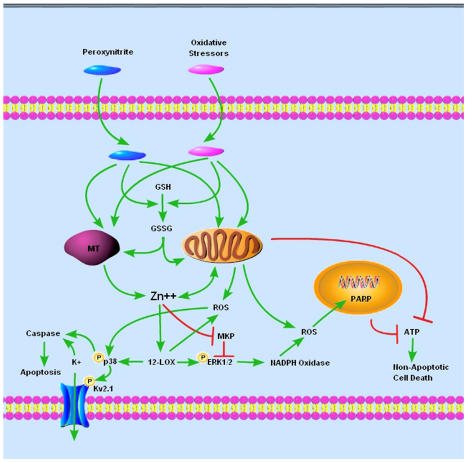
Figure 1.
Oxidative or nitrative stress-induced neuronal death pathways. Peroxynitrite or oxidative stressors (for example, DTDP, MIT) cause zinc release from metallothionein and/or mitochondria either directly or indirectly via the conversion of reduced glutathione (GSH) to oxidized glutathione (GSSG). Zinc activates 12-LOX and causes neuronal death via activation of p38 or ERK1/2. Phosphorylation of p38 results in caspase-dependent apoptosis, which is mediated in part by phosphorylation of the Kv2.1 potassium channel and subsequent K ± efflux. Phosphorylation of ERK1/2, which is downstream of 12-LOX activation and ERK1/2 directed phosphatase (MKP) inhibition, causes NADPH oxidase activation and ROS generation. ROS, which are also generated from activation of 12-LOX and from mitochondria, activate PARP leading to ATP depletion and caspase-independent neuronal death.
MECHANISMS OF ZINC TOXICITY TO OLIGODENDROCYTES
Similar to what has been observed in neurons, oxidizing and nitrating agents also can effectively trigger zinc release from astrocytes (18) and oligodendrocytes (32). Using primary cultures of mature oligodendrocytes expressing myelin basic protein, we found peroxynitrite causes sequential activation of ERK1/2, 12-LOX, and generation of ROS, which are all dependent upon the liberation of zinc from intracellular stores (32). In contrast to the observations made in neuronal systems (30), ERK1/2 activation triggered by zinc in mature oligodendrocytes occurs upstream of 12-LOX activation (32). As such, inhibition of ERK1/2 phosphorylation blocks 12-LOX activity and subsequent injurious ROS generation. We also have recently observed that glutathione depletion-induced toxicity in mature oligodendrocytes is blocked by zinc chelation (unpublished data) and 12-LOX inhibition (53), suggesting zinc release and 12-LOX activation might be a common pathway of the toxicity to mature oligodendrocytes triggered by various oxidizing and nitrating stressors. In more recent studies, we observed that glutathione depletion-induced toxicity in developing oligodendrocytes also occurs via the activation of 12-LOX (53). However, whether this enzyme also is activated by zinc liberation in these cells remains to be established. The pathway of peroxynitrite induced toxicity to mature oligodendrocytes is illustrated in Figure 2.
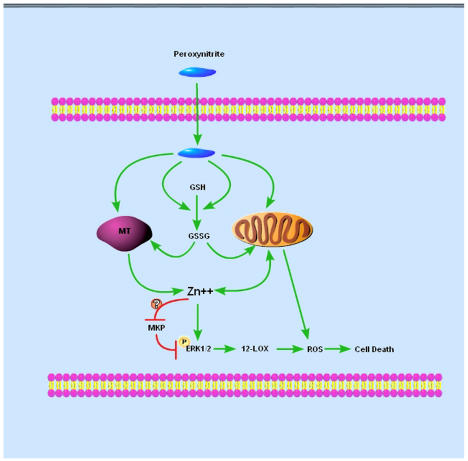
Figure 2.
Pathway of peroxynitrite-induced toxicity to mature oligodendrocytes. Peroxynitrite triggers zinc release from metallothionein and/or mitochondria either directly or indirectly via the conversion of reduced glutathione (GSH) to oxidized glutathione (GSSG). Zinc induces ERK1/2 phosphorylation, which may be due partially to the inhibition of MKP activity. In contrast to neurons, ERK1/2 phosphorylation occurs upstream of 12-LOX in mature oligodendrocytes. ROS generated from 12-LOX activation and mitochondria are toxic to mature oligodendrocytes.
ZINC DEPENDENT REGULATION OF ERK1/2 PHOSPHORYLATION IN NEURONS AND OLIGODENDROCYTES
Although it has been firmly established that zinc can activate ERK1/2 in neurons and oligodendrocytes, the mechanisms of activation of this MAPK are still not well understood. The regulation of ERK1/2 phosphorylation and activation during oxidative stress may in fact be reflective of a tight balance between ERK1/2-directed kinase and phosphatase activity. Indeed, several phosphatases have been shown to be either reversibly or irreversibly inhibited during oxidative injury, depending on the degree and the source of the stressors (54,55). We have demonstrated that ERK1/2-directed serine/threonine- and tyrosine- phosphatases are inhibited during glutamate-induced oxidative toxicity in primary immature neurons in culture (56), as well as in an animal model of global ischemia (57). This inhibition is tightly correlated with the increased phosphorylation of ERK1/2. Interestingly, we also have found that ERK phosphatase inhibition is mediated by liberated zinc: TPEN can readily reverse ERK-directed phosphatase inhibition caused by the oxidative agents (unpublished data, Figure 1). This phenomenon may be more widespread, as ERK1/2-directed phosphatase inhibition also is observed in mature oligodendrocytes treated with peroxynitrite (unpublished data, Figure 2). These results suggest that zinc-induced ERK1/2 activation may be mediated, or at least substantially facilitated, by ERK1/2-directed phosphatase inhibition.
CONCLUSIONS
Oxidative and nitrative stress have been closely associated with a large number of neurological disorders, including stroke, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, cerebral palsy, and multiple sclerosis. Approaches aimed at maintenance of zinc homeostasis, inhibition of the downstream signaling molecules activated by zinc, such as 12-LOX, ERK1/2 or p38 MAPK, and the blockade of ROS generation, provide novel therapeutic targets in the treatment of these disorders. However, due to the complexity of the multiple signaling pathways that may be involved following zinc liberation, and the large array of signaling molecules potentially involved in each of these diseases, the precise molecular target (i.e. ERK1/2 or p38 MAPK) responsive to increased zinc accumulation may differ in each condition (Figure 1). Understanding of the precise cell death signaling mechanisms in neurons and oligodendrocytes induced by increases in intracellular zinc may provide us with a rationale for specific treatment of those neurological disorders in which a pathogenetic role for zinc has been implicated.
ACKNOWLEDGMENTS
This work is supported by grants from the United Cerebral Palsy Foundation (R-759 to Y.Z.), the National Multiple Sclerosis Society (RG3741 to Y.Z.) and the National Institutes of Health (NS043277 to E.A., NS038319 to D.B.D., and NS038475 to P.A.R.).
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Weiss JH, Sensi SL, Koh JY. Zn(2+): a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- 2.Qian J, Noebels JL. Exocytosis of vesicular zinc reveals persistent depression of neurotransmitter release during metabotropic glutamate receptor long-term depression at the hippocampal CA3-CA1 synapse. J Neurosci. 2006;26:6089–95. doi: 10.1523/JNEUROSCI.0475-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fiber synapse. J Physiol. 2005;566:747–58. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–62. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 5.Sensi SL, Jeng JM. Rethinking the excitotoxic ionic milieu: the emerging role of Zn(2+) in ischemic neuronal injury. Curr Mol Med. 2004;4:87–111. doi: 10.2174/1566524043479211. [DOI] [PubMed] [Google Scholar]
- 6.Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- 7.Suh SW, et al. Detection of pathological zinc accumulation in neurons: methods for autopsy, biopsy, and cultured tissue. J Histochem Cytochem. 1999;47:969–72. doi: 10.1177/002215549904700715. [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Cole TB, Palmiter RD, Koh JY. Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: evidence against synaptic vesicle origin. J Neurosci. 2000;20:RC79. doi: 10.1523/JNEUROSCI.20-11-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frazzini V, Rockabrand E, Mocchegiani E, Sensi SL. Oxidative stress and brain aging: is zinc the link? Biogerontology. 2006;7:307–14. doi: 10.1007/s10522-006-9045-7. [DOI] [PubMed] [Google Scholar]
- 10.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–92. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 11.Krezel A, Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem. 2006;11:1049–62. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 12.Palmiter RD. Molecular biology of metallothionein gene expression. Experientia Suppl. 1987;52:63–80. doi: 10.1007/978-3-0348-6784-9_4. [DOI] [PubMed] [Google Scholar]
- 13.Masters BA, et al. Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J Neurosci. 1994;14:5844–57. doi: 10.1523/JNEUROSCI.14-10-05844.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quaife CJ, Findley SD, Erickson JC, et al. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994;33:7250–9. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- 15.Maret W. The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr. 2000;130:1455S–8S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- 16.Maret W, Vallee BL. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc Natl Acad Sci U S A. 1998;95:3478–82. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–88. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 18.Malaiyandi LM, Dineley KE, Reynolds IJ. Divergent consequences arise from metallothionein overexpression in astrocytes: zinc buffering and oxidant-induced zinc release. Glia. 2004;45:346–53. doi: 10.1002/glia.10332. [DOI] [PubMed] [Google Scholar]
- 19.Lee JY, Kim JH, Palmiter RD, Koh JY. Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol. 2003;184:337–47. doi: 10.1016/s0014-4886(03)00382-0. [DOI] [PubMed] [Google Scholar]
- 20.Land PW, Aizenman E. Zinc accumulation after target loss: an early event in retrograde degeneration of thalamic neurons. Eur J Neurosci. 2005;21:647–57. doi: 10.1111/j.1460-9568.2005.03903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dineley KE, Votyakova TV, Reynolds IJ. Zinc inhibition of cellular energy production: implications for mitochondria and neurodegeneration. J Neurochem. 2003;85:563–70. doi: 10.1046/j.1471-4159.2003.01678.x. [DOI] [PubMed] [Google Scholar]
- 22.Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–45. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai AL, Zipfel GJ, Sheline CT. Zinc neurotoxicity is dependent on intracellular NAD levels and the sirtuin pathway. Eur J Neurosci. 2006;24:2169–76. doi: 10.1111/j.1460-9568.2006.05110.x. [DOI] [PubMed] [Google Scholar]
- 24.Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH. Zn(2+) induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J Biol Chem. 2001;276:47524–9. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- 25.Sensi SL, et al. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A. 2003;100:6157–62. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng EH, Kirsch DG, Clem RJ, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–8. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 27.Jonas EA, Hickman JA, Hardwick JM, Kaczmarek LK. Exposure to hypoxia rapidly induces mitochondrial channel activity within a living synapse. J Biol Chem. 2005;280:4491–7. doi: 10.1074/jbc.M410661200. [DOI] [PubMed] [Google Scholar]
- 28.Bonanni L, et al. Zinc-dependent multi-conductance channel activity in mitochondria isolated from ischemic brain. J Neurosci. 2006;26:6851–62. doi: 10.1523/JNEUROSCI.5444-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin B, et al. p38 activation is required upstream of potassium current enhancement and caspase cleavage in thiol oxidant-induced neuronal apoptosis. J Neurosci. 2001;21:3303–11. doi: 10.1523/JNEUROSCI.21-10-03303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du S, McLaughlin B, Pal S, Aizenman E. In vitro neurotoxicity of methylisothiazolinone, a commonly used industrial and household biocide, proceeds via a zinc and extracellular signal-regulated kinase mitogen-activated protein kinase-dependent pathway. J Neurosci. 2002;22:7408–16. doi: 10.1523/JNEUROSCI.22-17-07408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J Neurosci. 2004;24:10616–27. doi: 10.1523/JNEUROSCI.2469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, et al. Intracellular zinc release and ERK phosphorylation are required upstream of 12-lipoxygenase activation in peroxynitrite toxicity to mature rat oligodendrocytes. J Biol Chem. 2006;281:9460–70. doi: 10.1074/jbc.M510650200. [DOI] [PubMed] [Google Scholar]
- 33.Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci. 2003;23:4798–802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal SK, Takimoto K, Aizenman E, Levitan ES. Apoptotic surface delivery of K+ channels. Cell Death Differ. 2006;13:661–7. doi: 10.1038/sj.cdd.4401792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redman PT, et al. Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proc Natl Acad Sci U S A. 2007;104:3568–73. doi: 10.1073/pnas.0610159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bossy-Wetzel E, et al. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41:351–65. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 37.Pal S, He K, Aizenman E. Nitrosative stress and potassium channel-mediated neuronal apoptosis: is zinc the link? Pflugers Arch. 2004;448:296–303. doi: 10.1007/s00424-004-1256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–31. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 39.Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur J Biochem. 2004;271:2060–6. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park JA, Koh JY. Induction of an immediate early gene egr-1 by zinc through extracellular signal-regulated kinase activation in cortical culture: its role in zinc-induced neuronal death. J Neurochem. 1999;73:450–6. doi: 10.1046/j.1471-4159.1999.0730450.x. [DOI] [PubMed] [Google Scholar]
- 41.Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci. 2000;20:RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YH, Koh JY. The role of NADPH oxidase and neuronal nitric oxide synthase in zinc-induced poly(ADP-ribose) polymerase activation and cell death in cortical culture. Exp Neurol. 2002;177:407–18. doi: 10.1006/exnr.2002.7990. [DOI] [PubMed] [Google Scholar]
- 43.Kohda Y, et al. Involvement of Raf-1/MEK/ERK1/2 signaling pathway in zinc-induced injury in rat renal cortical slices. J Toxicol Sci. 2006;31:207–17. doi: 10.2131/jts.31.207. [DOI] [PubMed] [Google Scholar]
- 44.Stanciu M, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275:12200–6. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- 45.Luo Y, DeFranco DB. Opposing roles for ERK1/2 in neuronal oxidative toxicity: distinct mechanisms of ERK1/2 action at early versus late phases of oxidative stress. J Biol Chem. 2006;281:16436–42. doi: 10.1074/jbc.M512430200. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- 47.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–63. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 49.van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–8. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- 50.Khanna S, et al. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 2005;36:2258–64. doi: 10.1161/01.STR.0000181082.70763.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–16. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 52.Stork CJ, Li YV. Intracellular zinc elevation measured with a “calcium-specific” indicator during ischemia and reperfusion in rat hippocampus: a question on calcium overload. J Neurosci. 2006;26:10430–7. doi: 10.1523/JNEUROSCI.1588-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, et al. 12-Lipoxygenase plays a key role in cell death caused by glutathione depletion and arachidonic acid in rat oligodendrocytes. Eur J Neurosci. 2004;20:2049–58. doi: 10.1111/j.1460-9568.2004.03650.x. [DOI] [PubMed] [Google Scholar]
- 54.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–99. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 55.Tonks NK. PTP1B: from the sidelines to the front lines! FEBS Lett. 2003;546:140–8. doi: 10.1016/s0014-5793(03)00603-3. [DOI] [PubMed] [Google Scholar]
- 56.Levinthal DJ, Defranco DB. Reversible oxidation of ERK-directed protein phosphatases drives oxidative toxicity in neurons. J Biol Chem. 2005;280:5875–83. doi: 10.1074/jbc.M410771200. [DOI] [PubMed] [Google Scholar]
- 57.Ho Y, Logue E, Callaway CW, Defranco DB. Different mechanisms account for extracellular-signal regulated kinase activation in distinct brain regions following global ischemia and reperfusion. Neuroscience. 2007;145:248–55. doi: 10.1016/j.neuroscience.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]