Abstract
Labeling for zinc transporter protein-3 (ZnT-3), which can be found localized to glutamatergic vesicles elsewhere in the nervous system, has revealed an unexpectedly high concentration of this transporter protein in the outer limiting membrane region of the murine retina, a region that contains the mitochondria-rich portion of photoreceptor inner segments and is not involved with vesicle release. Having suggested the possibility that Müller cell apical villi forming the outer limiting membrane may be associated with the labeling observed, we used immunohistochemical techniques to look for ZnT-3 labeling of Müller cells isolated from rat and mouse retinas. With DAB labeling, rat Müller cell apical villi, soma, and endfeet exhibited ZnT-3 reactivity. FITC label and confocal analysis revealed that ZnT-3 protein appeared throughout the length of mouse Müller cells. We conclude from these observations that the dense labeling for ZnT-3 in the photoreceptor inner segment region of murine retinal slices is due to labeling of ZnT-3 protein associated with Müller cell apical villi. Based on these findings we suggest that Müller cells utilize ZnT-3 to regulate retinal zinc homeostasis and that this role is important to mitochondrial function in the photoreceptor inner segments.
INTRODUCTION
Zinc transporter-protein-3 (ZnT-3) has 6 transmembrane domains with a histidine-rich cytoplasmic loop responsible for mediating zinc transport. Studies of the central nervous system shown that ZnT-3 is localized to glutamatergic- and tyrosine hydroxilase-positive vesicles. We initially used immunohistochemical techniques to examine the distribution of the ZnT-3 protein in vertical sections of the light-adapted mouse retina (1). The ZnT-3 protein appeared in lateral bands in the regions of the outer limiting membrane, photoreceptor inner segments, outer plexiform, inner nuclear, inner plexiform, and ganglion cell layers. The outer nuclear layer and photoreceptor outer segments did not exhibit immunoreactivity. In the present study, to clarify cellular ZnT-3 localization, we isolated murine retinal neurons and antibody-labeled them for ZnT-3.
The murine ZnT-3 gene has eight exons and maps to chromosome 5 in mice (2). The ZnT-3 protein has 388 amino acids, which form six transmembrane domains enclosing a pore lined with a histidine-rich loop active in zinc transport (3). The protein localizes primarily to glutamate and tyrosin-hydroxilase positive zinc-enriched vesicles in neural tissue (2,4). The concentration of zinc within ZnT-3 localized glutamatergic vesicles has been estimated to be up to 1 mM (5). Release of zinc into synaptic clefts of zinc-enriched terminals has been measured at concentrations of 10–30 μM with possible extreme rises to 300 μM (6). Regions in the mouse nervous system, which have labeled for the ZnT-3 protein, include hippocampus, amygdala, spinal cord, and superior cervical ganglia (4,7,8).
The transport mechanism of ZnT-3 functions via electrogenic antiport, exchanging one hydrogen for one zinc ion (3). Cellular zinc influx has been shown to exhibit pH dependence with a peak influx near pH 7.4 and inhibition of influx occurring at pH 6.0 (9). Neurons demonstrated decreased zinc uptake with extracellular acidification and increased zinc uptake with intracellular acidification (9).
The murine retina has been shown to localize zinc in photoreceptor soma in dark-adapted states and in photoreceptor inner segments in light-adapted states (10). Zinc with an unbound charge capable of being labeled is also found in rat retinal pigment epithelium, outer plexiform, inner plexiform, inner nuclear, and ganglion cell layers in both light- and dark-adapted conditions (10). The concentration of zinc in retinal cells other than photoreceptors remains relatively stable between light and dark conditions.
In our prior study labeling mouse retinal slices for ZnT-3, we noted that the lateral banding pattern of immunoreactivity mirrored regions containing Müller cell apical villi, soma, and endfeet (1). In the current study we demonstrate cellular ZnT-3 localization to Müller cells by antibody labeling of isolated rat and mouse retinal cells as described below.
MATERIALS AND METHODS
Microscope slide covers (Fisher-12-541A; Fisher Scientific, Pittsburgh, PA, USA) were prepared with poly-L-lysine and plated in 200 μL of Ringer solution (pH 7.4) containing [in mM] NaCl [137], KCl [5], CaCl2 [2], MgSO4 [1], Na2HPO4 [1], HEPES [10], and glucose [22]. Six adult C57BL\6 mice and four Long Evans rats were killed. Their eyes were removed and placed in ice-cold Ringer solution. By cutting along the ora serrata with a microscissor, we removed the cornea and lens. Eyecups were submerged in Ringer solution in 35×10 mm petri dishes, and retinas were teased out of the eyecups with forceps. Retinas were then rotated slowly for one hour in 35×10 mm dishes containing: papain (2 mg/mL) and L-cysteine (1 mg/mL) in 3 mL of Ringer solution. Retinas were transferred with a sterile 10-mL glass pipette to a 15-mL tube containing 3 mL of fresh Ringer solution and rinsed eight times.
After Ringer rinse, cells were isolated by repeating the triturations from 8 to up to 64 times and plating two to three drops of supernatant onto treated slide covers. From each dissociated retina, 10 slide covers of isolated cells were prepared for labeling. Cells were allowed to settle and adhere for one hour and then fixed with 4% paraformaldehyde. Cells were rinsed three times with 0.1 M PBS, pH 7.4. The cells were then rinsed with 0.6% Brij-58 (Sigma-P5884) for 20 min, then rinsed in PBS three times. Next, cells were rinsed two times in 0.05 M Tris-HCl buffer with 0.15 M NaCl, pH 7.4, (Fluka-93318).
Isolated cells were incubated in blocking buffer in TBS containing 3% BSA, 3% goat serum, and 0.025% DMSO for one hour, then rinsed with TBS for three 10-min periods. Cells were then incubated in affinity-purified rabbit anti-mouse ZnT-3 antibody at concentrations of 1:20–1:100 for one hour and rinsed with TBS for four 10-min periods. This procedure was followed by incubation in goat anti-rabbit FITC label for one hour (Sigma-F9887). Finally, cells were rinsed in TBS for three 10-min periods and then once in PBS for 10 min. Slide covers were sealed to slides, and cells imaged by use of a Zeiss confocal microscope.
RESULTS
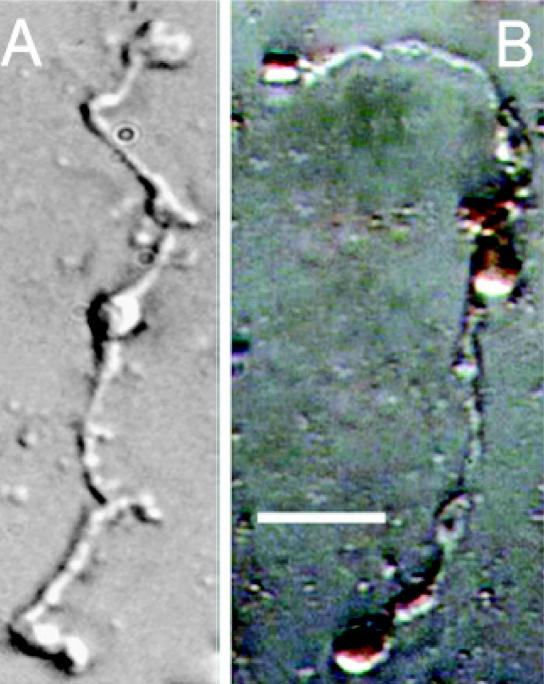
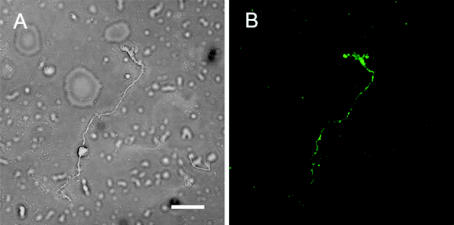
Of the isolated retinal cell types studied, only Müller cells showed significant labeling for the ZnT-3 antibody. In each slide with visible antibody reactivity, 6–8 labeled Müller cells were identifiable. In the majority of DAB-labeled rat retinal Müller cells, the protein localizations appeared slightly denser in the apical villi and endfeet regions (Figure 1). Processes and somas of Müller cells showed labeling with slightly lower concentration and intensity. FITC labeled mouse retinal Müller cells showed fluorescence along the entire length of these transretinal cells (Figure 2).
Figure 1.
DAB-labeled isolated rat Müller cells viewed using conventional light microscopy. (A) Control DAB-processed rat Müller cell in the absence of the ZnT-3 antibody showing no labeling. (B) ZnT-3-DAB-labeled Müller cell viewed using conventional light microscopy. Müller cell apical villi, soma, and endfeet show dark brown labeling indicating the presence of the ZnT-3 protein. Scale bar: 40 μm.
Figure 2.
FITC labeled ZnT-3 localization of mouse Müller cell viewed using confocal microscopy. (A) White light. (B) Fluorescent FITC. The ZnT-3 protein appears to be distributed ubiquitously in the FITC-labeled Müller cells. Slightly higher concentrations of fluorescent label appeared in endfoot and apical regions, while Müller cell somas typically labeled with lower intensities than other regions of the cell, as shown here. Scale bar: 40 μm.
In our earlier study of mouse-slice ZnT-3, antibody banding appeared primarily in regions in which Müller cell apical villi, soma, and endfeet are found. Outer and inner plexiform regions were also labeled with weaker labeling in the inner plexiform layer. In this study the ZnT-3 protein appeared to be expressed throughout the Müller cell. Confocal three-dimensional reconstruction showed that cells exhibited label in all areas of the cell membrane. This finding seems to indicate that ZnT-3 may be used in a novel way as a zinc transporter by Müller cells.
Presumed rod and cone somas treated under the identical labeling protocol as Müller cells exhibited no ZnT-3 localization. Presumptive photoreceptor cells were identified by small ~4-μm diameter soma and highly abundant distribution (11). Bipolar cell soma and processes also appeared to be clear of ZnT-3 reactivity. Other retinal neurons with indistinct morphology appeared less often and exhibited no reactivity.
In summary, in preparations of ZnT-3 DAB-labeled isolated retinal cells, Müller cell apical villi, soma, and endfeet exhibited reactivity. With FITC label and confocal analysis of isolated cells, ZnT-3 protein appeared to be distributed throughout the Müller cell. Neither bipolar cells nor photoreceptor somas appeared to label for the ZnT-3 protein.
DISCUSSION
ZnT-3 is a member of the cation diffusion facilitator (CDF) family of zinc transport proteins involved in zinc efflux out of the cytoplasm into intracellular compartments or across cell membranes (3). The ZIP (ZRT, IRT-like protein) family contains another group of zinc transporters responsible for influx. Evidence suggests that both transport families function at zinc concentrations less than 100 μM while neurons are at rest. In depolarized states with zinc concentrations above 100 μM, influx occurs through channel pathways (9). Our results indicate the presence of ZnT-3 on Müller cells. It is probable that this particular transport protein functions in concert with other zinc transporters and channels in the retina.
Based on these findings it seems plausible that Müller cells are utilizing the ZnT-3 protein to assist with mediating zinc transport. The glutamate GLAST (L-glutamate/L-aspartate transporter) localizes only to Müller cells in all retinal layers (12), and glutamate and zinc appear in corresponding retinal layers. Thus, Müller cell zinc transport may work in a fashion similar to glutamate transport by GLAST. Interestingly, in the rat retina, high levels of reactive zinc are observed in photoreceptor soma only in dark-adapted preparations, whereas high levels of reactive zinc in the region of the photoreceptor inner segments are observed only in light-adapted states (10). Müller cells are in constant contact with photoreceptors and may be involved in light-induced retinal zinc redistribution from proximal to more distal regions.
The band of reactive zinc noted in the region of the photoreceptor inner segments in light-adapted rat retina (10) is of special interest with respect to the ZnT-3–rich apical processes of Müller cells located in this region. Although this region is not known to have an abundance of vesicles that might be associated with ZnT-3 transporter, approximately 70% of retinal mitochondria are located in the photoreceptor inner segments (13). Zinc has been shown to enter mitochondria and modulate respiration via metallothionein (14). Mitochondria also release submicromolar levels of zinc in response to depolarization in a calcium-dependent manner (15,16). In the region of inner segments, mitochondria and high concentrations of zinc are colocalized (10,13), and the Müller cell apical processes are available to regulate zinc homeostasis.
Several electrophysiological studies have demonstrated zinc sensitivity in a variety of cell and receptor subtypes throughout the retina (17–20). It has been proposed that zinc is stored with glutamate in photoreceptor terminals and released to function as a neuromodulator (17). In regions where zinc is released from synaptic terminals, the ZnT-3 laden Müller cell may take in excess zinc to prevent zinc toxicity or simply to maintain a gradient of zinc concentration in the extracellular space.
Müller cells utilize K+ transporters in endfoot regions to establish homeostasis between vitreous and neural retina (21). Heavy ZnT-3 labeling in conical Müller cell endfeet suggests that Müller cells could also be involved in regulating zinc homeostasis between the vitreous and neural retina. Hence, our observation of ZnT-3 label associated with Müller cells isolated from rat and mouse retinas suggests that these cells may be actively involved retinal zinc transport. Elucidating the function of ZnT-3 localized to retinal Müller cells will require further investigation. On the basis of our current study results and those of related studies of zinc localization and physiology, it appears likely that Müller cells in the murine retina utilize the ZnT-3 transporter to facilitate retinal zinc localization. Based on these findings it seems possible that ZnT-3 transporter may be utilized by Müller cells to regulate retinal zinc homeostasis.
ACKNOWLEDGMENTS
The authors express their appreciation to Dr. Richard D Palmiter for providing the primary ZnT-3 antibody used in these studies. This study was supported in part by Fight for Sight, NSF grant 0615987, PSC/CUNY grant 68490-0037, and NCRR/NIH RCMI award RR-03037.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Redenti S, Chappell RL. Localization of zinc transporter-3 (ZnT-3) in mouse retina. Vision Res. 2004;44:3317–21. doi: 10.1016/j.visres.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 2.Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93:14934–9. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ZY, Danscher G, Dahlstrom A, Li JY. Zinc transporter 3 and zinc ions in the rodent superior cervical ganglion neurons. Neuroscience. 2003;120:605–16. doi: 10.1016/s0306-4522(03)00419-6. [DOI] [PubMed] [Google Scholar]
- 5.Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr. 2000;130:1471S–83S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RB, Peterson D, Mahoney W, et al. Fluorescent zinc indicators for neurobiology. J Neurosci Methods. 2002;118:63–75. doi: 10.1016/s0165-0270(02)00144-9. [DOI] [PubMed] [Google Scholar]
- 7.Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci U S A. 1997;94:12676–81. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danscher G, Wang Z, Kim YK, Kim SJ, Sun Y, Jo SM. Immunocytochemical localization of zinc transporter 3 in the ependyma of the mouse spinal cord. Neurosci Lett. 2003;342:81–4. doi: 10.1016/s0304-3940(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 9.Colvin RA. pH dependence and compartmentalization of zinc transport across the plasma membrane of rat cortical neurons. Am J Cell Physiol. 2002;282:C317–29. doi: 10.1152/ajpcell.00143.2001. [DOI] [PubMed] [Google Scholar]
- 10.Ugarte M, Osborne NN. The localization of free zinc varies in rat photoreceptors during light and dark adaptation. Exp Eye Res. 1999;69:459–61. doi: 10.1006/exer.1999.0727. [DOI] [PubMed] [Google Scholar]
- 11.Koulen P, Kuhn R, Wässle H, Brandstätter JH. Modulation of the intracellular calcium concentration in photoreceptor terminals by a presynaptic metabotropic glutamate receptor. Proc Natl Acad Sci U S A. 1999;96:9909–14. doi: 10.1073/pnas.96.17.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada T, Harada C, Watanabe M, et al. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc Natl Acad Sci U S A. 1998;95:4663–6. doi: 10.1073/pnas.95.8.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkins GA, Ellisman MH, Fox DA. Three-dimensional analysis of mouse rod and cone mitochondrial cristae architecture: bioenergetic and functional implications. Mol Vis. 2003;9:60–73. [PubMed] [Google Scholar]
- 14.Ye B, Maret W, Valee BL. Zinc metallothionein imported into liver mitochondria inhibits respiration. Proc Natl Acad Sci U S A. 2001;98:2317–22. doi: 10.1073/pnas.041619198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sensi SL, Ton-That D, Weiss JH. Mitochondrial sequestration and Ca(2+)-dependent release of cytosolic Zn(2+) loads in cortical neurons. Neurobiol Dis. 2002;10:100–8. doi: 10.1006/nbdi.2002.0493. [DOI] [PubMed] [Google Scholar]
- 16.Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A. 2003;100:6157–62. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu SM, Qiao X, Noebels JL, Yang XL. Localization and modulatory actions of zinc in vertebrate retina. Vision Res. 1993;33:2611–6. doi: 10.1016/0042-6989(93)90219-m. [DOI] [PubMed] [Google Scholar]
- 18.Qian H, Li L, Chappell RL, Ripps H. GABA receptors of bipolar cells from the skate retina: actions of zinc on GABA-mediated membrane currents. J Neurophysiol. 1997;78:2402–12. doi: 10.1152/jn.1997.78.5.2402. [DOI] [PubMed] [Google Scholar]
- 19.Han Y, Wu SM. Modulation of glycine receptors in retinal ganglion cells by zinc. Proc Natl Acad Sci U S A. 1999;96:3234–8. doi: 10.1073/pnas.96.6.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian H, Malchow P, Chappell RL, Ripps H. Zinc enhances ionic currents induced in skate Müller (glial) cells by the inhibitory neurotransmitter GABA. Proc R Soc (Lond) B Biol Sci. 1996;263:791–6. doi: 10.1098/rspb.1996.0118. [DOI] [PubMed] [Google Scholar]
- 21.Reichenbach A, Robinson SR. The involvement of Müller cells in the outer retina. In: Djamgoz MBA, Archer SN, Vallerga S, editors. Neurobiology and Clinical Aspects of the Outer Retina. Chapman & Hall; London: 1995. pp. 395–416. [Google Scholar]