Abstract
Ischemic stroke is one of the most pervasive life-threatening neurological conditions for which there currently exists limited therapeutic intervention beyond prevention. As calcium-focused neuroprotective strategies have met with limited clinical success, it is imperative that alternative therapeutic targets be considered in the attempt to antagonize ischemic-mediated injury. As such, zinc, which is able to function both as a signaling mediator and neurotoxin, has been implicated in cerebral ischemia. While zinc was first purported to have a role in cerebral ischemia nearly twenty years ago, our understanding of how zinc mediates ischemic injury is still in its relative infancy. Within this review, we examine some of the studies by which zinc has exerted either neuroprotective or neurotoxic effects during global and focal cerebral ischemia.
Worldwide, stroke is a leading cause of long-term disability and the most common life-threatening neurological disorder (1). While stroke is a heterogeneous condition that encompasses a variety of etiologies, most strokes result from the obstruction of an intra-cranial artery by a thrombus (2). To date, thrombolytic interventions, which aim to lyse clots and restore blood flow to compromised brain regions, represent the only effective treatment for ischemic strokes. Nevertheless, administration of tissue plasminogen activator (tPA), the only approved thrombolytic agent, is constrained by its limited therapeutic window of three hours and by complications derived from hemorrhagic risks, reperfusion injury, and its own intrinsic toxicity (3). Furthermore, the potential for neuroprotective therapies, which aim to antagonize glutamate-induced excitotoxicity or neuronal death mediated by calcium dyshomeostasis, have met with limited clinical success (4). However, effective and alternative therapeutic interventions may be unveiled from a more comprehensive understanding of the biochemical changes mediating ischemic brain injury. As such, calcium may not be the only divalent metal cation involved in ischemia. Rather, recent evidence suggests that calcium may serve as an accomplice to zinc, a possibly more potent ionic mediator of ischemic injury (5–8). While zinc and calcium may rely upon common pathways to penetrate and injure cells, recent data also suggests that toxic elevations in intracellular calcium levels, may in part or in its entirety, be induced by zinc (7,8).
Nearly two decades ago, zinc was first implicated in the pathogenesis of ischemia and more than twenty additional in vivo studies have since examined the role of zinc during both global and focal experimental paradigms. Some of these studies are summarized in Table 1 and are discussed below. From these studies, zinc has been reported to possess both neurotoxic and neuroprotective capabilities during experimentally-induced ischemia. However, as can be seen in Table 1, few of the findings from these studies are directly comparable, largely owing to considerable diversity in study design, such as variations in models and duration of ischemia, species and strain, and dosage, route, and regimen of either zinc chelators or zinc supplements.
Table 1.
Overview of in vivo Studies Investigating the Role of Zinc during Cerebral Ischemia
| Species/Model | Therapeutic Intervention | Assessment Methods/Main Finding(s) | Reference |
|---|---|---|---|
| Rat/Global ischemia; 4-VOa (20 min occlusion). | – | TSQb & acid fuschin staining between 2 and 24 h post-ischemia/At 2 h: TSQ-stained somata in CA4, diminished TSQ-signal in mossy fibers; at 18 h: TSQ-stained cells corresponded to acidophilic cells. | (9) |
| Rat/Focal ischemia; tMCAOc (60 min occlusion). | ZnPPd (1–10 μg); topical application. | Brain edema (wet/dry weight method) assessed at 24 h post-ischemia/Modest but significant reduction in brain edema with ZnPP treatment. | (24) |
| Rat/Global ischemia; 4-VO (20 min occlusion). | Pre-txe & intra-txf hypothermia (29°C) started 1h prior and maintained for ischemia. | TSQ staining 2 h to 7 days post-ischemia/Ischemia at 37°C: TSQ-stained CA3 neuronal bodies present at 2 to 24 h; ischemia at 29°C: TSQ-stained cell bodies absent. | (10) |
| Rat/Focal ischemia; pMCAOg or tMCAO (2 h occlusion). | ZnPP (50 mg/kg) i.p., pre-tx: 30 min(pMCAO & tMCAO); post-txh: 2 or 4 h (tMCAO). | Infarct volume & brain edema assessed 24 h post-ischemia/ZnPP pre-tx in pMCAO: no effect; ZnPP post-tx in tMCAO: no effect; ZnPP pre-tx in tMCAO: dramatic neuroprotective effect reducing infarct volume & brain edema. | (26) |
| Rat/Global ischemia; 2-VOi (15 min occlusion). | Ca-EDTA 300 mM (5 μL; i.c.v.), pre-tx: 30 min. | TSQ & acid fuschin staining 72 h post-ischemia/Znj accumulation leads to degeneration; Ca-EDTA reduced Zn accumulation & degeneration of CA1 neurons. | (11) |
| Rat/Focal ischemia; tMCAO (2 h occlusion) | ZnCl2k (10 mg/kg; i.p.), PPl (48.5 mg/kg; i.p.), ZnPP (50 mg/kg; i.p.), pre-tx: 30 min. | Infarct volume & brain edema assessed 24 h post-ischemia/ZnCl2 reduced infarct volume but had no effect on brain edema; ZnPP and PP: both significantly reduced infarct volume and brain edema. | (27) |
| Gerbil/Global ischemia; BCCAOm (3 min occlusion). | ZnCl2 (20 mg/kg; s.c), pre-tx: 1 h, or 48 and 24 h. | TUNELn and H-Eo at 3 and 4 days, respectively/1 h ZnCl2 pre-tx: no effect; 48 and 24 h ZnCl2 pre-tx: modest but significant protection of CA1 region. | (28) |
| Gerbil/Global ischemia; BCCAO (5 min occlusion). | – | Examined ZnT-1 mRNA expression between 12 h to1 week post ischemia/ZnT-1 mRNA expression was induced in CA1 neurons exhibiting Zn accumulation. Without subsequent ZnT-1 protein expression, these cells started to degenerate at 72 h. | (12) |
| Rat/Focal ischemia; pMCAO. | – | Neo-Timm stain to show synaptic Zn levels from 7 min to 7 days post-ischemia/Decrease in Zn staining at 7 min and continued throughout 7 days; at 1 h, Zn-positive cell bodies seen. | (31) |
| Rat/Global ischemia; 2-VO (15 min occlusion). | Ca-EDTA 300 mM (3 μL; i.c.v.); pre-tx: 30 min. | TFL-Znp and TUNEL staining 24, 48, and 72 h post-ischemia/Ca-EDTA markedly reduced Zn accumulation and degeneration of CA1 neurons at all time points. | (14) |
| Rat/Global ischemia; 2-VO (12 min occlusion). | Sodium Pyruvate (500 mg/kg; i.p.); pre-tx: 30 min; post-tx: 08, 0.5, 1, 2, 3 h. | TFL-Zn & TUNEL staining 3, 15, and 30 days post-ischemia/Sodium pyruvate almost completely blocked neuronal injury when given within 1 h of ischemia. | (16) |
| Rat/Focal ischemia; tMCAO (30 min occlusion – mild ischemia) or (60 min occlusion – severe ischemia). | Ca-EDTA 500 mM (5 μL; i.c.v.).; pre-tx: 15 min or post-tx: continuous infusion for 1 week (10–100 nmole/h) 1 μL/h; i.c.v. | TSQ, H-E, acid fuschin, CM1-IHCr staining 3 h to 14 days post-ischemia/Ca-EDTA pre-tx reduced intracellular Zn accumulation, infarct volume, and degeneration at 3 days but protective effects lost at 14 days or if insult intensity increased (30 min occlusion to 60 min occlusion) and if Ca-EDTA was continuously infused for 7 days. | (17) |
| Mouse/Global ischemia; BCCAO (20 min occlusion). | Hypothermia (33°C) initiated at occlusion; continued 35 min. | TSQ & H-E staining 72 h post-ischemia/Hypothermia markedly reduced Zn accumulation and degeneration of CA1, CA2, and CA4 neurons. | (18) |
| Mouse/Focal ischemia photothrombosis. | – | Infarct volumes & ZnSeAMG s assessed 30 min to 24 h post-ischemia/Infarct core devoid of Zn by 0.5 h until 24 h; 20% increase in Zn in peri-infarct region up to 6 h; Lesions markedly larger at later (12 and24 h) than at earlier (0.5–6 h) times. | (33) |
| Rat/Focal ischemia; Embolic MCAO. | ZnCl2 (80μmol/kg; i.p.); Bicuculline (48.5 μmol/kg; i.p.) or the above in combination; pre-tx: 30 min. | Infarct volume & brain edema assessed 48 h post-ischemia/ZnCl2 alone or ZnCl2 and Bicuculline: dramatically increased infarct volume, increased brain edema and worsened neurological deficits; Bicuculline alone: no effect. | (19) |
| Gerbil/Focal ischemia; right pMCAO. | – | Micro-dialysis collected 0–3 h post-ischemia; analyzed with GF-AASt/Zn levels dropped 75% of baseline and never recovered in ipsilateral hemisphere and only minimally changed in contralateral hemisphere. | (35) |
| Gerbil/Global ischemia BCCAO (5 min occlusion) and Rat/Global ischemia4-VO (10 min occlusion). | Ca-EDTA 300 mM (5 μL; i.c.v.); pre-tx: 30 min or post-tx: 3, 6, 48, 60, or 72 h. | GluR2 mRNA and protein expression, Caspase-3-activity, TSQ, TUNEL48 h to 5 days post-ischemia/Ca-EDTA pre-tx: reduced Zn levels, GluR2 down-regulation, and early stages of apoptosis in CA1 cells; Ca-EDTA Post-tx between 48 and 60 h: rescued CA1 cells by blocking the later stages of apoptosis (DNA fragmentation). | (22) |
| Rabbit/Global ischemia; inflatable neck tourniquet& systemic hypoperfusion(30 min occlusion). | – | Micro-dialysis collected 4 to 6 h post-ischemia; analyzed with pZn meter/Ischemia induced immediate rise in extracellular Zn levels from baseline (19 nM) in hippocampus. Reperfusion induced greater rise in extracellular Zn levels (~100 nM). Glutamate release was earlier and shorter in duration than Zn release | (38) |
| Rat/Focal ischemia tMCAO (60 min occlusion). | Ca-EDTA 100 mM (5 μL; i.c.v.); pre-tx: 30 min | Infarct volume assessed 3, 6, & 24 h post-ischemia/Ca-EDTA pre-tx: deleterious effect on early infarct development: larger infarct volumes at 3 and 6 h but similar to control at 24 h. | (30) |
| Rat/Global ischemia 4-VO(30 min occlusion). | – | TSQ staining 0 to 24 h post-ischemia/Zn accumulation in CA1 cells at 24 h but not before. Micro-dialysis collected 0 to 3h post-ischemia; analyzed with flameless AASu. Extracellular Zn levels in CA1 area increased to~600 nM within 15 min of occlusion, decreased during reperfusion, and returned to basal levels (~300 nM). | (36) |
| Rat/Focal ischemia tMCAO(60 min occlusion). | – | Micro-dialysis collected 0 to 3 h post-ischemia; analyzed with flameless AAS/Extracellular Zn levels increased to ~300 nM within 15 min of occlusion, decreased during reperfusion, and returned to basal levels (~150 nM). | (37) |
4-VO, 4-vessel occlusion.
TSQ, N-[6-methoxy-8-quinolyl]-P-toluenesulfonamide.
tMCAO, transient middle cerebral artery occlusion.
ZnPP, zinc protoporphyrin.
pre-tx, pre-treatment.
intra-tx, intra-treatment.
pMCAO, permanent middle cerebral artery occlusion.
post-tx, post-treatment.
2-VO, 2-vessel occlusion.
Zn, zinc.
ZnCl2, zinc chloride.
PP, protoporphyrin.
BCCAO, bilateral common carotid occlusion.
TUNEL, Terminal Deoxynucleotidyl Transferase Mediated dUTP Nick End Labeling.
H-E, hematoxylin and eosin stain.
TFL-Zn, N-(6-methoxy-8quinolyl)-P-carboxybenzoylsulfonamide.
“0” hr refers to the time coinciding with the onset of reperfusion
CM1-IHC, antibody against activated caspase-3 immunohistochemistry.
ZnSeAMG, zinc-selenium autometallography technique.
GF-AAS, graphite furnace atomic absorption spectroscopy.
AAS, atomic absorption spectroscopy.
NEUROTOXICITY OF ZINC IN ISCHEMIA
Tonder and colleagues (9) conducted the first study providing indirect evidence for the toxic translocation of zinc from presynaptic neurons into selective postsynaptic neurons during the experimental paradigm of global ischemia. TSQ (N-[6-methoxy-8-quinolyl]-P-toluenesulfonamide) and acid fuschin staining were used in conjunction to compare changes in zinc staining with the occurrence of degenerating or acidophilic cells between 2 and 24 h post-ischemia. Although degeneration of the cornu ammonis 1 (CA1) subfield was not observed due to the acute survival period of this study, the distribution of TSQ-cell stained bodies of CA4, which were observed as soon as 2 h post-ischemia, corresponded with the distribution of degenerating neurons observed beginning at 18 h post-ischemia. The concomitant decrease in TSQ fluorescence of the mossy fiber terminals and the intracellular accumulation in the CA4 neurons strongly implicated the toxic translocation of zinc.
Johansen and colleagues (10), as a follow-up to the original study conducted by Tonder and others (9) found that intra-ischemic hypothermia (29°C) prevented ischemic-induced intracellular zinc accumulation and subsequent cellular demise, likely by inhibiting zinc translocation.
More direct evidence for the translocation of zinc was provided by the findings of Koh and others (11), who demonstrated that during a brief period of global ischemia, intracellular zinc accumulation in vulnerable CA1 pyramidal hippocampal neurons preceded degeneration, which could be prevented with the intracerebroventricular administration of the high affinity, membrane-impermeable zinc-chelator, ethylenediaminetetraacetic acid (EDTA) saturated with calcium (Ca-EDTA). This finding convincingly postulated that selective neuronal death of CA1 neurons during global ischemia was mediated by the release of synaptic vesicle zinc from a subset of excitatory terminals and its subsequent translocation into vulnerable post-synaptic neurons. Furthermore, intracellular zinc accumulation preceded neuronal degeneration, which could be prevented with the administration of an extracellular zinc chelator.
In follow-up to the findings of Koh and others (11), Tsuda and colleagues (12) examined the induction of ZnT-1 mRNA expression in the CA1 subfield of the hippocampus following global ischemia. It was postulated that in response to the increased levels of intracellular zinc, vulnerable neurons would up-regulate ZnT-1, the plasma membrane-localized zinc transporter that facilitates zinc efflux (13). While ZnT-1 mRNA expression was enhanced as soon as 12 h post-ischemia, without subsequent ZnT-1 protein expression, cellular demise ensued by three days post-ischemia.
Park and colleagues (14) demonstrated that zinc-mediated neuronal death following ischemia may be achieved through the specific induction of p75NTR and its associated death executor, NADE. Following global ischemia, p75NTR and NADE induction was detected in degenerating CA1 pyramidal neurons exhibiting dense zinc accumulation. The co-induction of p75NTR and NADE was found to be dependent upon zinc levels because the administration of Ca-EDTA completely blocked the induction of p75NTR and NADE and subsequent neurodegeneration of CA1 pyramidal neurons.
Culture studies have demonstrated that zinc neurotoxicity may promote the disruption of different stages of cellular respiration through depletion of ATP and the oxidized form of the coenzyme, nicotinamide adenine dinucleotide (NAD+) (15). Animal studies have demonstrated that the administration of pyruvate, the end metabolite of glycolysis, can achieve neuroprotection by normalizing metabolic disturbances and antagonizing zinc neurotoxicity following global ischemia (16). Post-ischemic supplementation with pyruvate was found to provide remarkable, long-lasting neuroprotection when administered within 1 h after the onset of reperfusion (16).
Lee and colleagues (17) found that the intracerebroventricular administration of Ca-EDTA prior to mild focal ischemia achieved early neuroprotection against zinc accumulation and subsequent cellular demise. This effect, however, was lost if either the ischemic insult was more pronounced, if the survival period was extended to two weeks post-ischemia, or if Ca-EDTA treatment was continuously administered.
While Johansen and others (10) demonstrated that deep hypothermia (29°C) reduces interneuronal zinc movement and subsequent death, Tsuchiya and colleagues (18) investigated the impact of mild hypothermia (33°C) on zinc release and associated neuronal death following global ischemia. Mild intra-ischemic hypothermia was found to markedly reduce zinc accumulation and associated degeneration of hippocampal neurons 72 h following ischemia.
Shabanzadeh and colleagues (19) found that intraperitoneal pre-treatment of zinc alone or in conjunction with the GABAA antagonist, bicuculline had detrimental effects on neurological deficits and the development of the infarct following induction of focal ischemia.
It is now well established that there is an ischemic-mediated regulation of the subunit composition of calcium A/K channels (20). The GluR2 hypothesis postulates that reduced GluR2 expression allows for the toxic calcium, and even zinc entry during ischemia (5,21). Calderone and others (22) demonstrated that zinc plays an integral role in this regulation through its persistent downregulation of GluR2 mRNA (22). Increasing cytosolic zinc levels, for instance, have been shown to induce the expression of a zinc-finger transcription factor REST (restrictive element-1 silencing transcription factor), which is able to suppress neural-specific target genes, including GluR2 (22,23). Calderone and colleagues (22) further demonstrated that during ischemia, zinc triggers neuronal death through temporally distinct mechanisms. It was discovered that intracerebroventricular pre-treatment with Ca-EDTA significantly attenuated the ischemia-induced down-regulation of GluR2 mRNA and protein expression in the CA1 hippocampal subfield. Ca-EDTA pre-treatment further blocked the early stages of apoptosis in CA1 neurons by reducing levels of cytochrome c and caspase 3 activity and DNA fragmentation. Furthermore, Ca-EDTA, when administered between 48 and 60 h post-ischemia, also prevented zinc accumulation and degeneration of CA1 pyramidal neurons, likely by blocking the later stages of apoptosis, but not when administered at 3, 6, or 72 h post-ischemia.
While the above studies demonstrate that elevated intracellular zinc levels during ischemia serve as a critical mediator of neuronal death, zinc inhibition achieved through either early or late chelation paradigms may be effective in combating zinc neurotoxicity.
NEUROPROTECTION BY ZINC IN ISCHEMIA
In contrast to chelation-based therapeutic intervention, various studies have demonstrated neuroprotective benefits following the administration of various zinc compounds. Yamasaki and colleagues (24), for example, examined the effectiveness of zinc protoporphyrin (ZnPP) in mediating post-ischemic brain edema by selectively blocking the cytokine, interleukin-1 (IL-1). In this study, following transient focal ischemia, ZnPP was topically applied to the lateral ventricle at the onset of reperfusion. Brain edema was assessed 24 h later using the wet and dry method (25) and the topical application of ZnPP was found to reduce ischemic brain edema significantly by blocking IL-1 activity.
As a follow-up investigation into the reported anti-inflammatory effects of ZnPP demonstrated by Yamasaki and others (24), Kadoya and colleagues (26) demonstrated that ZnPP was limited in its level of neuroprotection by the temporal parameters of its administration and whether or not reperfusion followed the onset of ischemia. Specifically, intraperitoneal ZnPP pretreatment was found to significantly reduce infarct volume and post-ischemic brain edema in the transient model of ischemia (tMCAO). However, the neuroprotective effects of ZnPP were lost if either the severity of the ischemic insult increased (permanent MCAO) or if treatment was delayed two or four hours following ischemic onset.
In a follow-up study to Kadoya and others (26), Zhao and colleagues (27) set out to determine whether the zinc or protoporphyrin complement of ZnPP possessed neuroprotective capabilities. Equimolar doses of zinc chloride (ZnCl2), PP, and ZnPP were all found to reduce the lesion size, but only ZnPP and PP were found to ameliorate ischemic brain edema. As such, this study suggests that zinc ions, in comparison to protoporphyrin, provide neuroprotection by mechanisms other than reducing brain edema.
Matsushita and colleagues (28) later demonstrated that zinc supplementation could provide neuroprotection to the CA1 hippocampal subfield during global ischemia in the gerbil. Because the gerbil possesses isolated cerebral hemispheres and an incomplete circle of Willis, the bilateral occlusion of the common carotid arteries (BCCAO) results in pronounced global ischemia (29). It was found that while superacute (1 h) subcutaneous pretreatment with ZnCl2 had no effect, subacute (48 and 24 h) pretreatment afforded significant neuroprotection to vulnerable CA1 cells against delayed neuronal death.
Recently, Kitamura and colleagues (30) have found that reduction in zinc levels following intracerebroventricular injection of Ca-EDTA prior to focal ischemia accelerated the early development of the infarct, suggesting the need for a minimum complement of zinc to maintain cellular viability, even during cerebral ischemia.
CHANGES IN ZINC LEVELS DURING ISCHEMIA
Alternatively, other studies have attempted to monitor dynamic changes in zinc levels during cerebral ischemia in the absence of therapeutic interventions. Sorensen and colleagues (31) set out to examine if altered levels of synaptic vesicle zinc during the course of focal ischemia could be detected histochemically (32). Relying on the neo-Timm stain, it was demonstrated that as soon as 7 min following ischemic onset, zinc positive-terminal staining was visibly decreased within the ischemic region and was relatively absent for all remaining times examined, up to seven days post-ischemia. The rapid reduction and eventual absence of zinc staining was attributed to the likely release of zinc from synaptic vesicles. The emergence of zinc-stained neurons at one hour post-ischemia may reflect zinc accumulation following trans-synaptic movement of zinc from surrounding zinc-enriched terminals.
Similarly, our laboratory has examined the temporo-spatial changes in synaptic vesicle zinc levels following focal ischemia using the zinc-selenium autometallography technique (33). Utilizing the technique of photothrombosis (34), we found that the core of the infarct was devoid of zinc staining up until 24 h post-ischemia, the last time point examined, while the peri-infarct region showed a significant increase (at least 20%) in staining intensity up to six hours post-ischemia. Interestingly, infarct volumes were found to be significantly larger, at least double in size, at the latter time points (12 and 24 h) compared with infarct volumes assessed at the earlier time points (30 min, 1–6 h). We also verified these findings in a transient model of focal ischemia to confirm the vascular access of sodium selenite. The reason for the elevated levels of synaptic zinc staining within the periphery of the infarct is currently under investigation but may represent a compensatory mechanism to buffer the release of synaptic vesicle zinc and, moreover, may even delineate the putative penumbral region.
In addition to utilizing histochemical stains or fluorescent dyes to measure changes in zinc levels during ischemia, detection using micro-dialysis has also been employed. Using dual probe micro-dialysis coupled with graphite furnace atomic absorption spectroscopy (MD-GFAAS), Yang and colleagues (35) detected a significant decrease in zinc levels from baseline in the ipsilateral cortical hemisphere and slight changes in the contralateral hemisphere during focal ischemia. Although this study suggested that extracellular zinc levels drop during ischemia, more recent micro-dialysis studies suggest that while a temporal derangement in extracellular zinc levels during ischemia occurs, levels are increasing rather than decreasing.
Kitamura and colleagues (36) examined the temporal release profile of extra-cellular zinc using micro-dialysis and also examined subsequent intracellular zinc accumulation in the CA1 subfield of the hippocampus during global ischemia. Within 15 min post-ischemia, extracellular zinc levels reached a peak (~600 nM) that was double the basal level. Subsequently, extracellular zinc levels decreased and returned to baseline 15 min following reperfusion. A similar release profile also was found for glutamate, although glutamate levels reached peak level within 30 min and were more than 20× the basal level. Additionally, using TSQ fluorescence, evidence for intracellular zinc-accumulation was found within the CA1 pyramidal neurons but not before 24 h post-ischemia, suggesting that the release of zinc from synaptic vesicle stores is neither excessive nor immediately accumulated in vulnerable post-synaptic neurons. Similarly, Kitamura and colleagues (37) also found that extra-cellular zinc levels in the focal ischemic cortex increased within 15 min, peaked to twice the basal level (~300 nM) within 30 min post-ischemia, and returned to basal level within 15 min after reperfusion.
Recently, Frederickson and colleagues (38) also have used micro-dialysis to examine extracellular zinc levels during global ischemia and reperfusion. Following ischemic onset, extracellular zinc levels increased and were synchronous with glutamate release. However, the reperfusion-induced zinc release was more pronounced (> 100 nM, in some cases), in both intensity and duration than the initial ischemic-induced zinc release. Moreover, the delayed reperfusion-induced zinc release was unaccompanied by glutamate release, possibly reflecting a release of zinc from intracellular stores, such as metallothioneins (39–41) or mitochondria (42–44).
Although the predominance of available literature from in vivo ischemic studies has focused on the involvement of synaptic vesicle zinc, it is likely that intracellular zinc accumulation during ischemia is achieved through a possible synergism of zinc release from both synaptic and intracellular stores of zinc, as most recently demonstrated during global ischemia (38) and reported in other experimental paradigms (45–51).
CONCLUSION
Despite recognizing the involvement of zinc in cerebral ischemia nearly twenty years ago, we are only beginning to unravel the physiological functions of zinc during ischemia. Considering the multimodal impact that zinc has on cellular physiology, undoubtedly intricate, overlapping, and even synergistic mechanisms are liable to account for its toxic or protective capabilities (5,6,52–54; Figure 1).
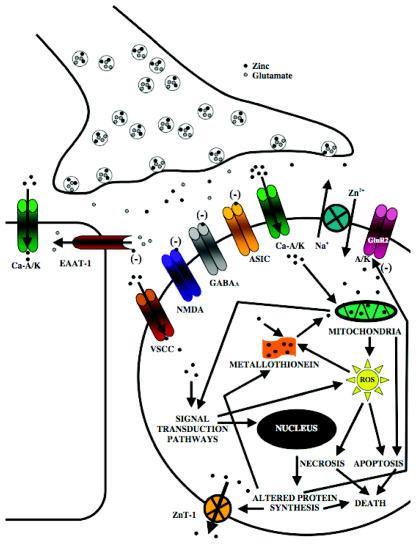
Figure 1. Schematic Overview of the Putative Toxic and Protective Mechanisms Elicited by Zinc during Cerebral Ischemia.
During ischemia, heightened release of zinc from a subset of glutamatergic terminals likely promotes the translocation and accumulation of zinc in vulnerable post-synaptic neurons. Following release, synaptic zinc is thought to achieve cellular access predominately through subpopulations of calcium-permeable AMPA and/or kainate channels (Ca-A/K) (56–58). Zinc entry may also be facilitated by the zinc-sodium exchanger and less predominately through voltage sensitive calcium channels (VSCC) or NMDA-type glutamate receptors (56,57,59,60). Intense cytosolic zinc overloads, likely mediated by Ca-A/K receptor channels, can promote pronounced mitochondrial dysfunction and reactive oxygen species (ROS) generation to trigger necrosis, whereas milder cytosolic zinc loads may augment apoptotic pathways (43,57,61–63). The cellular oxidative stress and acidosis achieved during ischemia may additionally promote the liberation of zinc from zinc-ligands, such as metallothioneins (39,49,64,65). In the attempt to confer resistance to the rising cytosolic zinc levels, the zinc transporter, ZnT-1 and metallothionien III can be upregulated during ischemia to promote zinc efflux and cytosolic buffering, respectively (12,66,67). Zinc also plays an integral role in the subunit expression of Ca-A/K channels during ischemia by altering transcriptional regulation that leads to GluR2 subunit downregulation, the presence of which renders A/K receptor channels calcium-impermeable (20–23). Zinc also can activate signal transduction pathways, such as protein kinase C, which can promote ROS generation (68,69). Zinc also can augment glutamate-induced neuronal injury by directly inhibiting GABAA channels and inhibiting glutamate re-uptake by blocking excitatory amino acid transporters (EAAT-1) expressed on glial cells (70–72). During ischemia, activation of Ca-A/K receptor channels, acidosis, and elevated zinc levels can also work synergistically to promote glial injury (73). Conversely, during ischemia, zinc also may achieve protective effects by substantially inhibiting calcium influx by blocking NMDA-type glutamate receptor channels or acid-sensing ion channels (ASICs) (74–80). Zinc also can exert anti-apoptotic efforts through the inhibition of various caspases, pro-apoptotic genes, and endonucleases (81–83).
While the mechanisms by which zinc mediates or prevents ischemic-induced injury are complex, conceivably, as is the case for calcium, cells are also expected to possess a specific zinc set-point, by which too little or too much zinc can promote cellular demise (5). As such, studies examining either zinc chelation or supplementation should heed the delicate balance by which zinc achieves its toxic or protective capabilities. Moreover, in view of the clinical importance of zinc in mediating ischemic injury, future investigation is warranted to develop more effective, potentially zinc-based therapies. To this effect, a novel lipophilic BAPTA diester, DP-b99 already has shown remarkable clinical promise in treating patients with ischemia. The success of DP-b99 in the laboratory and in the clinic, thus far, has, in part, been attributed to the delicate manner by which it buffers and redistributes zinc ions (55).
ACKNOWLEDGMENTS
This work was supported by operating grants from the Natural Sciences and Engineering Research Council of Canada (NSERC; RHD) and the Canadian Institutes of Health Research (RHD), and graduate scholarship from NSERC (SLG).
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Rothwell PM. The high cost of not funding stroke research: a comparison with heart disease and cancer. Lancet. 2001;357:1612–6. doi: 10.1016/s0140-6736(00)04730-9. [DOI] [PubMed] [Google Scholar]
- 2.Caplan LR. Caplan’s Stroke: A Clinical Approach. Butterworth-Heinemann; Boston, Massachusetts: 2000. 556. [Google Scholar]
- 3.Bambauer KZ, Johnston SC, Bambauer DE, Zivin JA. Reasons why few patients with acute stroke receive tissue plasminogen activator. Arch Neurol. 2006;63:661–4. doi: 10.1001/archneur.63.5.661. [DOI] [PubMed] [Google Scholar]
- 4.Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 5.Sensi SL, Jeng JM. Rethinking the excitotoxic ionic milieu: the emerging role of Zn(2+) in ischemic neuronal injury. Curr Mol Med. 2004;4:87–111. doi: 10.2174/1566524043479211. [DOI] [PubMed] [Google Scholar]
- 6.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–62. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 7.Stork CJ, Li YV. Intracellular zinc elevation measured with a “calcium-specific” indicator during ischemia and reperfusion in rat hippocampus: a question on calcium overload. J Neurosci. 2006;26:10430–7. doi: 10.1523/JNEUROSCI.1588-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin JL, Stork CJ, Li YV. Determining zinc with commonly used calcium and zinc fluorescent indicators, a question on calcium signals. Cell Calcium. 2006;40:393–402. doi: 10.1016/j.ceca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Tonder N, Johansen FF, Frederickson CJ, Zimmer J, Diemer NH. Possible role of zinc in the selective degeneration of dentate hilar neurons after cerebral ischemia in the adult rat. Neurosci Lett. 1990;109:247–52. doi: 10.1016/0304-3940(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 10.Johansen FF, Tonder N, Berg M, Zimmer J, Diemer NH. Hypothermia protects somatostatinergic neurons in rat dentate hilus from zinc accumulation and cell death after cerebral ischemia. Mol Chem Neuropathol. 1993;18:161–72. doi: 10.1007/BF03160030. [DOI] [PubMed] [Google Scholar]
- 11.Koh JY, et al. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–6. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 12.Tsuda M, et al. Expression of zinc transporter gene, ZnT-1, is induced after transient forebrain ischemia in the gerbil. J Neurosci. 1997;17:6678–84. doi: 10.1523/JNEUROSCI.17-17-06678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14:639–49. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JA, Lee JY, Sato TA, Koh JY. Co-induction of p75NTR and p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the rat. J Neurosci. 2000;20:9096–9103. doi: 10.1523/JNEUROSCI.20-24-09096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheline CT, Behrens MM, Choi DW. Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J Neurosci. 2000;20:3139–46. doi: 10.1523/JNEUROSCI.20-09-03139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JY, Kim YH, Koh JY. Protection by pyruvate against transient forebrain ischemia in rats. J Neurosci. 2001;21:RC171. doi: 10.1523/JNEUROSCI.21-20-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JM, et al. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience. 2002;115:871–8. doi: 10.1016/s0306-4522(02)00513-4. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya D, et al. Mild hypothermia reduces zinc translocation, neuronal cell death, and mortality after transient global ischemia in mice. J Cereb Blood Flow Metab. 2002;22:1231–8. doi: 10.1097/01.wcb.0000037995.34930.F5. [DOI] [PubMed] [Google Scholar]
- 19.Shabanzadeh AP, Shuaib A, Yang T, Salam A, Wang CX. Effect of zinc in ischemic brain injury in an embolic model of stroke in rats. Neurosci Lett. 2004;356:69–71. doi: 10.1016/j.neulet.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 20.Bennett MV, et al. The GluR2 hypothesis: Ca(++)-permeable AMPA receptors in delayed neurodegeneration. Cold Spring Harb Symp Quant Biol. 1996;61:373–84. [PubMed] [Google Scholar]
- 21.Pellegrini-Giampietro DE, Gorter JA, Bennett MV, Zukin RS. The GluR2 (GluR-B) hypothesis: Ca(2+)-permeable AMPA receptors in neurological disorders. Trends Neurosci. 1997;20:464–70. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- 22.Calderone A, et al. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J Neurosci. 2004;24:9903–13. doi: 10.1523/JNEUROSCI.1713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calderone A, et al. Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci. 2003;23:2112–21. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamasaki Y, et al. Possible involvement of interleukin-1 in ischemic brain edema formation. Neurosci Lett. 1992;142:45–7. doi: 10.1016/0304-3940(92)90616-f. [DOI] [PubMed] [Google Scholar]
- 25.Hatashita S, Hoff JT, Salamat SM. Ischemic brain edema and the osmotic gradient between blood and brain. J Cereb Blood Flow Metab. 1988;8:552–9. doi: 10.1038/jcbfm.1988.96. [DOI] [PubMed] [Google Scholar]
- 26.Kadoya C, Domino EF, Yang GY, Stern JD, Betz AL. Preischemic but not postischemic zinc protoporphyrin treatment reduces infarct size and edema accumulation after temporary focal cerebral ischemia in rats. Stroke. 1995;26:1035–8. doi: 10.1161/01.str.26.6.1035. [DOI] [PubMed] [Google Scholar]
- 27.Zhao YJ, Yang GY, Domino EF. Zinc protoporphyrin, zinc ion, and protoporphyrin reduce focal cerebral ischemia. Stroke. 1996;27:2299–303. doi: 10.1161/01.str.27.12.2299. [DOI] [PubMed] [Google Scholar]
- 28.Matsushita K, et al. Effect of systemic zinc administration on delayed neuronal death in the gerbil hippocampus. Brain Res. 1996;743:362–5. doi: 10.1016/s0006-8993(96)01112-2. [DOI] [PubMed] [Google Scholar]
- 29.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura Y, et al. Protective effect of zinc against ischemic neuronal injury in a middle cerebral artery occlusion model. J Pharmacol Sci. 2006;100:142–8. doi: 10.1254/jphs.fp0050805. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen JC, Mattsson B, Andreasen A, Johansson BB. Rapid disappearance of zinc positive terminals in focal brain ischemia. Brain Res. 1989;812:265–9. doi: 10.1016/s0006-8993(98)00943-3. [DOI] [PubMed] [Google Scholar]
- 32.Danscher G. Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electronmicroscopy. Histochemistry. 1981;71:1–16. doi: 10.1007/BF00592566. [DOI] [PubMed] [Google Scholar]
- 33.Subramaniam S, Barber PA, Hoyte L, Buchan AM, Dyck RH. Pre-synaptic Zinc Dynamics in Permanent and Transient Focal Ischemia. Ann Neurol. 2003;54:S65. [Google Scholar]
- 34.Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- 35.Yang DY, et al. The determination of brain magnesium and zinc levels by a dual-probe microdialysis and graphite furnace atomic absorption spectrometry. J Am Coll Nutr. 2004;23:552S–5S. doi: 10.1080/07315724.2004.10719402. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura Y, et al. In vivo measurement of presynaptic Zn2+ release during forebrain ischemia in rats. Biol Pharm Bull. 2006;29:821–3. doi: 10.1248/bpb.29.821. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura Y, et al. Release of vesicular Zn2+ in a rat transient middle cerebral artery occlusion model. Brain Res Bull. 2006;69:622–5. doi: 10.1016/j.brainresbull.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Frederickson CJ, et al. Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp Neurol. 2006;198:285–295. doi: 10.1016/j.expneurol.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Maret W. Metallothionein/disulfide interactions, oxidative stress, and the mobilization of cellular zinc. Neurochem Int. 1995;27:111–7. doi: 10.1016/0197-0186(94)00173-r. [DOI] [PubMed] [Google Scholar]
- 40.Erickson JC, Hollopeter G, Thomas SA, Froelick GJ, Palmiter RD. Disruption of the metallothionein-III gene in mice: analysis of brain zinc, behavior, and neuron vulnerability to metals, aging, and seizures. J Neurosci. 1997;17:1271–81. doi: 10.1523/JNEUROSCI.17-04-01271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Irie Y, Keung WM, Maret W. S-nitrosothiols react preferentially with zinc thiolate clusters of metallothionein III through transnitrosation. Biochemistry. 2002;41:8360–7. doi: 10.1021/bi020030+. [DOI] [PubMed] [Google Scholar]
- 42.Sensi SL, Yin HZ, Weiss JH. AMPA/kainate receptor-triggered Zn2+ entry into cortical neurons induces mitochondrial Zn2+ uptake and persistent mitochondrial dysfunction. Eur J Neurosci. 2000;12:3813–8. doi: 10.1046/j.1460-9568.2000.00277.x. [DOI] [PubMed] [Google Scholar]
- 43.Sensi SL, et al. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A. 2003;100:6157–62. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sensi SL, Ton-That D, Weiss JH, Rothe A, Gee KR. A new mitochondrial fluorescent zinc sensor. Cell Calcium. 2003;34:281–4. doi: 10.1016/s0143-4160(03)00122-2. [DOI] [PubMed] [Google Scholar]
- 45.Aizenman E, et al. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 46.Lee JY, Cole TB, Palmiter RD, Koh JY. Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: evidence against synaptic vesicle origin. J Neurosci. 2000;20:RC79. doi: 10.1523/JNEUROSCI.20-11-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frederickson CJ, Cuajungco MP, LaBuda CJ, Suh SW. Nitric oxide causes apparent release of zinc from presynaptic boutons. Neuroscience. 2002;115:471–4. doi: 10.1016/s0306-4522(02)00399-8. [DOI] [PubMed] [Google Scholar]
- 48.Lee JY, Kim JH, Palmiter RD, Koh JY. Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol. 2003;184:337–47. doi: 10.1016/s0014-4886(03)00382-0. [DOI] [PubMed] [Google Scholar]
- 49.Bossy-Wetzel E, et al. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41:351–65. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 50.Land PW, Aizenman E. Zinc accumulation after target loss: an early event in retrograde degeneration of thalamic neurons. Eur J Neurosci. 2005;21:647–57. doi: 10.1111/j.1460-9568.2005.03903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavoie N, et al. Extracellular chelation of zinc does not affect hippocampal excitability and seizure-induced cell death. J Physiol. 2006;578:275–89. doi: 10.1113/jphysiol.2006.121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss JH, Sensi SL, Koh JY. Zn(2+): a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- 53.Frederickson CJ, Bush AI. Synaptically released zinc: physiological functions and pathological effects. Biometal. 2001;14:353–66. doi: 10.1023/a:1012934207456. [DOI] [PubMed] [Google Scholar]
- 54.Capasso M, Jeng JM, Malavolta M, Mocchegiani E, Sensi SL. Zinc dyshomeostasis: a key modulator of neuronal injury. J Alzheimers Dis. 2005;8:93–108. doi: 10.3233/jad-2005-8202. [DOI] [PubMed] [Google Scholar]
- 55.Rosenberg G, Angel I, Kozak A. Clinical pharmacology of DP-b99 in healthy volunteers: first administration to humans. Br J Clin Pharmacol. 2005;60:7–16. doi: 10.1111/j.1365-2125.2005.02378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin HZ, Weiss JH. Zn(2+) permeates Ca(2+) permeable AMPA/kainate channels and triggers selective neural injury. Neuroreport. 1995;6:2553–6. doi: 10.1097/00001756-199512150-00025. [DOI] [PubMed] [Google Scholar]
- 57.Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A. 1999;96:2414–9. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin HZ, Sensi SL, Ogoshi F, Weiss JH. Blockade of Ca2+-permeable AMPA/kainate channels decreases oxygen-glucose deprivation-induced Zn2+ accumulation and neuronal loss in hippocampal pyramidal neurons. J Neurosci. 2002;22:1273–9. doi: 10.1523/JNEUROSCI.22-04-01273.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sensi SL, et al. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci. 1997;17:9554–64. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohana E, et al. A sodium zinc exchange mechanism is mediating extrusion of zinc in mammalian cells. J Biol Chem. 2004;279:4278–84. doi: 10.1074/jbc.M309229200. [DOI] [PubMed] [Google Scholar]
- 61.Wudarczyk J, Debska G, Lenartowicz E. Zinc as an inducer of the membrane permeability transition in rat liver mitochondria. Arch Biochem Biophys. 1999;363:1–8. doi: 10.1006/abbi.1998.1058. [DOI] [PubMed] [Google Scholar]
- 62.Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH. Zn(2+) induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J Biol Chem. 2001;276:47524–9. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- 63.Bonanni L, et al. Zinc-dependent multi-conductance channel activity in mitochondria isolated from ischemic brain. J Neurosci. 2006;26:6851–62. doi: 10.1523/JNEUROSCI.5444-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aravindakumar CT, Ceulemans J, De Ley M. Nitric oxide induces Zn2+ release from metallothionein by destroying zinc-sulphur clusters without concomitant formation of S-nitrosothiol. Biochem J. 1999;344:253–8. doi: 10.1042/0264-6021:3440253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maret W. The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr. 2000;130:1455S–8S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- 66.Yuguchi T, et al. Expression of growth inhibitory factor mRNA after focal ischemia in rat brain. J Cereb Blood Flow Metab. 1997;17:745–52. doi: 10.1097/00004647-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Yanagitani S, et al. Ischemia induces metallothionein III expression in neurons of rat brain. Life Sci. 1999;64:707–15. doi: 10.1016/s0024-3205(98)00612-2. [DOI] [PubMed] [Google Scholar]
- 68.Kim YH, Kim EY, Gwag BJ, Sohn S, Koh JY. Zinc-induced cortical neuronal death with features of apoptosis and necrosis: mediation by free radicals. Neuroscience. 1999;89:175–82. doi: 10.1016/s0306-4522(98)00313-3. [DOI] [PubMed] [Google Scholar]
- 69.Noh KM, Kim YH, Koh JY. Mediation by membrane protein kinase C of zinc-induced oxidative neuronal injury in mouse cortical cultures. J Neurochem. 1999;72:1609–16. doi: 10.1046/j.1471-4159.1999.721609.x. [DOI] [PubMed] [Google Scholar]
- 70.Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 71.Vandenberg RJ, Mitrovic AD, Johnston GA. Molecular basis for differential inhibition of glutamate transporter subtypes by zinc ions. Mol Pharmacol. 1998;54:189–96. doi: 10.1124/mol.54.1.189. [DOI] [PubMed] [Google Scholar]
- 72.Spiridon M, Kamm D, Billups B, Mobbs P, Attwell D. Modulation by zinc of the glutamate transporters in glial cells and cones isolated from the tiger salamander retina. J Physiol. 1998;506:363–76. doi: 10.1111/j.1469-7793.1998.363bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sensi SL, Rockabrand E, Canzoniero LM. Acidosis enhances toxicity induced by kainate and zinc exposure in aged cultured astrocytes. Biogerontology. 2006;7:367–74. doi: 10.1007/s10522-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 74.Xiong ZG, Chu XP, Simon RP. Acid sensing ion channels – novel therapeutic targets for ischemic brain injury. Front Biosci. 2007;12:1376–1386. doi: 10.2741/2154. [DOI] [PubMed] [Google Scholar]
- 75.Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987;236:589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- 76.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–3. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 77.Christine CW, Choi DW. Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. J Neurosci. 1990;10:108–16. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–7. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 79.Chu XP, et al. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24:8678–89. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hey JG, Chu XP, Seeds J, Simon RP, Xiong ZG. Extracellular zinc protects against acidosis-induced injury of cells expressing Ca2+-permeable Acid-sensing ion channels. Stroke. 2007;38:670–3. doi: 10.1161/01.STR.0000251443.68897.99. [DOI] [PubMed] [Google Scholar]
- 81.Ganju N, Eastman A. Zinc inhibits Bax and Bak activation and cytochrome c release induced by chemical inducers of apoptosis but not by death-receptor-initiated pathways. Cell Death Differ. 2003;10:652–61. doi: 10.1038/sj.cdd.4401234. [DOI] [PubMed] [Google Scholar]
- 82.Perry DK, et al. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J Biol Chem. 1997;272:18530–3. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 83.Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals. 2001;14:315–30. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]