Abstract
Auditory evoked potentials (AEPs) have become a widely utilized measure of hearing sensitivity. Most investigators use pharmacological paralysis to reduce myogenic noise and immobilize the animal for stable electrical recordings, but additional anesthesia is generally not used because the most commonly available fish anesthetic, the cholinergic antagonist tricane methanosulfate (MS222), is known to disrupt hair cell and primary afferent physiology. Anesthetic agents that do not interfere with auditory function would be a useful adjunctant to paralytic immobilization and would reduce any possible distress incurred by prolonged immobilization. In this report we tested the opiate anesthetic Fentanyl and compared hearing thresholds in immobilized versus immobilized and anesthetized animals. Short-term effects of mild MS222 anesthesia were also measured via evoked potential audiometry. Animals were tested before and after Fentanyl injection (100, 500 and 2500 μg g−1 fish body-weight) using standard evoked potential audiometry. Tone pips, 0.2 to 3 kHz, from an aerial loudspeaker served as stimuli. Fentanyl altered evoked potential waveforms slightly, but did not alter estimated threshold sensitivity. These results suggest Fentanyl be considered as a possible addition to AEP techniques in goldfish (Carassius auratus) and poikilothermic vertebrates generally.
Keywords: auditory evoked potential, auditory brainstem response, anesthesia, sound detection threshold, ABR, AEP
1. Introduction
Auditory evoked potential (AEP) physiology has become a widely used non-invasive method for the study of fish hearing (Corwin et al., 1982; Kenyon et al., 1998; Yan, 2001). In the AEP technique, electrical activity is recorded from the surface of the head without surgical implantation of electrodes and stimulus evoked responses are measured by time-locked averaging (Jewett, 1970). The ease and rapidity of collecting AEP data have made this a popular technique for comparing hearing abilities across species (Kenyon et al., 1998), or assessing hearing damage as a result of noise (Scholik and Yan, 2002; Amoser and Ladich, 2003; Smith et al., 2004), exposure to toxins (Lu and Tomchick, 2002), or experimental manipulations of the sensory periphery (Yan, 1998; Yan and Curtsinger, 2000; Fletcher and Crawford, 2001; Ladich and Wysocki, 2003).
Electrophysiological techniques, including AEP audiometry typically utilize anesthesia during the surgical preparation of the recording site and injection of a long-lasting immobilant such as Flaxedil (gallamine triethiodide). The most commonly-used anesthetic is tricaine methanesulfonate (MS-222), which is known to interfere with sensory function in fish (Hensel at al., 1975; Spath and Schweickert, 1977; Arnolds et al., 2002, Palmer and Mensinger, 2004). When used to prepare animals for single-unit recordings, experimenters routinely postpone data collection for approximately one hour following MS-222 administration. In some laboratories, brief MS-222 anesthesia is used in some laboratories during AEP studies prior to the administration of a paralytic or immobilant (e.g. Flaxedil) and during attachment to the head-holder. Paralytics act on neuromuscular synapses and are widely used to reduce myogenic noise during recording and prevent struggling when the animal recovers from anesthesia (Kenyon et al., 1998; Wysocki and Ladich, 2002, Casper et al., 2003). The commonly used paralytics do not appear to produce sensory deficits or stress (Smith and Schauf, 1981; Foutz et al., 1983). In fact, thresholds estimated by AEP are significantly lower when animals are pharmacologically immobilized (Kenyon et al. 1998), probably due to the reduction in myogenic noise. Measurements of evoked potentials are typically begun within 60 minutes after removal of the subject from MS-222 solution, by which time the animal is considered awake and recovered from MS-222 effects. Paralytic effects of Flaxedil persist for 6–8 hours following injection, depending on dosage, species, and ambient temperature.
While it has been assumed that MS-222 anesthesia has minimal lasting effects on the AEP, this has not been empirically assessed as during single unit recordings (Palmer and Mensinger, 2004). Further, prolonged pharmacological paralysis in the absence of anesthesia is potentially distressing to the subject and could induce stress-related changes in sensory function and/or evoked potential waveforms. An additional anesthetic agent co-administered with Flaxedil could be used to reduce distress during long recording sessions, but is only appropriate if it does not alter auditory physiology.
Fentanyl (N-Phenyl-N-(1–2-phenylethyl–4-piperidyl) propanamide) is a promising opioid agonist that has been used during physiological recordings in fishes (Bodnar and Bass, 1997), but the use of Fentanyl in AEP studies has not been reported. Fentanyl was first developed in 1960 (Janssen, 1962) as a narcotic analgesic for use during open heart surgery and an anesthetic in non-human animal experimentation (Lowenstein et al., 1969; Stanley, 1992, Flecknell and Waterman-Pearson, 2000). Compared with other opioids, Fentanyl provides excellent analgesic effects at acute durations and long-term sedation with increased dosages (Flecknell and Waterman-Pearson, 2000). Nolan (2000) states that the potential for respiratory depression limits Fentanyl use to “mechanically ventilated” animals, but most fish AEP studies use artificial respiration to compensate for Flaxedil-induced paralysis, making Fentanyl a feasible anesthetic adjutant if it does not affect hearing sensitivity. The present study reports on the changes in auditory evoked potentials following MS-222 administration and tests the hypothesis that Fentanyl does not interfere with auditory physiology, as assessed by AEPs. A subset of these results was previously presented in abstract form (Cordova et al., 2004).
2. Results
2.1. Tricaine effects on amplitude and threshold
Tricaine effects on response amplitude and threshold were assessed by replicate AEP collection over a time course of one hour following the removal of MS222 from the holding and respiratory water (Figure 1A). We used a 1000 Hz stimulus presented at 112 dB SPL (~30 dB above threshold). Peak to peak response amplitudes in four animals showed relatively little change over the 74 minutes following MS222 anesthesia (Figure 1A). Slight variation in amplitude is on the scale of tenths of microvolts which is typical for repeated ABR recordings (<20% of pk-pk amplitude). Further, there was no consistent trend of increasing amplitudes as possible MS-222 effects abated. Threshold estimates (to 1000 Hz tone pips) were also made for three of four animals at each time interval, but no change in threshold was observed over this time period (Figure 1B).
Figure 1.
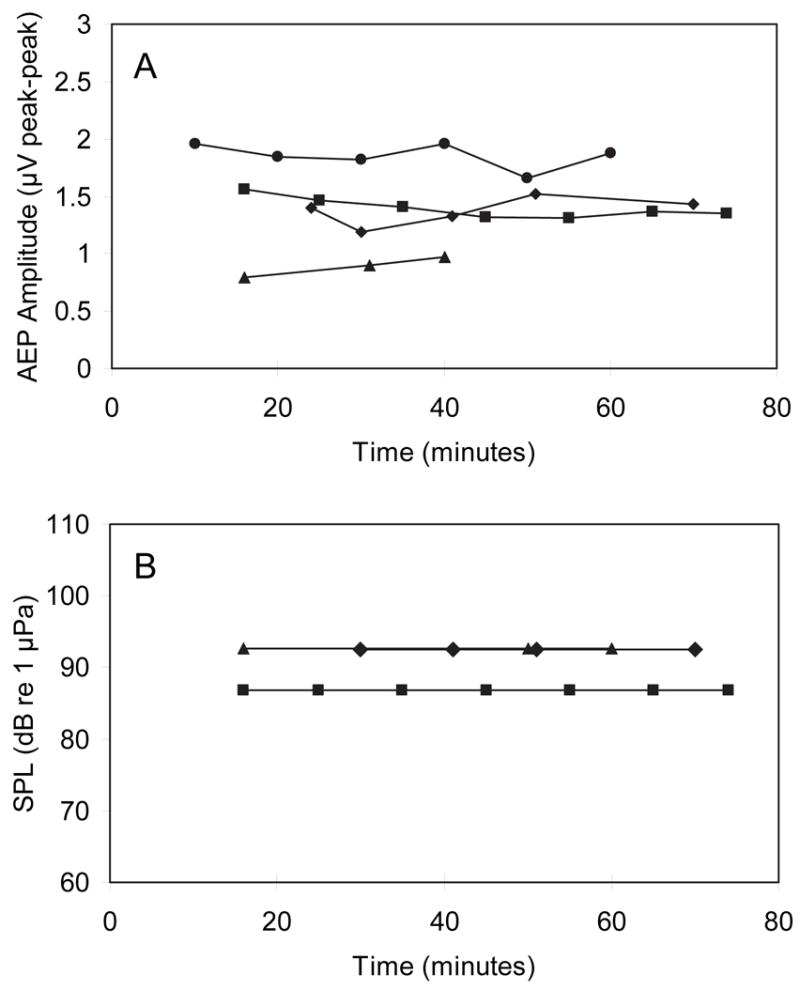
(A) Peak to peak amplitude of AEP responses to 112 dB tone pip (1000 Hz) for four animals in the 74 minutes following administration of fresh respiratory flow (following MS222 anesthesia). (B) 1000 Hz tone pip detection thresholds for three of these animals during this same time period. In both panels, different symbols indicate individual subjects and time 0 refers to removal from MS222 and application of clean respiratory flow.
2.2. Effects of Fentanyl on hearing sensitivity
An ANOVA of the magnitude and direction of the Fentanyl effect was conducted using the signed difference in threshold before and after Fentanyl administration for each of four dosages of Fentanyl (0 (no Fentanyl), 100, 500 and 2500 μg per g body weight). The magnitude of the effect was estimated by a significant departure from zero, and any directional effects could have been assessed using the sign of the difference. We did not demonstrate any statistically significant effects of any Fentanyl dosage at any frequency (F3,108 = 1.757, P = 0.160). That is, all four dosage groups, including vehicle alone, had statistically indistinguishable changes in threshold following Fentanyl administration (before-after differences are equal to zero).
Figure 2 illustrates the effect of 500 μg g−1 Fentanyl on AEP responses to a 1000 Hz tone pip. The AEP waveforms are nearly indistinguishable and, in this case, the estimated threshold is unchanged. The mean hearing thresholds at all frequencies for each of the four dosage groups are plotted as audiograms in figure 3. In individual animals, Fentanyl had no apparent effects on hearing thresholds. The vast majority (76%) of thresholds measured after Fentanyl (or vehicle) administration were within 5 dB of the pre-administration threshold estimate. The mean absolute difference in thresholds before and after Fentanyl administration across frequencies was ±0.42 dB, with a maximum mean difference of 9.97 dB. Of the 112 paired measurements (before and after Fentanyl), just over 50% (57 pairs) had no threshold change after Fentanyl administration. Of the remaining pairs of measures, only 27 (24%) had differences larger than 5 dB and these were split nearly evenly, with 15 instances of decreased sensitivity and 12 instances of increased sensitivity following drug treatment.
Figure 2.

AEP waveforms of subject mcgf34 before (A) and after (B) Fentanyl administration at 500 μg g-1 using a 1000 Hz stimuli (sound pressure levels = 71 to 106 dB re: 1 μPa). Response amplitude decreases as stimulus intensity decreases. The smallest detectable evoked response occurred with a 76 dB stimulus both before (A) and after Fentanyl (B).
Figure 3.
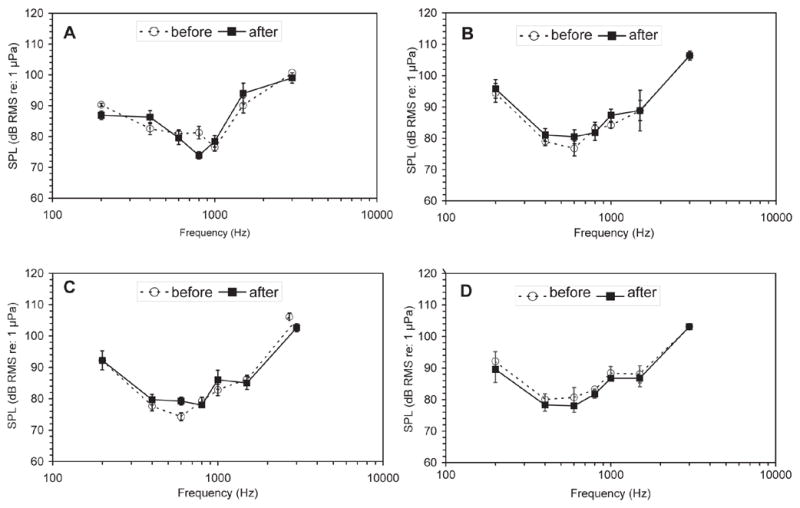
Mean audiograms for all Fentanyl dosage groups. In all panels, the mean thresholds prior to Fentanyl administration are plotted as open circles and dashed lines and the thresholds measured following Fentanyl administration are plotted as squares and solid lines. Error bars depict one standard error (n=4). (A) 0 ug g−1, (B) 100 μg g−1 (C), 500 μg g−1 (D) and 2500 μg g−1 Fentanyl.
3. Discussion
Based on the present results, Fentanyl is an appropriate supplement to pharmacological paralysis during measurements of AEPs in Carassius auratus. We were unable to find evidence that Fentanyl alters auditory sensitivity. One major advantage of AEP techniques is the non-invasive nature of the procedure. However, particularly for species with high-susceptibility to stress, the stress of immobilization and handling could have long-lasting detrimental consequences. These consequences could be alleviated with proper anesthesia and stress-reduction. For ethical considerations, we suggest Fentanyl anesthesia become a routine component of AEP audiometry in fishes.
Further, brief (less than 10 minutes) MS-222 sedation used during paralytic injection and positioning of the subject in the restraint apparatus does not appear to alter auditory sensitivity. Palmer and Mensinger (2004) showed that anesthesia with MS-222 at dosages greater than 0.01% decreases spontaneous and stimulus-evoked activity in anterior lateral line nerve afferents. We did not find a similar reduction of AEP amplitude following brief sedation using 0.01% MS222. The difference may be due to the compound nature of the AEP, as opposed to the single unit activity recorded by Palmer and Mensinger (2004). More likely, it is due to the brevity of MS222 exposure in the present report. Animals were immersed in MS222 only until respiration ceased (or just nearly so), usually fewer than 20 minutes. They were immediately injected with immobilant and mounted in the experimental chamber. Fresh-water respiration (no MS222) began within minutes of initial MS222 sedation. It is possible that any MS222-induced suppression had already abated by the time we began recording, while animals in Palmer and Mensinger’s study were maintained on a respiratory flow containing 0.01% MS222. It is important to note also that lower concentrations of MS222 did not suppress firing in lateral line nerve fibers (Palmer and Mensinger 2004). It may not be necessary, therefore, to wait long periods of time (~1 hour) following brief MS222 anesthesia before initiating AEP.
4. Experimental Procedures
4.1. Experimental subjects and anesthesia
Sixteen specimens of Carassius auratus [90–130 mm standard length (SL); 30–70 g] were obtained from a local aquarium fish supplier. Protocol for experimentation was approved by the Hunter College Institutional Animal Care and Use Committee (IACUC).
Subjects were briefly sedated by immersion in a 100 mg l−1 buffered (pH 6.5) solution of tricaine methanesulfonate (MS-222; Sigma). After the cessation of respiratory activity (approximately 15 minutes) the subject was immediately removed from anesthesia and two scales were carefully removed 2–3 cm lateral to the dorsal fin. The paralytic gallamine triethiodide (Flaxedil) was injected intramuscularly (10 μg g−1 of body weight) and subjects were secured to a head restraint in the experimental arena (44.5 cm x 39.5 cm x 14.8 cm) containing 10 cm of aquarium water (19 L). Aerated water was supplied by gravity-feed to the fish through a mouthpiece secured to the head restraint. The experimental arena rested on a vibration isolation table (Nano-K, Minus-K Technology) in a single-walled sound attenuating chamber (Industrial Acoustics Company, Inc.).
After estimating thresholds at all stimulus frequencies, an intramuscular injection of Fentanyl (0 (equivalent volume of vehicle), 100, 500 or 2500 μg g−1 body weight) was administered while the subject was still attached to the head restraint. In some cases, it was necessary to adjust the electrodes to administer the injection, but the micromanipulators were used to return them to their original recording position. All stimuli were presented a second time after injection and thresholds were estimated again. Following a completed test session animals were carefully removed from the holding tank and euthanized by an overdose of MS-222 until cessation of breath.
4.2. Stimulus generation and AEP recording
Sound stimuli and AEP waveforms were processed and acquired using TDT signal processing workstations and software packages (Tucker-Davis Technologies, Alachua FL). Stimulus signals were amplified (D-75A; Crown Audio) and presented by a loudspeaker (Control 22; JBL) suspended 0.6 m above the water surface. Stimuli were brief tone pips at frequencies of 200, 400, 600, 800, 1000, 1500 and 3000 Hz. To preserve spectral fidelity, all tone pips were gated by a cosine function whereby 90% of total amplitude was reached within a specified rise time. For frequencies between 200 and 800 Hz, rise/fall duration was one cycle with three cycles of plateau intensity (5 cycles total, 6.25– 25 msec). The 1000 Hz stimulus had a rise/fall time of two cycles, with three cycles of plateau intensity (7 msec). Two cycle rise-fall times were also used for 1500 and 3000 Hz stimuli, with plateau duration of four cycles (5.3 and 2.7 msec). These tone pips were presented at a rate of 12 sec−1 for the 200 Hz stimulus and 21 sec−1 for stimuli between 400 and 3000 Hz. Signal levels were controlled by a programmable attenuator (PA5; TDT) and calibrated using a pressure-hydrophone (8103; Brüel & Kjaer) in place of the fish. Stimulus intensity was determined following the method of Burkhard (1984). The root-mean-squared amplitude of the largest single cycle (360°) in the hydrophone recording was used as the calibrated sound pressure level (SPL) in dB re: 1 μPa.
AEP recording techniques have been described in detail elsewhere (Kenyon et al. 1998; Yan, 2001). Low impedance electrodes (<8 K ohms) were constructed from Teflon-insulated silver wire (0.25 mm diameter), with the final 1 mm stripped bare of insulation. The tips of two electrodes were pressed firmly against the surface of the head, with the recording electrode dorsal to the hindbrain and a reference electrode placed more rostrally, near the nares, and held in position with micromanipulators (YOU-1; Narishige). The electrical potential between these electrodes was amplified (RA4; TDT) 20X and band pass filtered between 20 and 3000 Hz before digitization at 25 kHz.
4.3. Threshold estimates and statistical analysis
Averaged responses to 1000 stimulus presentations were recorded as the AEP. Stimuli were presented in alternating (0° and 180° starting phase) polarities to reduce stimulus artifacts. Stimulus intensity was reduced in 5 dB increments until threshold was reached. Thresholds were defined as the lowest stimulus intensity that evoked a repeatable ABR. Visual inspection thresholds were determined by graphically overlaying replicate traces and visually selecting the smallest stimulus amplitude that evoked a repeatable response.
The effect of initial MS-222 anesthesia was assessed by recording AEPs evoked by 1000 Hz tone pips following removal from MS-222 and injection of Flaxedil. Response amplitudes (peak-peak) were compared in four animals at time intervals ranging from 16 to 74 minutes following removal from MS-222. Detection thresholds for three of these animals were also estimated at these same time intervals.
To assess dosage effects on hearing sensitivity, we calculated the difference in threshold before and after Fentanyl administration, for each subject at each frequency. Mean threshold differences were compared across dosage groups using a one-factor ANOVA on the difference in sensitivity after Fentanyl administration. For example, at a dose of 2500 μg, threshold for one goldfish was measured at 92.57 dB before injection and 86.8 dB after injection of Fentanyl. The difference is a decrease of 5.77 dB in threshold SPL or, a 5.77 dB increase in sensitivity. Mean signed differences were compared, testing the null hypothesis that Fentanyl has no affects on hearing sensitivity (i.e. mean difference of 0 and no difference in mean sensitivity changes between dosage groups). All ANOVA results were assessed at the P ≤ 0.05 significance level.
Table 1.
Mean (SD, n=4) sound pressure threshold (dB RMS re: 1 μPa) for each dosage before and after injection
| 0 μg/g | 100 μg/g | 500 μg/g | ||||
|---|---|---|---|---|---|---|
| Frequency | before | after | before | after | before | after |
| 200 | 90.3 (1.0) | 86.9 (2.8) | 94.5 (6.1) | 95.8 (6.8) | 92.2 (6.1) | 92.2 (6.1) |
| 400 | 82.6 (3.9) | 86.3 (4.3) | 79.0 (2.6) | 81.1 (5.6) | 77.7 (3.0) | 79.7 (3.4) |
| 600 | 80.9 (2.6) | 79.6 (4.3) | 76.8 (4.8) | 80.5 (5.0) | 74.3 (2.5) | 79.3 (2.5) |
| 800 | 81.3 (4.1) | 73.9 (2.4) | 83.1 (4.2) | 81.8 (6.4) | 79.3 (2.5) | 78.0 (0) |
| 1000 | 76.5 (2.6) | 78.4 (3.8) | 84.1 (1.8) | 87.4 (3.9) | 82.8 (3.6) | 86.0 (6.0) |
| 1500 | 90.1 (4.9) | 94.0 (6.6) | 88.8 (6.4) | 88.8 (6.4) | 86.0 (2.5) | 85.0 (4.1) |
| 3000 | 100.7 (0) | 99.0 (3.4) | 106.4 (2.8) | 106.4 (2.8) | 105.2 (2.5) | 102.6 (2.6) |
| 2500 μg/g | All Groups | |||||
|
| ||||||
| Frequency | before | after | before | after | Difference | |
| 200 | 92.1 (6.1) | 89.6 (8.3) | 92.3 (5.0) | 91.1 (6.6) | -1.2 | |
| 400 | 80.0 (3.5) | 78.3 (3.8) | 79.8 (3.5) | 81.3 (5.0) | 1.5 | |
| 600 | 80.7 (6.4) | 78.0 (4.1) | 78.2 (4.9) | 79.3 (3.8) | 1.1 | |
| 800 | 83.2 (0.4) | 81.7 (2.6) | 81.7 (3.3) | 78.8 (4.7) | -2.9 | |
| 1000 | 88.4 (4.2) | 86.7 (0.2) | 83.0 (5.2) | 84.6 (5.2) | 1.6 | |
| 1500 | 88.0 (5.4) | 86.8 (5.4) | 88.2 (4.7) | 88.7 (6.2) | 0.5 | |
| 3000 | 103.1 (1.6) | 103.1 (1.6) | 103.8 (2.9) | 102.8 (3.6) | -1.0 | |
Acknowledgments
We thank Jianqiang Xiao for helpful comments on earlier drafts of this paper. Suzanne Palmer and Johanna Berberena assisted in early phases of data collection and we are grateful for their contribution. Their participation was supported by NIH awards 5T34GM07823-24(MARC) and GM60665 (RISE), respectively. M.C. was also supported by NIH GM 60665. Financial support for this research was provided by NIH awards 1 S06 GM60654 and 1 R03 MH067808 to C.B.B.
The National Center for Research Resources of the NIH supports research infrastructure at Hunter College through the Research Centers in Minority Institutions (RCMI) award RR-03037. The contents of this publication are solely the responsibility of the authors however, and do not necessarily represent the official views of the NCRR/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amoser S, Ladich S. Diversity in noise-induced temporary hearing loss in otophysine fishes. J Acoust Soc Am. 2003;113:2170–2179. doi: 10.1121/1.1557212. [DOI] [PubMed] [Google Scholar]
- Amoser S, Ladich F. Are hearing sensitivities of freshwater fish adapted to the ambient noise in their habitats? J Exp Biol. 2005;208:3533–3542. doi: 10.1242/jeb.01809. [DOI] [PubMed] [Google Scholar]
- Arnolds DE, Zottoli SJ, Adams CE, Dineen SM, Fevrier S, Guo Y, Pascal AJ. Physiological effects of tricaine on the supramedullary/dorsal neurons of the cunner, Tautogolabrus adspersus. Biol Bull. 2002;203:188–189. doi: 10.2307/1543388. [DOI] [PubMed] [Google Scholar]
- Bodnar DA, Bass AH. Temporal coding of concurrent acoustic signals in auditory midbrain. J Neurosci. 1997;17:7553–7564. doi: 10.1523/JNEUROSCI.17-19-07553.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard R. Sound pressure level measurement and spectral analysis of brief acoustic transients. Electroencephalogr Clin Neurophysiol. 1984;57:83–91. doi: 10.1016/0013-4694(84)90010-5. [DOI] [PubMed] [Google Scholar]
- Casper BM, Lobel PS, Yan HY. The hearing sensitivity of the little skate, Raja erincea: a comparison of two methods. Environ Biol Fish. 2003;68:371–379. [Google Scholar]
- Cordova MS, Berberena J, Palmer SA, Braun CB. Auditory brainstem responses to loudspeaker and dipole sources in goldfish (Carassius auratus): Source comparisons and effects of Fentanyl anesthesia. Society for Neuroscience. 20042004 online. [Google Scholar]
- Corwin JT, Bullock TH, Schweitzer J. The auditory brain stem response in five vertebrate classes. Electroencephalogr Clin Neurophysiol. 1982;54:629–641. doi: 10.1016/0013-4694(82)90117-1. [DOI] [PubMed] [Google Scholar]
- Flecknell PA, Waterman-Pearson A. Pain Management in Animals. W.B. Saunders; London: 2000. [Google Scholar]
- Fletcher LB, Crawford JD. Acoustic detection by sound-producing fishes (Mormyridae): The role of gas-filled tympanic bladders. J Exp Biol. 2001;204:175–183. doi: 10.1242/jeb.204.2.175. [DOI] [PubMed] [Google Scholar]
- Foutz AS, Dautheir C, Kerdelhue B. β-endorphin plasma levels during neuromuscular blockade in unanesthetized cat. Brain Res. 1983;263:119–123. doi: 10.1016/0006-8993(83)91207-6. [DOI] [PubMed] [Google Scholar]
- Hensel H, Bromm B, Nier K. Effect of ethyl m-aminobenzoate (MS-222) on ampullae of lorenzini and lateral-line organs. Experientia. 1975;31:958–960. doi: 10.1007/BF02358876. [DOI] [PubMed] [Google Scholar]
- Janssen PAJ. A review of the chemical features associated with strong morphine-like activity. Br J Anaesth. 1962;34:260. doi: 10.1093/bja/34.4.260. [DOI] [PubMed] [Google Scholar]
- Jewett DL. Volume conducted potentials in response to auditory stimuli as detected by averaging in the cat. Electroencephalogr Clin Neurophysiol. 1970;28:609–618. doi: 10.1016/0013-4694(70)90203-8. [DOI] [PubMed] [Google Scholar]
- Kenyon TN, Ladich F, Yan HY. A comparative study of hearing ability in fishes: the auditory brainstem response approach. J Comp Physiol, A. 1998;182:307–318. doi: 10.1007/s003590050181. [DOI] [PubMed] [Google Scholar]
- Ladich F, Wysocki LE. How does tripus extirpation affect auditory sensitivity in goldfish? Hearing Res. 2003;182:119–129. doi: 10.1016/s0378-5955(03)00188-6. [DOI] [PubMed] [Google Scholar]
- Lowenstein E, Hallowell P, Levin FH, Daggett WM, Austen WG, Laver MB. Cardiovascular response to large doses of intravenous morphine in man. New Engl J Med. 1969;281:1389. doi: 10.1056/NEJM196912182812503. [DOI] [PubMed] [Google Scholar]
- Lu Z, Tomchik SM. Effects of red-tide toxin on fish hearing. J Comp Physiol. 2002;188:807–813. doi: 10.1007/s00359-002-0369-8. [DOI] [PubMed] [Google Scholar]
- Martin P, Bateson P. Measuring Behaviour: An Introductory Guide. 2. Cambridge University Press; Cambridge: 1993. [Google Scholar]
- Nolan AM. Pharmacology of analgesic drugs. In: Flecknell PA, Waterman-Pearson A, editors. Pain Management in Animals. W.B Saunders; London: 2000. 2001. pp. 21–52. [Google Scholar]
- Ozdamar O, Delgado RE, Eilers RE, Urbano RC. Automated electrophysiologic hearing testing using a threshold-seeking algorithm. J Am Acad Audiol. 1994;5:77–88. [PubMed] [Google Scholar]
- Palmer LM, Mensinger AF. The effect of the anesthetic tricaine (MS-222) on nerve activity in the anterior lateral line of the oyster toadfish, Opsanus tau. J Neurophysiol. 2004;92:1034–1041. doi: 10.1152/jn.01151.2003. [DOI] [PubMed] [Google Scholar]
- Scholik AR, Yan HY. Effects of boat engine noise on the auditory sensitivity of the fat head minnow, Pimephales promelas. Environ Biol Fish. 2002;63:203–209. [Google Scholar]
- Smith KJ, Schauf CL. Effects of gallamine triethiodide on membrane currents in amphibian and mammalian peripheral nerve. J Pharacol Exp Ther. 1981;217:719–726. [PubMed] [Google Scholar]
- Smith ME, Kane AS, Popper AN. Acoustical stress and hearing sensitivity in fishes: does the linear threshold shift hypothesis hold water? J Exp Biol. 2004;207:3591–3602. doi: 10.1242/jeb.01188. [DOI] [PubMed] [Google Scholar]
- Spath M, Schweickert W. The effect of metacaine (MS-222) on the activity of the efferent and afferent nerves in the teleost lateral-line system. Naunyn Schmiedebergs Arch Pharmacol. 1977;297:9–16. doi: 10.1007/BF00508804. [DOI] [PubMed] [Google Scholar]
- Stanley TH. The history and development of the Fentanyl series. J Pain Symp Manag. 1992;7(3 Suppl):S3–S7. doi: 10.1016/0885-3924(92)90047-l. [DOI] [PubMed] [Google Scholar]
- Wysocki LE, Ladich F. Can fishes resolve temporal characteristics of sounds? New insights using auditory brainstem responses. Hearing Res. 2002;169:36–46. doi: 10.1016/s0378-5955(02)00336-2. [DOI] [PubMed] [Google Scholar]
- Yan HY. Auditory role of the suprabranchial chamber in gourami fish. J Comp Physiol A. 1998;183:325–333. doi: 10.1007/s003590050259. [DOI] [PubMed] [Google Scholar]
- Yan HY. A non-invasive electrophysiological study on the enhancement of hearing ability in fishes. Proc Instit Acoust. 2001;23:15–25. [Google Scholar]
- Yan HY, Curtsinger WS. The otic gasbladder as an ancillary auditory structure in a mormyrid fish. J Comp Physiol A. 2000;186:595–602. doi: 10.1007/s003590000114. [DOI] [PubMed] [Google Scholar]


