Abstract
Assays of angiogenesis in vitro are critical to the study of vascular morphogenesis and to the evaluation of therapeutic compounds that promote or inhibit vascular growth. Culture of explanted aortic segments from rats or mice in a 3-dimensional extracellular matrix (ECM) is one of the most effective ways to generate capillary-like endothelial sprouts in vitro. We have modified the classic aortic explant model by placing the aortic segments from mice within small (5.6 mm diameter, 30μl volume) lenticular hydrogels of type I collagen supported at the edge by nylon mesh rings. This method of culture, referred to as the "miniature ring-supported gel" (MRSG) assay, optimizes handling, cytological staining, and conventional imaging of the specimen and permits use of minimal volumes of reagents in a 96-well tissue culture format. We have used the MRSG assay to quantify the impaired angiogenic response of aged mice relative to young mice and to show that aged mice have significantly decreased sprout formation, but have similar levels of invasion of vascular smooth muscle cells into the supportive ECM. The MRSG assay, which combines low volume, physically robust gels in conjunction with mouse aortic segments, may prove to be a highly useful tool in studies of the process and control of vascular growth.
Keywords: aging, angiogenesis, aorta, assay, collagen gel, explant, in vitro, microvessel, mouse
Introduction
Models of vascular growth (angiogenesis) in vitro have proven useful in the study of vascular morphogenesis and for screening compounds for therapeutic regulation of blood vessel growth. Typically, angiogenesis in vitro involves the organization of endothelial cells (ECs) into capillary-like structures – a process that occurs best in 3-dimensional extracellular matrix (ECM) hydrogels comprised of collagen, fibrin, or basement membrane material that simulate the 3-dimensional connective tissue environment in vivo. In addition to the composition of the ECM support, an important consideration in angiogenic models in vitro is the source of ECs, which offer different advantages according to the species of origin. For example, bovine aortic ECs are easily obtained and hardy in tissue culture, whereas human ECs develop tubes with wide, patent lumens that closely resemble capillaries in vivo (Davis and Camarillo 1996; Koike et al. 2003). Rats offer a wide range of well-characterized disease models and, consequently, have been the focus of a number of in vitro models of angiogenesis. Previous studies have shown that explanted segments of rat aortae placed in 3-dimensional ECM gels will give rise to arborizing, capillary-like sprouts (Nicosia et al. 1982; Mori et al. 1988; Diglio et al. 1989; Nicosia and Ottinetti 1990; Nicosia et al. 1992; Zhu and Nicosia 2002). The capability to perform sophisticated genetic manipulation in mice has led to the use of mouse aortic segments as alternatives to rat segments. Under permissive conditions in vitro, mouse aortic segments will generate microvascular sprouts similar to sprouts formed by rat segments (Masson et al. 2002; Zhu et al. 2003).
Although there is consensus that 3-dimensional ECM hydrogels are optimal for support of angiogenesis in vitro, the fragility of these gels can make analyses (i.e., staining, imaging) problematic. Moreover, large sized ECM gels, which are typically easier to handle and image, are wasteful of scarce and expensive reagents. The present study reports a novel "miniature ring-supported gel" (MRSG) assay of angiogenesis in vitro that places isolated mouse aortic segments within low volume (30μl) 3-dimensional collagen hydrogels supported at the edges by nylon mesh rings. This assay combines the extensive capabilities of the mouse experimental system with a culture method that optimizes specimen handling, staining, imaging, and the economical use of reagents.
Materials and Methods
Preparation of aortic segments
Young (4–5 months old, n = 4) and aged (20–22 months old, n = 4–5) C57Bl/6 male mice were obtained from the NIA Rodent Colony at Harlan Sprague Dawley (Chicago, IL) and housed in an SPF facility at the University of Washington. The University of Washington Animal Welfare Committee approved all animal procedures. To prepare aortic segments, mice were sacrificed by isoflurane overdose and the thoracic aortae were removed and rinsed in MCDB 131 culture medium (Invitrogen Corp. Grand Island, NY) with 100U/ml penicillin, 100μg/ml streptomycin, and 0.25μg/ml amphotericin. The isolated aortae were cleaned of perivascular adipose tissue and cut into segments 1mm in length with a #20 scalpel, care being taken to avoid damage to the endothelial lining of the segments.
Preparation of MRSG assemblies
Custom made nylon (Nitex) mesh rings (mesh type 03-100/44 with an outer diameter of 5.6 mm and an inner diameter of 3 mm – Sefar America, Inc., Kansas City, MO) were placed on a hydrophobic surface (Parafilm) and each flooded with 10 μl of a solution consisting of 1 volume of rat tail collagen stock (BD Biosciences, Two Oak Park, Bedford, MA), 1/9 volume of 10-strength NaHCO3-saturated Medium 199 (Invitrogen), and sufficient MCDB 131 and fetal bovine serum (FBS) to yield a gel with final collagen and FBS concentrations of 1.5 mg/ml and 2.5%, respectively. After dispensing the collagen solution, the preparations were incubated at 37°C for 30 min to gel the collagen. Subsequently, one aortic segment was placed (by pipette) onto the center of each collagen gel and each segment then overlaid with 20 μl of collagen solution. After polymerization of the overlayed collagen (at 37°C for 30 min), the MRSG assemblies were transferred to 96-well plates filled with 100 μl of MCDB 131, 2.5% FBS, antibiotics, and 0, 5, or 10ng/ml of vascular endothelial growth factor (VEGF – PeproTech, Rocky Hill, NJ). After 7–8 days of culture (with culture media changed every 2 days), the MRSG assemblies were rinsed in Dulbecco's phosphate-buffered saline, fixed in 10% neutral-buffered formalin for 20 min, and stored in tris-buffered saline (TBS).
Measurement of microvascular sprouting from aortic segments
Fixed MRSG assemblies were stained with a 2% crystal violet solution for 10 min followed by multiple washes in TBS until the collagen gels became transparent. For collection of images, the stained assemblies were mounted on standard microscope slides with coverslips. Images were captured using a Nikon Eclipse 50i and Q-Imaging Digital Camera with Q-capture software (Q-Imaging, Burnaby, BC, Canada). Images were imported into ImageJ (Abramoff 2004) and analyzed for total outgrowth using color-based threshholding to register only microvascular outgrowths and not the parent aortic segment, invasive single cells, or the Nitex support ring. After thresholding, vascular outgrowth was measured as the percent of total image area occupied by vascular structures.
Immunofluorescent labeling of microvascular sprouts
MRSG assemblies were blocked 1hour in TBS/2% horse serum, and then exposed to 4μg/ml of a goat polyclonal antibody to mouse CD31 (Item SC-1506 – Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and 50–100 μg/ml of a mouse monoclonal antibody to the synthetic N-terminal decapeptide of α-smooth muscle (SM) actin (multi-species cross reactivity) (Clone 1A4, Item A2547, Sigma Chemical Co., St. Louis, MO). Bound primary antibodies were visualized with Alexa Fluor 594-conjugated rabbit anti-goat IgG (for CD31) (Molecular Probes, Inc./Invitrogen, Eugene, OR) and the Mouse on Mouse immunodetection kit (Vector Laboratories, Inc., Burlingame, CA) in conjunction with fluorescein isothiocyanate-conjugated streptavidin (for SM actin). After rinsing with TBS, nuclei were labeled with 1μg/ml of 4',6-diamidino-2-phenylindole (DAPI) (Molecular Probes). Labeled preparations were visualized by conventional epifluorescence microscopy.
The number of actin positive cells that had invaded the collagen and were not in sprouts was determined by hand counting of 2–4 10X fields per assembly in 8 MRSG assemblies obtained from 4 young mice and 10 MRSG assemblies obtained from 5 aged mice.
Results
The MRSG assemblies (diagrammed in Figure 1A) readily supported outgrowth of capillary-like sprouts from embedded mouse aortic vascular segments within 7 days (Figure 1B). There were minimal sprouts in the absence of VEGF, but similar levels of outgrowths were obtained with both 5 and 10ng/ml of VEGF (Figure 1B). The limited thickness of the collagen gels (1–2 mm) allowed sprouts to be visualized easily by phase contrast or brightfield microscopy. Notably, the Nitex ring support enabled the assemblies to be easily transported from culture wells to staining and wash solutions without damage to the collagen gel or embedded vascular sprouts. Moreover, when an assembly was mounted on a slide, the Nitex ring acted as a spacer to protect the collagen gel from excessive compression and damage by the coverslip. After collection of images, slide-mounted assembles could be demounted for storage in TBS and remounted for additional imaging. Multiple cycles of mounting and demounting on slides could be performed without damage to the specimen.
Figure 1.
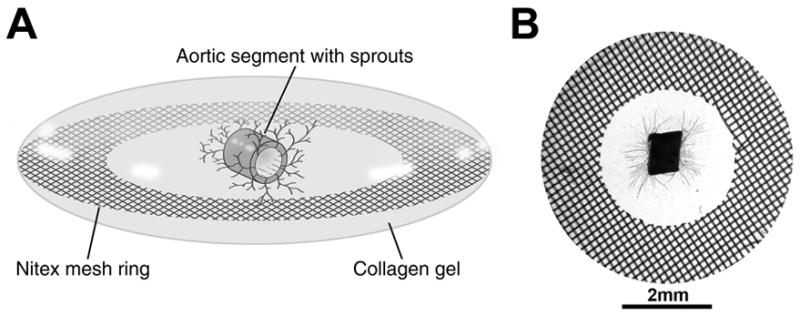
Depiction of the MRSG angiogenesis assay. A) Diagram showing an oblique view of an MRSG assembly comprised of a lenticular collagen gel, supportive Nitex mesh ring, and mouse aortic segment. B) View of an actual MRSG preparation after 7 days of culture. Microvascular sprouts are visible within the collagen gel.
Although the MRSG method offered clear advantages in specimen handling compared to 3-dimensional collagen gel cultures cast directly in tissue culture wells, it was important to demonstrate that the MRSG method could perform as an assay of angiogenesis in a manner similar to established models of vascular growth in vitro and in vivo. In a number of established models, the angiogenic responses of aged rodents are consistently 30–50% less than responses in young rodents (Puolakkainen et al. 1995; Reed et al. 1998; Rivard et al. 1999; Swift et al. 1999; Agah et al. 2004). In accordance with such observations, angiogenesis assays utilizing the MRSG method showed a 40% decrease in sprout formation from aortic segments from aged mice (Figure 2B) relative to segments from young mice (Figure 2A) (data are shown quantitatively in Figure 2C). As expected, sprout formation in aortic segments from middle-aged mice (14 months old) was less than that of young mice and more than that of aged mice (data not shown).
Figure 2.
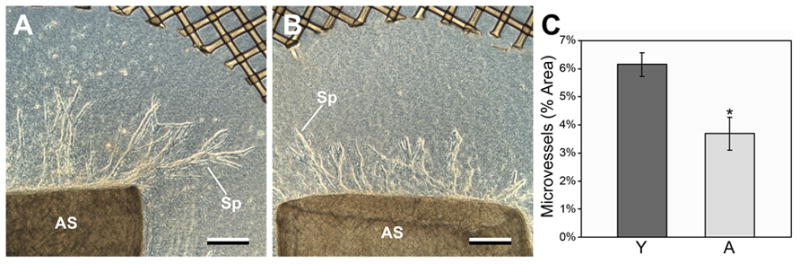
Aortic segments from young mice show significantly more sprouting than segments from aged mice in MRSG assays. A,B) Representative aortic segments (AS) cultured 7 days and viewed by phase contrast. Sprouts (Sp) from the young segment (panel A) are significantly longer and more branched than are sprouts from the aged segment (panel B). In A and B, scale bars = 250μm. This trend is represented quantitatively in panel C (vertical bars = standard deviations; asterisk = significance, p = 0.017, n = 4 for both young and aged aortic segments).
Vascular outgrowths were readily immunostained in MRSG assays, as demonstrated by dual immunofluorescent labeling of sprouts from aortic segments of aged mice (Figure 3). Cells were also labeled effectively by low molecular weight dyes (e.g., DAPI). CD31-positive ECs comprised the body of each sprout, with SM actin-positive cells irregularly distributed on the external surface of the sprouts (e.g., Figure 3). SM actin-positive cells were also observed migrating individually within the collagen gel. It has been reported that unlike ECs, vascular smooth muscle cells from aged individuals exhibit minimal deficiencies in proliferation and migration (Stemerman et al. 1982; Porreca et al. 1993; Li et al. 1997; Barandier et al. 2005; Zhang et al. 2005). Accordingly, MRSG assays showed that cultures from young and aged mice had similar degrees of outgrowth by SM actin-positive cells both on sprouts and within the surrounding collagen gel (Figure 4).
Figure 3.
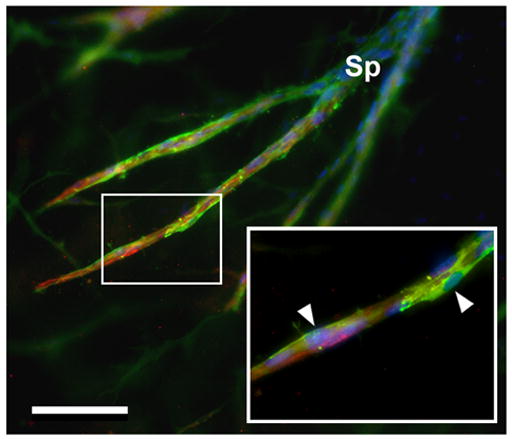
Immunofluorescent imaging of ECs and supportive cells in sprouts from an aged mouse aortic segment. A sprout (Sp) from the segment bifurcates into 2 branches. ECs are labeled for CD31 (red) and supporting cells are labeled for SM actin (green). Cell nuclei are stained with DAPI (blue). Inset shows a portion of a branch (rectangle) that is enlarged. Green SM actin-positive cells (arrowheads) form an incomplete layer on the outside of the red CD31-positive ECs. Scale bar = 200μm.
Figure 4.
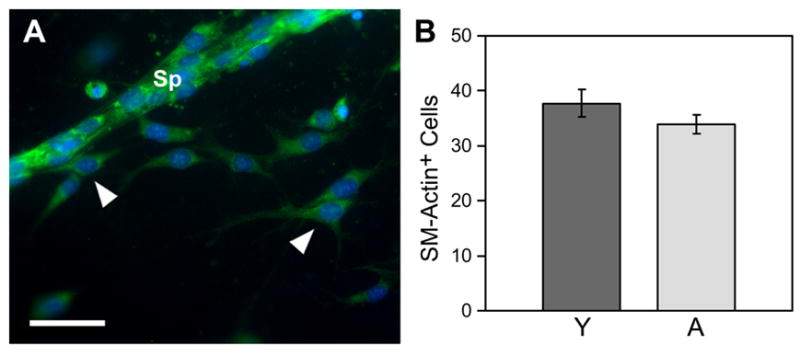
Outgrowth of SM actin-positive cells from young and aged aortic segments is similar. A) Sprout (Sp) from an aged aortic segment exhibits substantial numbers of SM actin-positive cells (green). Additionally, many SM actin-positive cells have migrated as single cells into the collagen gel (arrowheads). Cell nuclei are labeled with DAPI. The average number of SM actin-positive cells per 10X field that had invaded the collagen and were not in sprouts was similar in aortic segments from aged mice as that of segments from young mice, as represented quantitatively in panel B (vertical bars = standard error; p=>0.05, n = 4 for young and n = 5 for aged aortic segments). In A, scale bar = 100μm.
Discussion
The present study describes a novel modification of the traditional rodent aortic segment angiogenesis assay that combines the use of mouse aortic segments with low volume, robust, 3-dimensional collagen gel supports. The advantages of the MRSG assay can be summarized as follows: 1) The nylon mesh rings support the specimens for easy transport from culture wells to other incubation media. 2) For imaging, the mesh rings act as spacers that prevent damage to the specimen during slide mounting and coverslipping, which allows specimens to be demounted for storage, additional imaging, or other procedures. 3) The mesh rings support the collagen gel against cell-mediated distortion and contraction. 4) The collagen gel has an adequate depth to permit sprouting, but is flat enough to enable good imaging with conventional wide field microscopy. Moreover, the flatness of the gel improves penetration and removal of stains and wash solutions. 5) The volume of collagen per assay disk is 30μl and the disks can be cultured in a 96-well plate in as little as 50μl of culture medium, thereby minimizing the use of scarce or expensive reagents. The 96-well format is also useful for large scale automated scanning procedures; consequently, the MRSG assay might be adaptable to high throughput screening of pro- or anti-angiogenic compounds. 6) Use of aortic segments from mice enables the role of specific molecular mediators of angiogenic morphogenesis to be studied using sophisticated genetic (e.g., knock-out, knock-in) approaches. Significantly, the short life span of mice make these animals well suited to studies of the influence of aging on vascular growth.
In our previous studies with other models of angiogenesis, we found that tissues of aged mice exhibited many of the same deficits in vascular growth as aged rats (Puolakkainen et al. 1995; Arthur et al. 1998; Reed et al. 1998; Sadoun and Reed 2003). In this context, it is noteworthy that the MRSG assay described here identified an impaired angiogenic response in aged mice that correlated highly with results from previous studies of impaired angiogenesis in aged rodents (Rivard et al. 1999; Swift et al. 1999; Agah et al. 2004).
In addition to quantification of angiogenic responses, the MRSG assay may also be employed to analyze the behavior of cells that comprise the sprouts or that are dispersed within the surrounding ECM. Sprouts from both young and aged aortic segments were comprised of an inner layer of CD31-positive ECs with irregularly distributed SM actin-positive cells on the outer surface. Whereas most of the CD31-positive ECs were associated with sprouts, there were numerous individual SM actin-positive cells that were scattered throughout the collagen gel in both young and aged specimens. Prior studies have suggested that SM actin-positive cells (e.g., smooth muscle cells) do not demonstrate age-related deficits in proliferation (Stemerman et al. 1982; Porreca et al. 1993; Barandier et al. 2005; Zhang et al. 2005). Whether preservation of replicative function by smooth muscle cells contributes to atherosclerosis or other age-related diseases of the vasculature remains to be determined.
In conclusion, the advantages of the MRSG assay with respect to specimen handling, requirement for minimal reagent volumes, and utilization of mouse tissue may yield significant benefits in future studies of the biology and regulation of vascular growth.
Acknowledgments
This work was supported by National Institutes of Health AG024458.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramoff MD. Image Processing with ImageJ. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- Agah A, Kyriakides TR, Letrondo N, Bjorkblom B, Bornstein P. Thrombospondin 2 levels are increased in aged mice: consequences for cutaneous wound healing and angiogenesis. Matrix Biol. 2004;22(7):539–47. doi: 10.1016/j.matbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Arthur WT, Vernon RB, Sage EH, Reed MJ. Growth factors reverse the impaired sprouting of microvessels from aged mice. Microvasc Res. 1998;55(3):260–70. doi: 10.1006/mvre.1998.2078. [DOI] [PubMed] [Google Scholar]
- Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289(5):H1807–13. doi: 10.1152/ajpheart.01259.2004. [DOI] [PubMed] [Google Scholar]
- Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224(1):39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- Diglio CA, Grammas P, Giacomelli F, Wiener J. Angiogenesis in rat aorta ring explant cultures. Lab Invest. 1989;60(4):523–31. [PubMed] [Google Scholar]
- Koike T, Vernon RB, Gooden MD, Sadoun E, Reed MJ. Inhibited angiogenesis in aging: a role for TIMP-2. J Gerontol A Biol Sci Med Sci. 2003;58(9):B798–805. doi: 10.1093/gerona/58.9.b798. [DOI] [PubMed] [Google Scholar]
- Li Z, Cheng H, Lederer WJ, Froehlich J, Lakatta EG. Enhanced proliferation and migration and altered cytoskeletal proteins in early passage smooth muscle cells from young and old rat aortic explants. Exp Mol Pathol. 1997;64(1):1–11. doi: 10.1006/exmp.1997.2204. [DOI] [PubMed] [Google Scholar]
- Masson VV, Devy L, Grignet-Debrus C, Bernt S, Bajou K, Blacher S, Roland G, Chang Y, Fong T, Carmeliet P, Foidart JM, Noel A. Mouse Aortic Ring Assay: A New Approach of the Molecular Genetics of Angiogenesis. Biol Proced Online. 2002;4:24–31. doi: 10.1251/bpo30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Sadahira Y, Kawasaki S, Hayashi T, Notohara K, Awai M. Capillary growth from reversed rat aortic segments cultured in collagen gel. Acta Pathol Jpn. 1988;38(12):1503–12. doi: 10.1111/j.1440-1827.1988.tb02290.x. [DOI] [PubMed] [Google Scholar]
- Nicosia RF, Bonanno E, Villaschi S. Large-vessel endothelium switches to a microvascular phenotype during angiogenesis in collagen gel culture of rat aorta. Atherosclerosis. 1992;95(2–3):191–9. doi: 10.1016/0021-9150(92)90022-9. [DOI] [PubMed] [Google Scholar]
- Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63(1):115–22. [PubMed] [Google Scholar]
- Nicosia RF, Tchao R, Leighton J. Histotypic angiogenesis in vitro: light microscopic, ultrastructural, and radioautographic studies. In Vitro. 1982;18(6):538–49. doi: 10.1007/BF02810077. [DOI] [PubMed] [Google Scholar]
- Porreca E, Di Febbo C, Pandolfi A, D'Orazio A, Martelli N, Mezzetti A, Cuccurullo F, Poggi A. Differences in the glutathione system of cultured aortic smooth muscle cells from young and aged rats. Atherosclerosis. 1993;100(2):141–8. doi: 10.1016/0021-9150(93)90200-e. [DOI] [PubMed] [Google Scholar]
- Puolakkainen PA, Reed MJ, Gombotz WR, Twardzik DR, Abrass IT, Sage EH. Acceleration of wound healing in aged rats by topical application of transforming growth factor-β1. Wound Rep Reg. 1995;3:330–339. doi: 10.1046/j.1524-475X.1995.t01-1-30314.x. [DOI] [PubMed] [Google Scholar]
- Reed M, Corsa A, Penn P, Pendergrass W, Sage E, Abrass I. Neovascularization of sponge implants in aged mice: delayed angiogenesis is coincident with decreased levels of TGF-β1 and type I collagen. Am J Path. 1998;152:113–123. [PMC free article] [PubMed] [Google Scholar]
- Rivard A, Fabre J, Silver M, Chen D, Murohara T, Kearney M, Magner M, Ashara T, Isner JM. Aged-dependent impairment of angiogenesis. Circ. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51(9):1119–30. doi: 10.1177/002215540305100902. [DOI] [PubMed] [Google Scholar]
- Stemerman MB, Weinstein R, Rowe JW, Maciag T, Fuhro R, Gardner R. Vascular smooth muscle cell growth kinetics in vivo in aged rats. Proc Natl Acad Sci U S A. 1982;79(12):3863–6. doi: 10.1073/pnas.79.12.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. lab Invest. 1999;79(12):1479–1487. [PubMed] [Google Scholar]
- Zhang H, Fazel S, Tian H, Mickle DA, Weisel RD, Fujii T, Li RK. Increasing donor age adversely impacts beneficial effects of bone marrow but not smooth muscle myocardial cell therapy. Am J Physiol Heart Circ Physiol. 2005;289(5):H2089–96. doi: 10.1152/ajpheart.00019.2005. [DOI] [PubMed] [Google Scholar]
- Zhu WH, Iurlaro M, MacIntyre A, Fogel E, Nicosia RF. The mouse aorta model: influence of genetic background and aging on bFGF- and VEGF-induced angiogenic sprouting. Angiogenesis. 2003;6(3):193–9. doi: 10.1023/B:AGEN.0000021397.18713.9c. [DOI] [PubMed] [Google Scholar]
- Zhu WH, Nicosia RF. The thin prep rat aortic ring assay: a modified method for the characterization of angiogenesis in whole mounts. Angiogenesis. 2002;5(1–2):81–6. doi: 10.1023/a:1021509004829. [DOI] [PubMed] [Google Scholar]


