Abstract
Overactivation of glutamate receptors and subsequent deregulation of the intraneuronal calcium ([Ca2+]i) levels are critical components of the injurious pathways initiated by cerebral ischemia. Another hallmark of stroke is parenchymal acidosis, and we have previously shown that mild acidosis can act as a switch to decrease NMDAR-dependent neuronal loss while potentiating the neuronal loss mediated by AMPARs. Potentiation of AMPAR-mediated neuronal death in an acidotic environment was originally associated only with [Ca2+]i dyshomeostasis, as assessed by Ca2+ imaging; however, intracellular dyshomeostasis of another divalent cation, Zn2+, has recently emerged as another important co-factor in ischemic neuronal injury. Rises in [Zn2+]i greatly contribute to the fluorescent changes of Ca2+-sensitive fluorescent probes, which also have great affinity for Zn2+. We therefore revisited our original findings (Mcdonald et al., 1998) and investigated if AMPAR-mediated fura-2 signals we observed could also be partially due to [Zn2+]i increases. Fura-2 loaded neuronal cultures were exposed to the AMPAR agonist, kainate, in a physiological buffer at pH 7.4 and then washed either at pH 7.4 or pH 6.2. A delayed recovery of fura-2 signals was observed at both pHs. Interestingly this impaired recovery phase was found to be sensitive to chelation of intracellular Zn2+. Experiments with the Zn2+ sensitive (and Ca2+-insensitive) fluorescent probe FluoZin-3 confirmed the idea that AMPAR activation increases [Zn2+]i, a phenomenon that is potentiated by mild acidosis. Additionally, our results show that selective Ca2+ imaging mandates the use of intracellular heavy metal chelators to avoid confounding effects of endogenous metals such as Zn2+.
INTRODUCTION
Excitotoxicity is a key phenomenon that promotes neuronal demise in cerebral ischemia (1). Evidence cumulated in the past 30 years has linked ischemic neuronal death to the overactivation of glutamatergic ionotropic receptors (NMDA, N-Methyl-D-aspartate and AMPA, α-amino3-hydroxy-5-methyl-4-isoxazolepropionic-acid), a process that leads to massive Ca2+ entry into neurons and activation of a plethora of Ca2+-dependent injurious pathways. NMDAR associated channels are highly Ca2+ permeable and seem to play a prominent role in ischemic neuronal death. Most of the AMPAR associated channels are Ca2+ impermeable; nevertheless, AMPAR activation is also able to mediate neurotoxic rises of intracellular Ca2+ ([Ca2+]i) via indirect opening of the voltage sensitive Ca2+ channels (VSCC). Parenchymal acidosis is another key component of the injurious pathways set in motion by cerebral ischemia (2), as well as an important modulator of excitotoxicity. NMDAR-mediated currents are attenuated by moderate extracellular acidity in the range of pH 6.2–7.2 (3–4), and in vitro, reduction of extracellular pH below 6.5 reduces both glutamate and oxygen-glucose deprivation induced neurotoxicity (5–7). While acidosis can reduce NMDAR-dependent neuronal loss, we previously have shown that lowering the extracellular pH can enhance AMPAR-mediated neuronal injury (8). Such enhanced toxicity originally was explained partially by increased [Ca2+]i deregulation. In the Mcdonald study, we also found that neurons challenged with AMPA (under non-desensitizing conditions) undergo a phase of delayed recovery of [Ca2+]i levels when washed in an acidic (pH 6.6) buffer. Using Ca2+ imaging experiments, this delayed recovery was attributed to deregulation of [Ca2+]i homeostatic mechanisms, as evaluation of Ca2+ influx by 45Ca2+ uptake showed that influx of the cation via VSCC was decreased by extracellular acidity.
Excitotoxic neuronal loss also has been linked to the dyshomeostasis of another divalent cation, Zn2+. A large amount of data supports the idea that Zn2+ can in fact act not only as a cellular messenger, but also as a potent trigger of neuronal death through several mechanisms –including energy depletion, reactive oxygen species (ROS) generation, and mitochondrial dysfunction, reviewed in (9). Injurious [Zn2+]i rises have been linked to excitotoxic conditions such as transient global ischemia, head trauma, and epilepsy (10). Elevations of [Zn2+]i are due to a combination of influx of the cation via glutamate receptors and VSCC activation, as well as its release from intracellular sites. Intracellularly Zn2+ is loosely bound to proteins such as metal-lothioneins (MTs) and several studies have indicated that the cation can be mobilized from MTs under conditions of oxidative stress. Further studies have shown that Zn2+ also can be mobilized in a Ca2+-dependent fashion from mitochondria (11–12). Interestingly, changes in the intracellular proton concentration appear to act as a co-factor in ROS-mediated [Zn2+]i rises as a decrease in pH rapidly destabilizes Zn2+ binding to MTs (13) and promotes an overall release of the cation. This phenomenon has now been confirmed in neurons, where mild acidosis has been found to dramatically increase [Zn2+]i rises induced by MT oxidation (12).
A very intriguing debate has recently emerged in the excitotoxic field: the “Calcium-Zinc dilemma” (9,12,14–16). While for many years excitotoxicity has been interpreted as a purely Ca2+-dependent process, most of the evidence for the Ca2+-dependency derives from experimental paradigms that made use of either Ca2+ chelators or intracellular Ca2+ imaging with Ca2+ sensitive fluorescent indicators. One problem with this “Ca2+-centered” view comes from the fact that both Ca2+ fluorescent probes and chelators also bind Zn2+, and in fact do so with higher affinity. The potential confounding effect of Zn2+ on Ca2+ imaging has been neglected, moving from the assumption that [Zn2+]i levels are very low. However, as mentioned above, a wealth of evidence has been gathered in recent years demonstrating that upon patho-physiological conditions (i.e. oxidative and nitrosative stress; NMDAR activation and oxygen-glucose deprivation) [Zn2+]i rises can reach levels high enough to greatly interfere with fluorescent measurements of [Ca2+]i and/or chelation by divalent chelators (12,14,16–17). Thus, in this study, we critically reexamined our previous results (8) and investigated whether the AMPAR-mediated changes in fura-2 fluorescence signals that we originally interpreted as purely Ca2+-dependent could be also linked to [Zn2+]i rises.
MATERIALS AND METHODS
Materials
FluoZin-3 AM, fura-2 AM, and hydroethidine (Het) were purchased from Molecular Probes (Invitrogen, Milan, Italy). Carbonyl cyanide 3-chlorophenyl-hydrazone (CCCP), and N,N,N′,N′-Tetrakis-(2-pyridylmethyl)ethylene-diamine (TPEN) were obtained from Sigma-Aldrich (Milan, Italy). Tissue culture media and serum were from Gibco (Invitrogen, Milan, Italy). All other chemicals and reagents were obtained from common commercial sources.
Animal Cell Cultures
All animal procedures were approved by the institutional animal care and use committee. Murine cortical cultures were prepared from embryonic CD1 mice and neurons plated upon astrocytic monolayers on poly-lysine + laminin coated cover slips as previously described (18).
Fura-2, FluoZin-3, and HEt Microfluorimetric Studies
Fluorescence imaging was carried out using an inverted microscope with a xenon lamp, filter wheel, at 40X, epifluorescence oil immersion objective and fluorescent cubes. For ratiometric fura-2 imaging, cultures were loaded in the dark, with 3 μM of the acetoxymethyl ester form of the probe in a HEPES-buffered medium (HCSS) whose composition was (in mM): 120 NaCl, 5.4 KCl, 0.8 MgCl2, 20 HEPES, 15 glucose, 1.8 CaCl2, 10 NaOH, pH 7.4 for 30 min at 25°C, then washed in HCSS and kept in the dark for an additional 30 min, excitation was at 340 and 380 nm, with emission at 510 nm. For Zn2+ imaging, cells were loaded with the same protocol using 5 μM of FluoZin-3 AM, excitation was at 490 nm and emission at 530 nm. Experiments were carried out at room temperature (25°C). Drugs were applied by bath application and removed through a rapid flow exchange system. Images were acquired with a 12-bit digital CCD camera and analyzed (after background subtraction from a cell-free region of the dish) with Metafluor 6.0 software (Universal Imaging, West Chester, PA, USA).
Oxygen radical production was monitored using the oxidation sensitive dye, HEt. Stock HEt (1 mg/mL) was prepared as previously described (11) in dry DMSO and stored in frozen aliquots for use within eight weeks. Cultures were loaded in the dark with 5 μM HEt in HCSS (45 min, 25°C). After loading, cultures were washed (4×) in a static bath of HCSS containing 5 μM HEt. Cells were excited at 510–560 nm and emission monitored at > 590 nm.
Fura-2, FluoZin-3, and HEt fluorescence measurements for each cell (Fx) were normalized to either the fluorescence intensity (Fluozin-3 and HEt) or 340/380 ratio (fura-2) for that cell at the beginning of the experiment (F0) and changes over time expressed as percentage increase over baseline.
Experiment Replication and Statistics
All experiments reported represent at least three independent replications. Comparisons were obtained by Student-Newman-Keuls’ test (P < 0.01).
RESULTS
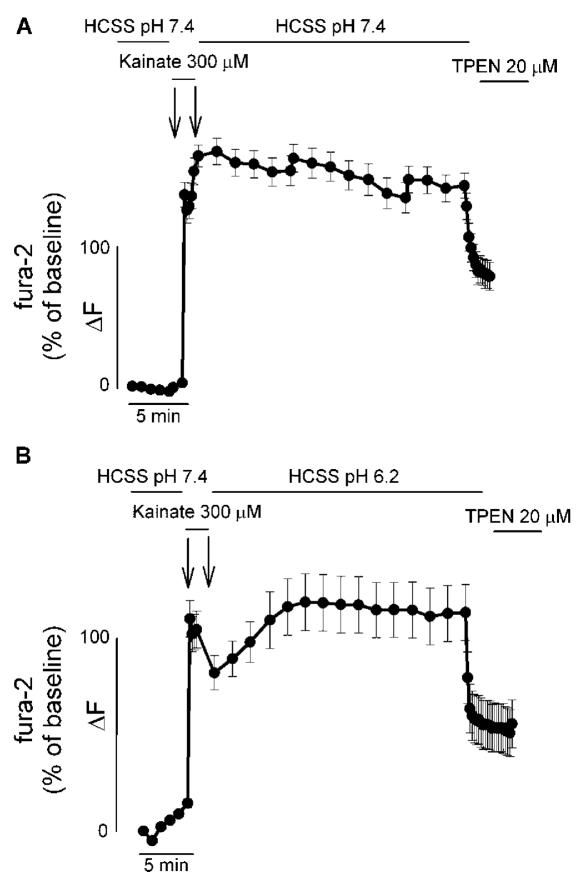
We employed an experimental paradigm similar to the one used in our original study (8). Cortical neurons were loaded with the Ca2+- (and Zn2+-) sensitive ratiometric fluorescent probe fura-2 and fluorescence changes evaluated both during a 1 min exposure to 300 μM kainate and in the washout phase (30 min at pH 7.4). At the end of the 30 min washout phase, neurons were exposed for 5 min to the cell-permeant heavy metal (and high affinity Zn2+) chelator TPEN [20 μM; Kd 10-26 (19)]. In a second set of experiments, fura-2 fluorescence changes were investigated in neurons that, after the kainate challenge at pH 7.4, were washed in an acidic buffer (pH 6.2) for 30 min and then exposed to TPEN. Surprisingly, both in the case of post-kainate washout at pH 7.4 and pH 6.2, we observed a delayed recovery in the fura-2 signal. This delayed recovery in fura-2 fluorescence was found to be largely reduced upon TPEN exposure (Figure 1A and B). When we evaluated the extent of the decrease in the fura-2 signal produced by the TPEN exposure, we found no statistical difference between the two conditions. TPEN can decrease fura-2 fluorescence by 58% (SEM ± 4.33) in neurons washed at pH 7.4, while the same maneuver at pH 6.2 produced a 53% (SEM ± 2.68) fluorescence drop. When analyzing these [Zn2+]i rises, one has to consider that potential differences could be difficult to be evaluated considering the high affinity for Zn2+of fura-2 (Kd 0.5–3 nM).
Figure 1.
AMPAR activation increases both [Ca2+]i and [Zn2+]i levels. A, B. Time course of Ca2+ and Zn2+-dependent changes in fura-2 fluorescence upon activation of AMPARs: Neuronal cultures loaded with fura-2 were imaged before, during, and after a 1 min exposure to 300 μM kainate. After the kainate challenge, neurons were washed for 30 min in a physiological buffer either at pH 7.4 (A) or pH 6.2 (B). At the end of the washout period, neurons were exposed for 5 min to the cell permeant Zn2+ chelator TPEN (20 μM). Traces show mean fura-2 ΔF (expressed as % over baseline of 340/380 nm ratios) (±SEM) of 13–24 neurons from one experiment representative of 5–6, respectively.
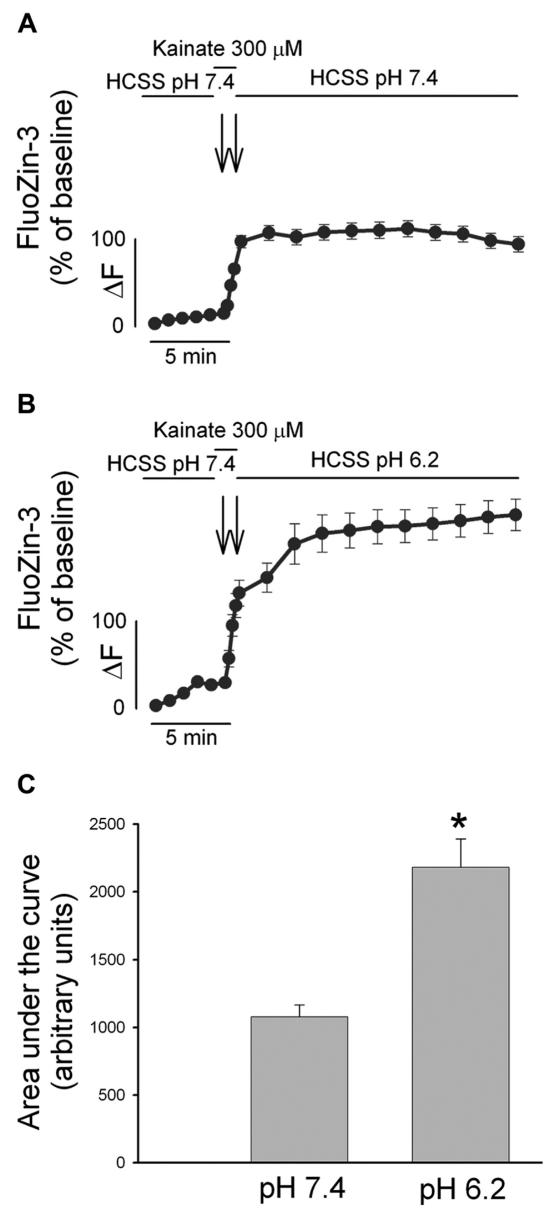
To better analyze AMPAR-mediated [Zn2+]i dyshomeostasis, we therefore loaded our cortical neurons with the Zn2+-sensitive (and Ca2+-insensitive) fluorescent probe FluoZin-3 (20). As with the fura-2 experiments, FluoZin-3 loaded neurons were imaged upon a brief excitotoxic pulse (300 μM kainate for 1 min at pH 7.4) and during a 20 min washout period either at pH 7.4 or pH 6.2 (Figure 2A and B). Integral analysis of [Zn2+]i overload occurring in the washout period revealed that post-kainate Zn2+ dyshomeostasis is enhanced by extracellular acidosis (Figure 2C).
Figure 2.
Mild acidosis enhances AMPAR-mediated [Zn2+]i rises. A, B. FluoZin-3 loaded cultures were exposed to kainate (300 μM) for 1 min and washed out in a physiological buffer either at pH 7.4 (A) or pH 6.2 (B) for 20 min. Traces show mean FluoZin-3 ΔF (±SEM) of > 50 neurons from 4–7 experiments. C. Bar graph depicts the area under the curve during the recovery phase in the two conditions, * indicates differences between washout at pH 7.4 versus washout at pH 6.2 (P < 0.01). Note that AMPAR-mediated [Zn2+]i rises are greatly enhanced upon exposure to an acidotic environment.
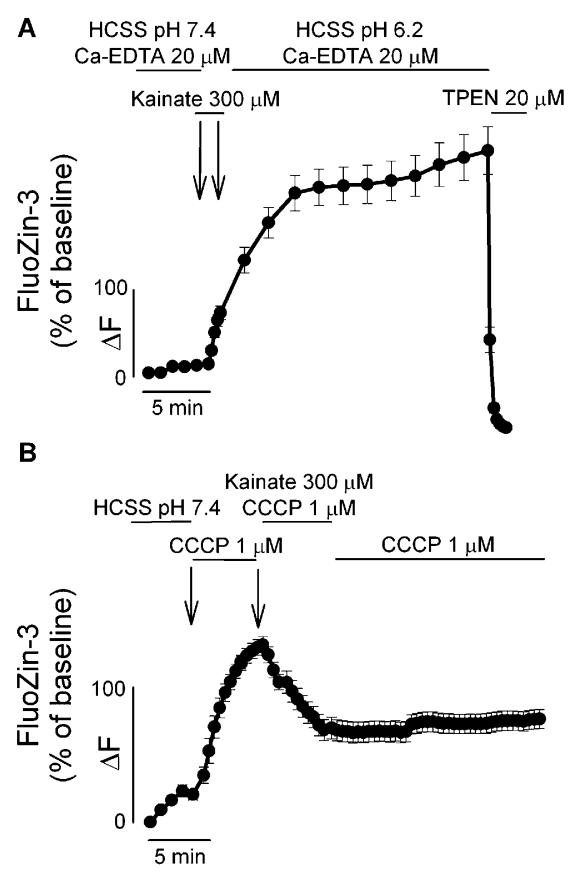
To elucidate the source of the [Zn2+]i rises triggered by kainate exposure, we performed a control experiment in which FluoZin-3 loaded neurons were challenged with kainate and washed in an acidotic buffer in the presence of the extracellular Zn2+ chelator Ca2+-EDTA (20 μM). Indicating that AMPAR activation promotes a process of intracellular mobilization of Zn2+, which is enhanced by low extracellular pH, chelation by Ca2+-EDTA of any possible contaminant Zn2+ in the extracellular medium did not attenuate the FluoZin-3 fluorescent rises observed in the washout period (Figure 3A).
Figure 3.
AMPAR-mediated [Zn2+]i rises are mainly dependent on intracellular Zn2+ mobilization. A. AMPAR-mediated [Zn2+]i rises are not modulated by extracellular Zn2+ chelation. FluoZin-3 loaded neurons were exposed, as in Figure 1, to kainate in the presence of the extracellular Zn2+ chelator Ca2+ EDTA (20 μM). Note that the [Zn2+]i rises occurring both during and after kainate exposure are not affected by extracellular chelation of contaminant Zn2+, indicating that the source of these rises is intracellular. B. AMPAR-mediated release of intra-mitochondrial Zn2+. FluoZin-3 loaded cultures were exposed for 5 min to the mitochondrial protonophore, CCCP (1 μM), challenged with 300 μM kainate for 5 min and washed at pH 7.4, in the presence of CCCP. Note that prior exposure to CCCP greatly inhibits subsequent kainate-triggered [Zn2+]i rises, indicating that these two manipulations target a common intracellular Zn2+ pool. Traces show time course of FluoZin-3 Delta;F (±SEM), and are derived from > 15 neurons from 3–5 experiments.
Mobilization of [Zn2+]i can occur from MT oxidation as well as from Zn2+ release from mitochondria (12,14,17). We first explored the possibility that AMPAR activation can induce release of Zn2+ present in mitochondria. In FluoZin-3 loaded cultures, exposure to a protonophore (CCCP; 1 μM) that collapses the mitochondrial membrane potential and, as shown before (11–12) promotes Zn2+ release from mitochondria, produced a large increase in cytosolic FluoZin-3 fluorescence. Suggesting that mitochondria are a likely source for AMPAR-mediated [Zn2+]i rises, CCCP exposure also occluded subsequent responses to kainate (Figure 3B).
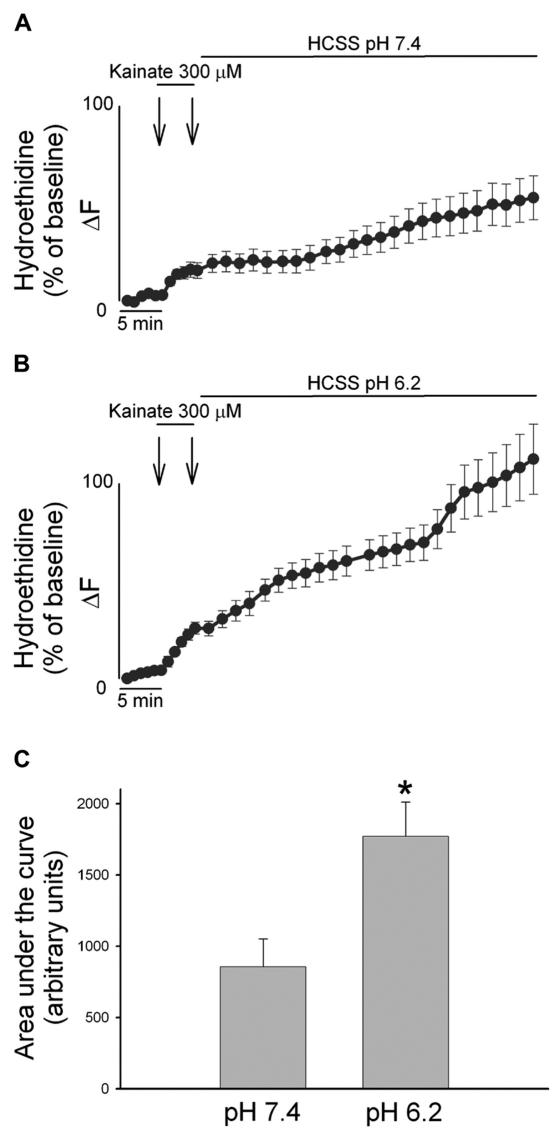
On the contrary of NMDAR, AMPAR activation has been shown previously to generate very moderate oxidative stress of mitochondrial origin (21); therefore, a ROS-dependent Zn2+ mobilization seems less likely. We examined the possibility that AMPAR stimulation (at physiological and acidotic pH) can produce different levels of cytosolic ROS in neurons loaded with the super-oxide sensitive fluorescent probe hydroethidine (HEt). Confirming the expected low oxidative stress produced by AMPAR activation under physiological conditions, Het-loaded neurons undergoing a 5 min kainate exposure showed very little increase in ROS production in the following 50 min when washed out in a buffer at pH 7.4 (Figure 4A). Interestingly, when the same excitotoxic challenge was followed by a 50 min wash at pH 6.2, ROS generation was found to be significantly higher (Figure 4B and C).
Figure 4.
AMPAR-mediated ROS generation is enhanced by low extracellular pH. A, B. Cortical cultures were loaded with HEt, and exposed for 5 min to 300 μM kainate and washed for 50 min with a buffer at pH 7.4 (A) or pH 6.2 (B). HEt fluorescence changes for each neuron are expressed as the ratio of fluorescence at each time point (Fx) to its own baseline fluorescence (F0). Traces show mean (± SEM) of 29 (A) or 40 (B) neurons from 3 experiments. C. Bar graph depicts the cumulative ROS production as area under the curve during the recovery phase in the two conditions, * indicates differences between washout at pH 7.4 versus washout at pH 6.2 (P < 0.01) Note the marked increase in HEt fluorescence only in the case of kainate exposures followed by an acidotic wash out.
DISCUSSION
Our present study complements previous Ca2+ imaging studies in both neuronal cultures and hippocampal slices and clearly indicates that excitotoxic activation of glutamate receptors leads not only to [Ca2+]i dyshomeostasis but also to a significant [Zn2+]i mobilization. This idea is substantiated by the interpretation of the increases in both fura-2 ratios and FluoZin-3 fluorescence (quenched by TPEN) that we observed. Fura-2 has a low affinity for Mg2+ (Kd 6–10 mM) and exhibits an affinity for Zn2+ that is nearly 100-fold higher than its affinity for Ca2+(Kd 0.5–3 nM). Upon Zn2+ binding, fura-2 fluorescence undergoes changes similar to what is observed with Ca2+ (i.e. increases of the 340/380 nm excitation ratio). On the other hand, fura-2 binding to transition metals likely to be intracellularly released in significant amounts, such as manganese, ferrous and ferric iron, and copper results in different levels of quenching of the probe fluorescence.
FluoZin-3 is a Zn2+ selective indicator that exhibits high Zn2+ binding affinity (Kd for Zn2+ ~15 nM), that is unperturbed by Ca2+ and Mg2+ concentrations up to at least 0.1 mM and 1 M respectively (20). As with fura-2, iron and copper binding to FluoZin-3 produce quenching of the probe fluorescence. Thus, the only metal that could promote TPEN-sensitive increases in both fura-2 ratios and FluoZin-3 fluorescence is Zn2+.
Our findings reinforce the idea that glutamate receptor overactivation can trigger a synergistic injurious interplay between Ca2+ and Zn2+. Considering the fact that Zn2+ is able to induce neuronal loss with greater potency compared with Ca2+, the cation might soon emerge as the major ionic determinant of excitotoxicity. Indeed, given the fact that significant [Zn2+]i release from mitochondria can result from large, NMDAR-triggered [Ca2+]i rises, and the likely probability that Ca2+-induced mitochondrial ROS generation could also trigger Zn2+ mobilization from MTs, we might envision a pathological setting in which glutamate receptor-driven [Ca2+]i rises actually play a more modest injurious role than previously thought. Instead of being the executioner, [Ca2+]i dyshomeostasis can actually serve as an “accomplice” to deregulate the intracellular concentrations of the primary ionic mediator of neuronal injury, Zn2+.
This study also integrates the main results of our previous experiments with the novel finding that reduction of extracellular pH to levels of parenchymal acidosis associated with cerebral ischemia (2) enhances AMPAR-mediated Zn2+ dyshomeostasis.
The connection between Zn2+ and acidosis is intriguing as pH reduction can affect many Zn2+ homeostatic systems. The permeability of major routes of Zn2+ entry, such as the VSCC and Ca2+-permeable AMPAR, is enhanced at acidic pH, while the Ca2+ permeability of these channels (as well as NMDAR permeability) has been shown to be decreased under the same conditions (22–23). Zn2+ uptake and compartmentalization by Zinquin-positive cytosolic organelles is blocked by intracellular acidification, indicating the presence of a putative intracellular Zn2+/proton antiporter (24), and previous studies have also indicated that an acidotic environment can greatly destabilize Zn2+ binding to MTs and therefore promote [Zn2+]i accumulation (13). Interestingly, Zn2+ dyshomeostasis itself can alter the intracellular acid-base equilibrium and promote a feed-forward dyshomeostatic loop. Findings in cultured neurons suggest that Zn2+ can in fact favor intracellular acidification in a Ca2+-dependent fashion through an increased activity of the Na+/Ca2+ exchanger, and also maintain such acidification by Zn2+-dependent inhibition of proton efflux via the Cl-/HCO3- exchanger (25). Finally, as observed in our study, prolonged intracellular acidosis could further evoke [Zn2+]i dyshomeostasis by interfering with the overall neuronal redox state, either by reducing the activity of cellular antioxidant enzymes or increasing hydroxyl radical formation (26).
In our study, it appears that the acidotic/AMPAR-mediated [Zn2+]i rises observed in neuronal cultures can be attributed to combined mechanisms of mitochondrial release of the cation together with impaired buffering by MTs. The fact that mitochondrial depolarization prior to the kainate exposure completely occludes AMPAR-mediated [Zn2+]i increases seems to indicate that most of the Zn2+ is released from that subcellular compartment (Figure 3B). On the other hand, the impaired Zn2+ buffering under acidotic conditions that follows AMPAR activation appears to be related to a synergistic inhibition on Zn2+ binding to MTs that is promoted by protons either directly (13), or indirectly, by enhancing AMPAR-mediated ROS production. This process eventually leads to MT oxidation and further decreased Zn2+ buffering.
On a speculative note, our model, which emphasizes the role played by acidosis and AMPAR-mediated Zn2+ dyshomeostasis in excitotoxicity, might be relevant for the timing and selection of pharmacological intervention in neurological conditions associated with both acidosis and alteration of [Zn2+]i content such as stroke, status epilepticus, and trauma. While hours away from the is-chemic attack when the acid-base equilibrium is restored, NMDAR antagonists maintain a very important therapeutic value, AMPAR antagonists might result as a better option immediately after the insult given the fact that NMDARs are already blocked by post-ictal parenchymal acidosis (27). The importance of AMPAR antagonism has been demonstrated recently by an elegant series of studies in which blockade of the Ca2+-permeable AMPARs has been found to be neuroprotective both in “in vitro” and “in vivo” models of cerebral ischemia (28–30). Work by our laboratory and the groups of Suzanne Zukin and Mike Bennett has convincingly demonstrated that, in the post-ischemic period, blockade of Ca-A/K channels by spermine derivatives can greatly inhibit neuronal death in the CA1 hippocampal region by counteracting both [Zn2+]i accumulation and Zn2+-dependent injurious processes.
Targeting Zn2+ dyshomeostasis might also require further exploration, as recent animal studies involving the use of pyruvate to restore Zn2+-dependent injurious depletion of cytosolic NAD+ levels showed a dramatic neuroprotective effect in the context of both global and focal cerebral ischemia (31–32).
A final technical issue raised by our findings concerns the need for highly selective Ca2+-sensitive fluorescent probes. The warning contained in the paper by Grynkiewicz, Poenie and Tsien (33) indicating the high sensitivity of Ca2+-sensitive fluorescent probes to heavy metals has been neglected. It is now clear that mobilization of intracellular Zn2+ can be a significant contributor to the fluorescence changes of Ca2+ fluorescent probes. This effect should no longer be ignored nor Ca2+ dependency claimed without a prior careful exclusion of Zn2+-dependent phenomena.
ACKNOWLEDGMENTS
We thank Tiziana D’Ettorre and Francesca Faricelli for expert assistance with cell cultures. This work was supported by PRIN 2004, FIRB 2003, and PRIN 2006 (SLS).
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 2.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 3.Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+ Proc Natl Acad Sci U S A. 1990;87:6445–9. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traynelis S, Cull-Candy S. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–50. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- 5.Giffard RG, Monyer H, Christine CW, Choi DW. Acidosis reduces NMDA receptor activation, glutamate neurotoxicity, and oxygen-glucose deprivation neuronal injury in cortical cultures. Brain Res. 1990;506:339–42. doi: 10.1016/0006-8993(90)91276-m. [DOI] [PubMed] [Google Scholar]
- 6.Tombaugh GC, Sapolsky RM. Mild acidosis protects hippocampal neurons from injury induced by oxygen and glucose deprivation. Brain Res. 1990;506:343–5. doi: 10.1016/0006-8993(90)91277-n. [DOI] [PubMed] [Google Scholar]
- 7.Kaku DA, Giffard RG, Choi DW. Neuro-protective effects of glutamate antagonists and extracellular acidity. Science. 1993;260:1516–8. doi: 10.1126/science.8389056. [DOI] [PubMed] [Google Scholar]
- 8.McDonald JW, et al. Extracellular acidity potentiates AMPA receptor-mediated cortical neuronal death. J Neurosci. 1998;18:6290–9. doi: 10.1523/JNEUROSCI.18-16-06290.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sensi SL, Jeng JM. Rethinking the excitotoxic ionic milieu: the emerging role of Zn(2+) in ischemic neuronal injury. Curr Mol Med. 2004;4:87–111. doi: 10.2174/1566524043479211. [DOI] [PubMed] [Google Scholar]
- 10.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–62. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 11.Sensi SL, Ton-That D, Weiss JH. Mitochondrial sequestration and Ca(2+)-dependent release of cytosolic Zn(2+) loads in cortical neurons. Neurobiol Dis. 2002;10:100–8. doi: 10.1006/nbdi.2002.0493. [DOI] [PubMed] [Google Scholar]
- 12.Sensi SL, et al. Modulation of mitochondrial function by endogenous Zn2+ pools. PNAS. 2003;100:6157–62. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang LJ, Vasak M, Vallee BL, Maret W. Zinc transfer potentials of the alpha- and beta-clusters of metallothionein are affected by domain interactions in the whole molecule. Proc Natl Acad Sci U S A. 2000;97:2503–8. doi: 10.1073/pnas.97.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossy-Wetzel E, et al. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41:351–65. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 15.Devinney MJ, 2nd, Reynolds IJ, Dineley KE. Simultaneous detection of intracellular free calcium and zinc using fura-2FF and FluoZin-3. Cell Calcium. 2005;37:225–32. doi: 10.1016/j.ceca.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Stork CJ, Li YV. Intracellular zinc elevation measured with a “calcium-specific” indicator during ischemia and reperfusion in rat hippocampus: a question on calcium overload. J Neurosci. 2006;26:10430–7. doi: 10.1523/JNEUROSCI.1588-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aizenman E, et al. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–88. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 18.Yin HZ, Weiss JH. Zn(2+) permeates Ca(2+) permeable AMPA/kainate channels and triggers selective neural injury. Neuroreport. 1995;6:2553–6. doi: 10.1097/00001756-199512150-00025. [DOI] [PubMed] [Google Scholar]
- 19.Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+ J Biol Chem. 1985;260:2719–27. [PubMed] [Google Scholar]
- 20.Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium. 2002;31:245–51. doi: 10.1016/S0143-4160(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 21.Dugan LL, et al. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci. 1995;15:6377–88. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerchner G, Canzoniero L, Yu S, Ling C, Choi DW. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J Physio. 2000;528:39–52. doi: 10.1111/j.1469-7793.2000.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeng J-M, Jia Y, Bonanni L, Weiss JH. SFN Annual Meeting. Washington, DC: Society for Neuroscience, Orlando, FL; 2002. Divergent effects of pH on Zn2+ and Ca2+ flux through Ca2+-permeable AMPA/kainate channels (CAKR) p. 539. [Google Scholar]
- 24.Colvin RA. pH dependence and compartmentalization of zinc transported across plasma membrane of rat cortical neurons. Am J Physiol Cell Physiol. 2002;282:C317–29. doi: 10.1152/ajpcell.00143.2001. [DOI] [PubMed] [Google Scholar]
- 25.Dineley KE, Brocard JB, Reynolds IJ. Elevated intracellular zinc and altered proton homeostasis in forebrain neurons. Neuroscience. 2002;114:439–49. doi: 10.1016/s0306-4522(02)00294-4. [DOI] [PubMed] [Google Scholar]
- 26.Ying W, Han SK, Miller JW, Swanson RA. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J Neurochem. 1999;73:1549–56. doi: 10.1046/j.1471-4159.1999.0731549.x. [DOI] [PubMed] [Google Scholar]
- 27.Gill R, Lodge D. Pharmacology of AMPA antagonists and their role in neuroprotection. Int Rev Neurobiol. 1997;40:197–232. doi: 10.1016/s0074-7742(08)60721-7. [DOI] [PubMed] [Google Scholar]
- 28.Yin HZ, Sensi SL, Ogoshi F, Weiss JH. Blockade of Ca2+-permeable AMPA/kainate channels decreases oxygen-glucose deprivation-induced Zn2+ accumulation and neuronal loss in hippocampal pyramidal neurons. J Neurosci. 2002;22:1273–1279. doi: 10.1523/JNEUROSCI.22-04-01273.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calderone A, et al. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J Neurosci. 2004;24:9903–13. doi: 10.1523/JNEUROSCI.1713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noh KM, et al. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc Natl Acad Sci U S A. 2005;102:12230–5. doi: 10.1073/pnas.0505408102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JY, Kim YH, Koh JY. Protection by pyruvate against transient forebrain ischemia in rats. J Neurosci. 2001;21:RC171. doi: 10.1523/JNEUROSCI.21-20-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi JS, Kim TY, Kyu Kim D, Koh JY. Systemic pyruvate administration markedly reduces infarcts and motor deficits in rat models of transient and permanent focal cerebral ischemia. Neurobiol Dis. 2007;26:94–104. doi: 10.1016/j.nbd.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]