Abstract
This investigation was undertaken to evaluate whether mitochondrial energy metabolism is altered in a malnutrition model associated with dexamethasone treatment (1.5mg/kg/day for 5 days). Gastrocnemius and liver mitochondria were isolated from dexamethasone (DEX)-treated, pair-fed (PF) and control (CON) rats. Body weight was significantly more reduced in DEX-treated group (−16%) than in PF group (−9%). Dexamethasone increased the liver mass (+59% vs. PF and +23% vs. CON) and decreased gastrocnemius mass. Moreover, in DEX-treated rats, liver mitochondria exhibited an increased rate of non-phosphorylative oxygen consumption with all substrates (approximately +42%). There was no difference in enzymatic complex activities in liver mitochondria between rat groups. Collectively, these results suggest an increased proton leak and/or redox slipping in liver mitochondria of DEX-treated rats. In addition, dexamethasone decreased the thermodynamic coupling and efficiency of oxidative phosphorylation. We therefore suggest that this increase in the proton leak and/or of redox slip in liver is responsible for the decrease in the thermodynamic efficiency of energy conversion. In contrast, none of the determined parameters of energy metabolism were altered by dexamethasone in gastrocnemius mitochondria. Therefore, it appears that dexamethasone specifically affects mitochondrial energy metabolism in liver.
Keywords: Adipose Tissue; drug effects; physiology; Animals; Body Weight; drug effects; physiology; Citrate (si)-Synthase; metabolism; Dexamethasone; pharmacology; Eating; physiology; Energy Metabolism; drug effects; physiology; Glucocorticoids; pharmacology; Glutamates; metabolism; Male; Mitochondria, Liver; drug effects; enzymology; metabolism; Muscle, Skeletal; drug effects; metabolism; Nutrition Disorders; chemically induced; metabolism; Organ Size; drug effects; physiology; Oxidative Phosphorylation; drug effects; Oxygen Consumption; drug effects; physiology; Rats; Rats, Sprague-Dawley; Succinates; metabolism
Keywords: glucocorticoid, mitochondrion, oxidative phosphorylation, respiratory chain complexes
Introduction
Malnutrition is highly prevalent among patients, especially elderly people. It is responsible for an increased mortality, significant hospital expenditure, reduced muscle and immune function, and decreased quality of life (Wallace et al. 1995; Tucker & Miguel, 1996; Chima et al. 1997; Lesourd & Mazzari, 1997; Landi et al. 2000). Malnutrition results from a negative energy balance, a situation where energy intake fails to meet energy requirements. Although anorexia and a reduced energy intake are always associated with malnutrition, in some clinical circumstances, an increased resting oxygen consumption rate (referred to as an increased energy requirements) can be shown (Nguyen et al. 1999). On the contrary, most human and non-human studies show that energy restriction decreases energy expenditure (Ramsey et al. 2000). Therefore it appears that in these clinical states, adaptive mechanisms which lead to a reduction of energy requirements, fail to operate. However, the biochemical nature of this negative energy balance phenomenon is poorly understood at the present time. A wasting of energy may be a possible explanation. In other words, oxidative phosphorylation may be less efficient.
High-dose glucocorticoid treatment affects body weight and body composition (Kochakian & Robertson, 1951; Hausberger & Hausberger, 1958). It has also been found to induce a hypercatabolic state which leads to a reduced muscle mass (Marone et al. 1994; Minet-Quinard et al. 1999), suppressed protein synthesis, a transient increase in protein degradation and a negative nitrogen balance (Bowes et al. 1996; Max et al. 1988; Odedra et al. 1983). Moreover, it is a model of hypercortisolism, which occurs during metabolic stress in humans and which is associated with an increased energy expenditure (Woodward & Emery, 1989; Brillon et al. 1995; Tataranni et al. 1996). On the other hand, acute high-dose treatment (<1 week) of rats with dexamethasone decreases food intake (Kaur et al. 1989; Minet-Quinard et al. 1999). Therefore, a negative energy balance ensues, both because of an increased energy expenditure and a decreased energy intake. The mechanisms leading to this increased energy expenditure are not fully understood. There are arguments to suggest that mitochondrial energy production could be affected. However, the effects of glucocorticoids on cellular energy metabolism depend on the tissues being investigated and on the duration of treatment. In the liver for example, short-term administration (<24 hours) of dexamethasone appears to increase oxidative phosphorylation while having no effect on non-phosphorylative respiration (Wakat & Haynes, 1977; Allan et al. 1983). On the contrary, longer administration (<1 week) of glucocorticoids, results in decreased liver oxidative phosphorylation and ATP synthesis when fuelled through complex I, however remaining unchanged when fuelled through complex II or IV (Kerppola, 1960; Kimura & Rasmussen, 1977; Jani et al. 1991). In isolated skeletal muscle mitochondria, studies show either no change, a decrease or an increase in oxidative capacity (Cytochrome c oxidase activity) or in oxidative phosphorylation (Vignos & Greene, 1973; Koski et al. 1974; Capaccio et al. 1985; Marone et al. 1994; Weber et al, 2002).
Since liver and muscle contribute approximately 50% of body oxygen consumption (Rolfe & Brown, 1997), most of it being coupled with ATP synthesis, we undertook this study to investigate the effects of 5 days of high dose (1.5mg/kg/day) dexamethasone on energy metabolism particularly in muscle and liver mitochondria. The comparison with caloric restriction helps in the understanding of the increased energy expenditure observed at the whole body level.
Materials and methods
Animals
The present investigation was performed in accordance with the French guiding principles in the care and use of animals. Thirty-two male Sprague-Dawley rats, born and bred in our animal facility, were housed in individual cages at 9 weeks of age (300–350g). Animals were provided with water ad libitum and a standard diet (U.A.R A04) consisting (% weight) of 16% protein, 3% fat, 60% carbohydrate and 21% water, fibre, vitamins and minerals. The metabolizable energy content was 12 kJ/g. Rats were divided into 4 groups of 8 as follows: dexamethasone(DEX)-treated rats received a daily intraperitoneal injection of 1.5 mg/kg of dexamethasone for 5 days. Due to the fact that dexamethasone treatment induces anorexia, pair-fed (PF) rats were used to discriminate between the effect of anorexia and the effect of dexamethasone itself on the parameters measured. PF rats were pair-fed with DEX-treated animals (rats received the same food quantity consumed by DEX-treated rats the previous day) and were injected daily with an isovolumic solution of 0.9% NaCl. Rats from the control group (CON) were healthy, received no treatment, and were fed ad libitum. Animals of the control injected group (CI) were fed ad libitum and were injected with an isovolumic solution of 0.9% NaCl. This group was used to study the effects of NaCl injection. As results were similar between the two control groups, the CI group was omitted in the presentation of data. Experiments were conducted over a 5-day period. The dose and duration of the dexamethasone treatment was chosen with reference to the literature and is known to induce a reproducible maximum hypercatabolic state (Minet-Quinard et al. 2000). On the 4th day, following an overnight fast, the animals were killed by decapitation. Gastrocnemius, liver and interscapular brown adipose tissue were removed rapidly and weighed. Some tissue samples were immediately used for respiratory measurements and the remainder were frozen in liquid nitrogen and stored at −80°C in order to measure enzyme activity levels. Gastrocnemius muscle was chosen because this mixed-fibers tissue is representative of muscle fiber types contained in the hindlimb of the Sprague-Dawley rat (Armstrong & Phelps, 1984).
Mitochondrial enzyme activities
Frozen liver and gastrocnemius (10–30 mg) were thawed and homogenized with a Potter-Elvehjem homogeniser (7 strokes) in an isolation medium consisting of 220 mM mannitol, 75 mM sucrose, 10 mM Tris and 1 mM EGTA, pH 7.2. Each homogenate was centrifuged at 600 g for 10 min and the resulting supernatants were filtered through cheesecloth. All procedures were performed at 4°C. The activity of citrate synthase, succinate dehydrogenase and complexes I, III and IV was measured spectrophotometrically at 37°C in the supernatant fraction via an adaptation of that as described by Malgat et al. 1999, and in agreement with the Mitochondrial Diseases Group of the Association Française de Myopathie (AFM). Protein concentration was determined using the Bicinchoninic acid Assay kit (Interchim, Montluson, France) with bovine serum albumin (BSA) used as a control.
The activity of citrate synthase (CS) was measured in a reaction medium consisting of 100 mM Tris/HCl, 40 μg/ml 5,5′-dithio-bis(2-nitrobenzoic acid), 1 mM oxaloacetate, 0.3 mM acetyl Co A and 4 % of Triton X 100, pH 8.1. After 3 min of incubation, the reaction was initiated by adding the homogenate (20 to 50 μg proteins) and the change in optical density at 412 nm was recorded for 3 min.
The activity of succinate dehydrogenase was measured by following the reduction of 2,6--dichlorophenolindophenol (DCPIP), in the presence of phenazine methosulfate (PMS) at 600 nm. Homogenate (20 to 50 μg proteins) was preincubated in a buffer containing 50 mM KH2PO4, 16 mM succinate, 1.5 mM KCN, 100 μM PMS, pH 7.5 for 5 min. The reaction began with the addition of 103 μM DCPIP and the optical density was recorded for 3 min.
The activity of complex I was determined by monitoring the oxidation of NADH at 340 nm. Homogenate (40 to 100 μg proteins) was preincubated for 3 min in 820 μ1 of distilled water. Following this, 10 mM KH2PO4, 2 mM KCN, 5 μg/ml antimycin A, 100 μM decylubiquinone, 1.3 mg/ml BSA, 5 mM MgCl, pH 7.5 were added. The reaction was initiated by the addition of 200 μM NADH and the change in the optical density was analysed for 3 min. The NADH decylubiquinone reductase activity was also measured in the presence of 12.7 μM rotenone. The specific activity of complex I represented the difference between NADH oxidation activity, both with and without the rotenone.
The activity of complex III was determined by monitoring the reduction of cytochrome c at 550 nm. Homogenate (20 to 50 μg proteins) was incubated for 30 sec in a reaction medium consisting of 35 mM KH2PO4, 5 mM MgCl2, 2.5 mg/ml BSA, 1.8 mM KCN, 125 μM oxidized cytochrome c, 12.5 μM rotenone and 62.5 mM EDTA, pH 7.5. The reaction was initiated by adding 80 μM decylubiquinol and the optical density was measured for 3 min. The nonenzymatic reduction of cytochrome c was measured under identical conditions after the addition of 10 μg/ml antimycin A. The specific activity of complex III was calculated by subtracting the activity of the nonenzymatic reaction from that of the total activity of complex III.
The activity of complex IV was measured by monitoring the oxidation of reduced cytochrome c at 550 nm. A 50 μM solution of reduced cytochrome c (92 to 97% reduced using dithionite) in 10 mM KH2PO4 pH 7.0 was preincubated for 5 min. The reaction was initiated by adding the homogenate (20 to 50 μg proteins) and the change in optical density was measured for 1.5 min.
Mitochondrial isolations
Gastrocnemius muscle and liver were removed, weighed and immediately placed in an ice-cold isolation medium consisting of 250 mM sucrose, 1 mM EGTA and 10 mM Tris/HCl, pH 7.4. Muscle mitochondria were isolated from gastrocnemius via an adaptation of the differential centrifugation procedure, as used previously by Roussel et al. 2000. All steps were performed at 4°C.
Muscles (2–2.5 g) were cut with scissors, minced using a Polytron (4 to 5 sec.) in an isolation medium (20 ml/g tissue) then homogenized with a Potter-Elvehjem homogeniser (7 strokes). The homogenate was centrifuged at 600 g for 10 min. The resulting supernatant was filtered through cheesecloth and then centrifuged at 10 000 g for 10 min. The pellet was resuspended in the isolation medium and then centrifuged at 10 000 g for 10 min. Finally, the pellet was resuspended in a minimal volume of respiratory medium consisting of 120 mM KCl, 5 mM KH2PO4, 3 mM HEPES, 1 mM EGTA, 2 mM MgCl2 and 0.3 % (w/v) BSA, pH 7.4. Liver mitochondria were isolated by using a modification of the differential centrifugation procedure used previously by Krahenbuhl et al. 1994. The liver (6–7 g) was cut with scissors and homogenized using a Potter-Elvehjem homogeniser (7 strokes) in the isolation medium (8 ml/g tissue). The homogenate was centrifuged at 600 g for 10 min. The resulting supernatant was filtered through cheesecloth, then centrifuged at 7 000 g for 10 min. The pellet was resuspended in the isolation medium (10 ml/g tissue) and then centrifuged at 3 500 g for 10 min. The resulting pellet was resuspended in a minute volume of respiratory medium. The lower g force of the last centrifugation step (3500 g) gives a more homogeneous mitochondrial preparation and is known to minimize the cytoplasmic contamination and mitochondrial damage done to the pellet (< 10% in the present study) in addition to maximizing the respiratory parameters (Goglia et al. 1988; Lanni et al. 1996).
Mitochondrial respiration
Oxygen was measured using a Clark oxygen electrode (oxygraph Hansatech), in a 2 ml glass cell, via continuous stirring at a constant temperature of 30°C. Mitochondria (0.4–0.6 mg protein/ml) were incubated in the respiratory reaction medium as described above and saturated with room air. Substrate concentrations from liver measurements were 5 mM glutamate, 5 mM succinate and 5 mM ascorbate + 0.5 mM N,N, N′ N′-tetramethyl-p-phenylenediamine (TMPD). With regards to gastrocnemius measurements, 5 mM pyruvate + 5 mM malate, 5 mM succinate and 2 mM ascorbate + 0.5 mM TMPD were used. Inhibitor concentrations included 5 μM rotenone (to inhibit complex I of the respiratory chain), and 3 mM myxothiazole (to inhibit complex III). The active state of respiration (state 3) was initiated by the addition of ADP (150 μM to the liver mitochondria or 200 μM to the gastrocnemius mitochondria). The basal non-phosphorylating respiration rate (state 4) was obtained by the addition of 3 μg/ml of oligomycin. RCR was the ratio of oxygen consumed after the addition of ADP to that consumed in the presence of oligomycin. The uncoupled state of respiration was initiated by the addition of 2 μM of FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone). The respiratory parameters measured in isolated mitochondria were normalized in relation to the specific activity of citrate synthase.
Calculation of thermodynamic coupling and efficiency of oxidative phosphorylation
Parameters were calculated using the methodology of Cairns et al 1998. The thermodynamic coupling of the energy conversion is designated by the dimensionless parameter q, known as the degree of coupling of oxidative phosphorylation.
Jsh is the net oxygen consumption in state 4-oligomycin respiration, in the presence of oligomycin that inhibits ATP synthase. Junc is the uncoupled rate of oxygen uptake induced by the addition of FCCP, which dissipates the transmitochondrial proton gradient, and as a result ATP production becomes nil.
Kedem and Caplan (1965) have defined the efficiency of the energy conversion for oxidative phosphorylation (η). Between state 4-oligomycin and state 3-uncoupled respiration, which represent two steady states, an optimal thermodynamic efficiency of the energy conversion (ηopt) can be discerned for any value of q (Stucki, 1980).
Oxidative phosphorylation should operate at a steady state for optimal efficiency for any given degree of coupling. In addition, q can represent several well-defined values depending on the energetic needs of the cell (Stucki, 1980). This theory is based on the thermodynamic trade-off of reducing efficiency to produce the maximum phosphate potential or increasing the efficiency to economize phosphate potential. Stucki (1980) has defined some physiological meanings for the degrees of mitochondrial oxidative coupling. The specific thermodynamic degrees of coupling correspond to the following set points with an unique maximal value of q: qecp (0.972) which is the economic net output power (phosphate potential) at optimal efficiency, qecf (0.953) being the economic net output flow (ATP), qp (0.910) as the maximal net output power and qf (0.786) as the maximal net output flow at optimal efficiency.
In comparison to conventional measurements (RCR, ATP/O), non-equilibrium thermodynamics analysis provides a quantitative description and a better estimation of stoichiometry and the efficiency of energy conversion.
Statistical analysis
Results were expressed as mean ± standard deviation (SD). Means were compared by ANOVA using a Fisher Post-hoc test. A P value of < 0.05 was considered significant in all cases. All analyses were performed using StatView version 5.0 (SAS Institute, Gary, NC, USA).
Results
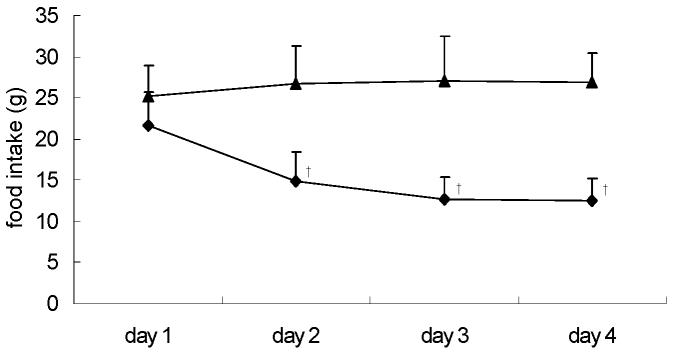
Dexamethasone induced a significant reduction in food intake from day 2 (Fig. 1).
Figure 1.
Daily food intake during the last 4 days of treatment, for control (CON) (▲) and dexamethasone (DEX)-treated (◆) rats. For details of procedure see Materials and methods section. Values expressed as the mean for eight rats per group (standard deviation denoted by vertical bars). Significant differences indicated: † P < 0.01 vs. CON.
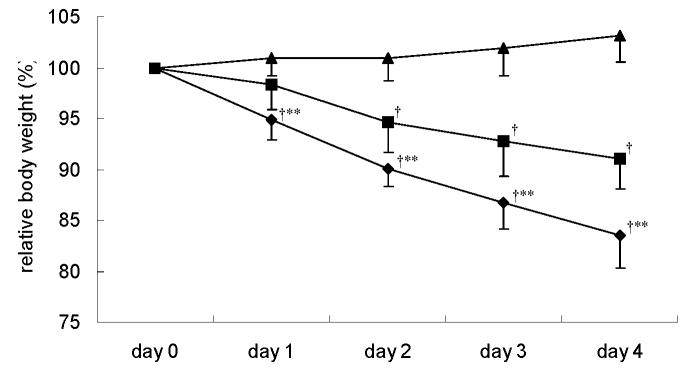
Animals in the 3 groups did not differ, with respect to body weight, at the beginning of the experimental procedure. Body weight decreased in DEX-treated rats from day 1, and PF animals from day 2 (Fig. 2). This decrease was significantly greater in DEX-treated rats than in PF animals, corresponding to 16% (DEX-treated) and 9% (PF) of initial body mass on the 4th day of treatment. At the same time, CON rats increased their body mass by 3.2% (Fig. 2).
Figure 2.
Body weight changes during the first 4 days of treatment for pair-fed (PF) (■), control (CON) (▲) and dexamethasone (DEX)-treated (◆) rats. For details of procedure see Materials and methods section. Values expressed as the mean for eight rats per group (standard deviation denoted by vertical bars). Significant differences indicated: † P < 0.01 vs. CON; ** P < 0.05 vs. PF.
The overnight fasting decreased body weight in the 3 rats groups (Table 1). Liver weight was increased in DEX-treated animals by 23% vs. CON (P<0.01) and by 59% vs. PF (P<0.01) (Table 1). Conversely food restriction significantly decreased liver mass by 20% (PF rats vs. CON rats). This difference in liver mass was not related to a change in hydration, as the dexamethasone-treatment or the food restriction did not affect relative water contents (62.0 ±3.0 vs. 66.0 ± 8.4 vs. 62.1 ± 4.3 % per liver in DEX-treated, PF and CON rats). In DEX-treated rats, gastrocnemius mass was significantly decreased in comparaison with PF rats (−19%) and CON animals (−19%) (Table 1). There was a significant increase in interscapular BAT mass in the DEX-treated group (+117% compared to PF and 90% compared to CON) (Table 1).
Table 1.
Body weight and organ mass: liver, gastrocnemius muscle and interscapular brown adipose tissue in pair-fed (PF), control (CON) and dexamethasone (DEX)-treated rats. (Values expressed as mean ± standard deviation for eight rats per group)
| DEX-treated | PF | CON | ANOVA | |
|---|---|---|---|---|
| Initial body weight, g | 310 ± 28 | 329 ± 38 | 329 ± 32 | NS |
| Sacrifice body weight, g | 242 ± 19 †* | 285 ± 37 | 315 ± 33 | P = 0.0006 |
| Liver, g | 13.38 ± 2.30 †* | 8.58 ± 1. 12 † | 10.84 ± 1.47 | P < 0.0001 |
| g/100g body weight | 5.49 ± 0.66 †* | 3.04 ± 0.28 | 3. 44 ±0.24 | P < 0.0001 |
| Gastrocnemius, g | 3.27 ±0.21 †* | 4.04 ± 0.50 | 4.25 ± 0.27 | P < 0.0001 |
| g/100g body weight | 1.36 ±0.07 | 1.41 ±0.06 | 1.36 ±0.09 | NS |
| Brown adipose tissue, g | 0.76 ± 0.20 †* | 0.35 ±0.10 | 0.40 ±0,14 | P < 0.0001 |
| g/100g body weight | 0.3 1± 0.08 †* | 0.12 ±0.04 | 0.12 ±0.04 | P < 0.0001 |
P < 0.01 vs. PF,
P < 0.01 vs. CON;
NS: not significant.
For details of procedure see Materials and methods.
In the liver, complex I activity was significantly higher in the DEX-treated group than in the PF group (+120%), but it was not different in comparison to the CON group (Table 2). Dexamethasone treatment significantly decreased the specific activity of complex IV (−28% compared to PF rats), although it was not different when compared to controls. There were no significant difference in the specific activities of citrate synthase, succinate dehydrogenase and complex III between the DEX-treated and other groups (Table 2).
Table 2.
Mitochondrial enzyme activities in liver 600 g homogenate from pair-fed (PF), control (CON) and dexamethasone (DEX)-treated rats. (Values expressed as mean ± standard deviation for eight rats per group)
| Enzymatic activity (nmol/min per mg of protein) | DEX-treated | PF | CON | ANOVA |
|---|---|---|---|---|
| Citrate Synthase | 151 ± 27 | 158 ± 23 | 152 ± 26 | NS |
| Succinate Dehydrogenase | 101 ± 26 | 107 ± 30 | 109 ± 26 | NS |
| NADH-ubiquinone reductase (Complex I) | 44 ± 11* | 20 ± 8† | 44 ± 20 | P < 0.01 |
| Ubiquinol-cytochrome c reductase (Complex III) | 88 ± 57 | 86 ± 52 | 101 ± 81 | NS |
| Cytochrome c oxidase (Complex IV) | 91 ± 31** | 126 ± 29 | 100 ± 21 | P<0.05 |
P < 0.01 vs. PF,
P < 0.01 vs. CON;
P < 0.05 vs. PF;
NS: not significant.
For details of procedures see materials and methods section.
In gastrocnemius, none of the enzymatic activities were significantly affected by glucocorticoid treatment (Table 3).
Table 3.
Mitochondrial enzyme activities in gastrocnemius muscle 600 g homogenate from pair-fed (PF), control (CON) and dexamethasone (DEX)-treated rats. (Mean values with their standard deviation for eight rats per group)
| Enzymatic activity (nmol/min per mg of protein) | DEX-treated | PF | CON | ANOVA |
|---|---|---|---|---|
| Citrate Synthase | 602 ± 239 | 653 ±314 | 538 ±183 | NS |
| Succinate Dehydrogenase | 130 ±65 | 147 ± 63 | 125 ± 49 | NS |
| NADH-ubiquinone reductase (Complex I) | 111±57 | 99 ±76 | 85 ±32 | NS |
| Ubiquinol-cytochrome c reductase (Complex III) | 382 ± 190 | 363 ± 208 | 409 ± 170 | NS |
| Cytochrome c oxidase (Complex IV) | 166 ±66 | 213 ±112 | 194 ± 84 | NS |
NS: not significant.
For details of procedures see materials and methods section.
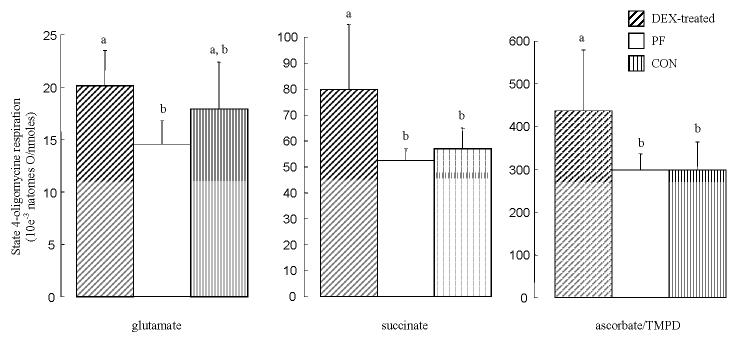
In the isolated liver mitochondria, the specific citrate synthase activity was significantly lower in the DEX-treated group than in the CON group (−28%) and PF group (−20%) (data not shown). Basal non-phosphorylative respiration (state 4-oligomycin respiration) rates, normalized by the specific citrate synthase activity, are shown in Figure 3. With succinate and TMPD/ascorbate used as substrates, the state 4-oligomycin respiration was significantly increased (+46%) in isolated liver mitochondria from DEX-treated rats compared to other groups. In the liver glutamate-respiring mitochondria, dexamethasone treatment significantly increased (+33%) the state 4-oligomycin oxygen consumption in comparison to PF rats, but not when compared to CON rats.
Figure 3.
State 4-oligomycin respiration normalized by the specific citrate synthase activity in liver isolated mitochondria from pair-fed (PF), control (CON) and dexamethasone (DEX)-treated rats. For details of procedure see Materials and methods section. Values expressed as the mean for eight rats per group (standard deviation denoted by vertical bars). Significant differences indicated: ‡ P < 0.05 vs. CON; ** P < 0.05 vs. PF.
Table 4 shows that the respiratory parameters in the liver, expressed per mg of mitochondria protein, were similar, across groups regardless of the respiratory substrate.
Table 4.
Respiratory parameters of liver isolated mitochondria from pair-fed (PF), control (CON) and dexamethasone (DEX)-treated rats. (Values expressed as mean ± standard deviation for eight rats per group)
| Respiratory substrates | Parameters | DEX-treated | PF | CON | ANOVA |
|---|---|---|---|---|---|
| Glutamate | State 3 | 35 ± 16 | 26 ± 13 | 43 ± 19 | NS |
| State 4 | 5 ± 1 | 5 ± 1 | 6 ± 1 | NS | |
| RCR | 7 ± 3 | 5 ± 3 | 7 ± 3 | NS | |
| Uncoupled state | 29 ± 17 | 24 ± 13 | 46 ± 24 | NS | |
| Succinate | State 3 | 76 ± 29 | 79 ± 28 | 98 ± 16 | NS |
| State 4 | 18 ± 5 | 14 ± 3 | 18 ± 2 | NS | |
| RCR | 4.2 ± 1.4 | 5.6 ± 1.4 | 5.4 ± 0.8 | NS | |
| Uncoupled state | 93 ± 26 | 103 ± 31 | 121 ± 21 | NS | |
| TMPD/ascorbate | State 3 | 131 ± 34 | 122 ± 13 | 147 ± 27 | NS |
| State 4 | 98 ± 26 | 81 ± 16 | 96 ± 16 | NS | |
| RCR | 1.3 ± 0.1 | 1.5 ± 0.2 | 1.5 ± 0.1 | NS | |
| Uncoupled state | 167 ± 39 | 163 ± 30 | 190 ± 41 | NS |
NS: not significant.
State 3, state 4 and uncoupled state respiration expressed as natomes of oxygen/min per mg mitochondrial protein. For details of procedures see materials and methods section.
In gastrocnemius none of the respiratory parameters were affected by dexamethasone treatment whatever the substrate used (Table 5). When gastrocnemius respiratory parameters were normalized by the CS activity, no difference was observed between groups (data not shown).
Table 5.
Respiratory parameters of gastrocnemius isolated mitochondria from pair-fed (PF), control (CON) and dexamethasone (DEX)-treated rats. (Values expressed as mean ± standard deviation for eight rats per group)
| Respiratory substrates | Parameters | DEX-treated | PF | CON | ANOVA |
|---|---|---|---|---|---|
| Pyruvate + malate | State 3 | 70 ± 39 | 105 ± 48 | 85 ± 30 | NS |
| State 4 | 8 ± 4 | 9 ± 2 | 7 ± 2 | NS | |
| RCR | 9 ± | 11.7 ± | 12.8 ± | NS | |
| Uncoupled state | 123 ± 74 | 174 ± 37 | 133 ± 41 | NS | |
| Succinate | State 3 | 88 ± 29 | 118 ± 26 | 120 ± 44 | NS |
| State 4 | 24 ± 13 | 35 ± 21 | 27 ± 16 | NS | |
| RCR | 3.7 ± | 3.4 ± | 4.4 ± | NS | |
| Uncoupled state | 123 ± 46 | 156 ± 53 | 128 ± 59 | NS | |
| TMPD/ascorbate | State 3 | 260 ± 117 | 289 ± 75 | 231 ± 61 | NS |
| State 4 | 172 ± 84 | 186 ± 58 | 156 ± 39 | NS | |
| RCR | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.3 | NS | |
| Uncoupled state | 385 ± 128 | 354 ± 108 | 403 ± 121 | NS |
NS: not significant.
State 3, state 4 and uncoupled state respiration expressed as natomes of oxygen/min per mg mitochondrial protein. For details of procedures see materials and methods section.
In liver succinate-respiring mitochondria, q was significantly decreased by dexamethasone treatment. With glutamate, q was marginally (P=0.12) lower in DEX-treated and PF than in CON rats (Table 6). Similar results were obtained for the determined thermodynamic optimal efficiency of oxidative phosphorylation (ηopt).
Table 6.
Thermodynamic degree of coupling (q) and optimal efficiency (ηopt) of the oxidative phosphorylation in liver isolated mitochondria from pair-fed (PF), control (CON) and dexamethasone (DEX)-treated rats. (Values expressed as mean ± standard deviation for eight rats per group)
| Respiratory substrates | Parameters | DEX-treated | PF | CON | ANOVA |
|---|---|---|---|---|---|
| Glutamate | q | 0.903 ± 0.044 | 0.896 ± 0.040 | 0.942 ± 0.013 | NS |
| ηopt | 0.413 ± 0.092 | 0.396 ± 0.080 | 0.500 ± 0.039 | NS | |
| Succinate | q | 0.896 ± 0.019‡** | 0.923 ± 0.022 | 0.922 ± 0.011 | P < 0.05 |
| ηopt | 0.387 ± 0.038‡** | 0.451 ± 0.052 | 0.439 ± 0.028 | P < 0.05 | |
| Ascorbate/TMPD | q | 0.656 ± 0.028†* | 0.708 ± 0.024 | 0.706 ± 0.028 | P < 0.005 |
| ηopt | 0.140 ± 0.016†* | 0.173 ± 0.016 | 0.173 ± 0.018 | P < 0.005 |
P < 0.05 vs. PF;
P < 0.05 vs. CON;
P < 0.01 vs. PF;
P < 0.01 vs. CON.
NS: not significant.
For details of procedures see materials and methods section.
In gastrocnemius, q and ηopt were similar in the 3 rats groups (Table 7).
Table 7.
Thermodynamic degree of coupling (q) and optimal efficiency (ηopt) of the oxidative phosphorylation in gastrocnemius isolated mitochondria from pair-fed (PF), control (CON) and dexamethasone (DEX)-treated rats. (Values expressed as mean ± standard deviation for eight rats per group)
| Respiratory substrates | Parameters | DEX-treated | PF | CON | ANOVA |
|---|---|---|---|---|---|
| Pyruvate + malate | q | 0.966 ± 0.011 | 0.972 ± 0.016 | 0.972 ± 0.011 | NS |
| ηopt | 0.593 ± 0.059 | 0.635 ± 0.089 | 0.628 ± 0.059 | NS | |
| Succinate | q | 0.889 ± 0.039 | 0.880 ± 0.052 | 0.887 ± 0.036 | NS |
| ηopt | 0.400 ± 0.076 | 0.374 ± 0.105 | 0.377 ± 0.064 | NS | |
| Ascorbate/TMPD | q | 0.749 ± 0.073 | 0.713 ± 0.025 | 0.774 ± 0.050 | NS |
| ηopt | 0.214 ± 0.083 | 0.176 ± 0.017 | 0.229 ± 0.051 | NS |
NS: not significant.
For details of procedures see materials and methods section.
Discussion
The present study reports that the induction of a catabolic state, by dexamethasone, results in an increased liver mass and increased non-phosphorylative oxygen consumption in liver mitochondria. In addition, we found a decreased thermodynamic coupling and efficiency of the oxidative phosphorylation in the complex I and II respiratory pathways in the liver mitochondria of DEX-treated rats. In contrast, dexamethasone induced gastrocnemius mass atrophy without affecting mitochondrial energy metabolism.
While the citrate-synthase activity was not modified in the 600 g homogenate it was decreased in isolated mitochondria of liver in DEX-treated rats. Moreover, we found a lower percentage of intact mitochondria in isolated liver mitochondria of DEX-treated rats (91% compared to 96% and 94% in CON and PF groups). Therefore, for the measurement of parameters in isolated mitochondria preparations, we used the specific citrate synthase activity as a mitochondrial marker enzyme. These parameters/CS ratios may reflect a change originating from the mitochondria themselves, rather than from the homogeneization or measurement procedures.
Of particular interest is the finding that dexamethasone treatment significantly increased liver non-phosphorylative oxygen consumption using succinate (+46%) and ascorbate as substrates (+46%; versus CON and PF). With regard to glutamate, state 4-oligomycin respiration was not different compared to control rats, but it was higher (+33%) in DEX-treated relative to PF animals. Therefore, it appears that there is a specific catabolic state-related increase (+42%) in the non-phosphorylative oxygen consumption of liver mitochondria. Indeed, state 4-oligomycin respiration was increased in DEX-treated rats compared to CON rats, except in complex I (effect of food restriction and/or hypercatabolism), and consistent increases were found when DEX-treated animals were compared to PF animals (effect of hypercatabolism). On the other hand, food restriction appears to reduce state 4-oligomycin oxygen consumption (via complex I) in PF compared to CON rats. Such a change in these non-phosphorylative conditions could be due to modifications in respiratory chain activity, inner membrane conductance (proton leak) or the intrinsic coupling of the respiratory chain (H+/2e−). The present acute treatment using dexamethasone did not modify respiratory chain complex activities in the liver. As a result, this strongly suggests that basal proton conductance and/or the efficiency at the level of the respiratory chain did change in the liver mitochondria. In the latter case, we can speculate an effect located on the cytochrome oxidase as state 4-oligomycin respiration by using ascorbate/TMPD as substrate was increased. Moreover, it has been demonstrated that cytochrome oxidase H+/2e− stoichiometry is variable and represents a possible location for intrinsic uncoupling at the level of the respiratory chain (Capitanio et al. 1991; Papa et al. 1991; Piquet et al. 2000). However, further experiments are necessary to confirm these possibilities. Whatever the mechanism involved, this represents a substantial increase in state 4 energy wastage. Moreover, it is interesting to note that proton leak and redox slipping may affect the oxidative phosphorylation yield. Our results agree with data obtained from the long-term administration (3–7 days) of high-dose glucocorticoids, which are reported to decrease liver oxidative phosphorylation (state 3 respiration) via complex I respiratory substrates while remaining unchanged when fuelled through complex II or IV (Kerppola, 1960; Kimura & Rasmussen, 1977; Jani et al. 1991). Our findings that state 4 respiration is increased are therefore complementary to those studies since it has never been studied in DEX-treated rats, although inconsistencies were shown in corticosterone-treated rats (Jani et al. 1991).
Mitochondria can vary the efficiency of oxidative phosphorylation in order to respond to one of four physiological missions: 1) maximizing ATP production with a corresponding value qf for the thermodynamic degree of coupling, 2) maximizing the cellular phosphate potential (qp), 3) minimizing the cost of production (qecf) and 4) a combination of all three (qecp) (Stucki JW, 1980). In the control animals used in our study, the experimentally derived q value for the complex I and II pathways were between that of qp and qecf. Therefore, the liver mitochondria of the control animals adapted their function for the economic production of ATP in addition to maintaining the phosphate potential. Dexamethasone decreases the degree of thermodynamic coupling of oxidative phosphorylation in both complex I and II respiratory pathways. Their values were between qf and qp and nearly that of qp. In relation to this result, the thermodynamic optimal efficiency of energy conversion (ηopt;) was also decreased by dexamethasone. These results therefore suggest that in DEX-treated rats, liver mitochondria adapt their function for maximum ATP production and also to maintain cellular phosphate potential at the expense of the energy conversion efficiency. Similar adaptive reductions in the efficiency of oxidative phosphorylation has already been noted by Nogueira et al 2001 who showed that cellular respiratory rate increases in liver mitochondria of either hyperthyroid rats or animals exposed to a polyunsatured fatty acid deficient diet.
It is well known that liver mass is decreased during energy restriction, which in turn could partly explain why energy expenditure falls in response to a reduced food intake (Ramsey et al. 2000). In the present study, liver weight was decreased by food restriction (PF versus control rats) and higher (23 to 59%) in DEX-treated rats compared with other rat groups. Such an increase in liver mass could result from an increased glycogen content (Weber & Kletzien, 1982; Michaels & Cardell, 1997; Bollen et al. 1998) although we did not find any difference in hydratation. Alternatively, hepatic lipid content is increased in DEX-treated rats, but is insufficient to fully explain the increased liver mass (Kaur N et al. 1989; Palacios et al. 1995; Franco-Colin et al. 2000). Finally, dexamethasone treatment increase liver protein synthesis and therefore metabolic tissue (Odedra et al. 1983; Savary et al. 2001). This suggests that both maintenance and growth costs may be increased.
Our study demonstrated that a dexamethasone-induced hypercatabolic state results in skeletal muscle atrophy without any change in mitochondrial energy metabolism. Indeed, none of the respiratory complex activities, oxygen consumption rates or the thermodynamic degree of coupling of oxidative phosphorylation, were altered in the mitochondria of DEX-treated rats. This is in agreement with previously reported effects of glucocorticoid treatment (Vignos & Greene, 1973; Capaccio et al. 1985; Marone et al, 1994). These results suggest that the mitochondrial metabolism of skeletal muscle produces enough ATP to fulfil either the cellular energy requirement and/or the energy-dependent pathways induced by glucocorticoids, such as the energy-ubiquin-dependent proteolytic pathway (Tiao G et al. 1996; Mitch et al. 1999), and the energy-dependent glutamine synthase activity pathway (Max SR et al, 1988; Minet-Quinard et al. 1999, 2000).
The main thermogenic tissue in rats is brown adipose tissue, the weight of which is largely increased (+100%, present study) by glucocorticoid injection. Previous studies have clearly demonstrated that such an increase in the BAT mass was due to increased lipid storage rather than an increased thermogenic capacity of this tissue (Mazzuccheli et al. 1960; Strack et al. 1995). Furthermore, glucocorticoids are known to reduce the activity or the gene expression of UCP1 (Tokuyama & Himms-Hagen, 1989; Moriscot et al. 1993; Strack et al. 1995). Moreover, in our study we found no effect of dexamethasone on mitochondrial oxidative capacity (cytochrome c oxidase activity; data not shown). Therefore, it is unlikely that interscapular BAT increases energy expenditure in DEX-treated rats.
A reduction in body size (a 16% weight loss in the present study) generally results in lowering of energy expenditure per whole rat, because of the reduced maintenance requirement (Ramsey et al. 2000). In the present study, the dexamethasone-related decrease in the body weight was greater than that observed in the PF rats, highlighting an increased whole body energy expenditure. This is paradoxical in view of the reducing effect of food restriction on energy expenditure (Ramsey et al. 2000). Furthermore, in our study, dexamethasone treatment increased the liver-to-body weight ratio while the relative skeletal muscle mass remained unchanged. Since liver and muscle are the main contributors to standard metabolic rate in rats (Rolfe & Brown, 1997), it is likely that the liver would effectively contribute to the increased energy expenditure despite the body weight loss (Woodward & Emery, 1989). Indeed, if we assume that liver contributes 20% to the metabolic rate of a rat (Rolfe & Brown, 1997), then the 60–80% gain in the liver-to-body weight ratio reported herein, could increase total energy expenditure by 12–16%. Glucocorticoids administered in humans and rats results in an increased energy expenditure by 10–20% (Woodward & Emery, 1989; Brillon et al. 1995; Tataranni et al. 1996). Obviously, we can not rule out the influence of other biochemical mechanisms or possible determinants of energy balance which have accounted also for the weight loss.
In conclusion, 5 days of high-dose dexamethasone treatment induced a significant increase in liver mass, an increase in liver mitochondrial non-phosphorylative oxygen consumption rate from all substrates used, and a decrease in the thermodynamic coupling of oxidative phosphorylation in liver respiratory pathways. We suggest therefore that dexamethasone increases proton leak and/or redox slipping in liver mitochondria, which in turn is probably responsible for the decrease in the thermodynamic efficiency of energy conversion. Thus, rats would adapt their mitochondrial energy functions to a dexamethasone-induced hypermetabolic state by maximizing ATP production in addition to maintaining their cellular energy state, regardless of the cost. This treatment has no effect on energy metabolism in the muscle. Together with a decreased food intake, the increase in energy expenditure induced by high dose of dexamethasone results in a negative energy balance and thus weight loss.
Acknowledgments
Authors thank Pierre Legras, Jerome Roux and Dominique Gilbert for animal care, Antoine Augeraud for technical assistance and to Miriam Ryan for her assistance in correcting the english. This work was supported by grants from Contrat de Plan Etat Region 2000–2004.
Text footnotes
- BAT
brown adipose tissue
- CS
citrate synthase
- DCPIP
2,3-dichlorophenollindophenol
- FCCP
carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- PMS
phenazine methosulfate
- RCR
respiratory control ratio
- TCA
tricarboxylic acid
- TMPD
N,N,N′,N′-tetramethyl-p-phenylenediamine
- TNB
thio-bis(2-nitrobenzoic acid)
References
- Allan EH, Chisholm AB, Titheradge MA. The stimulation of hepatic oxidative phosphorylation following dexamethasone treatment of rats. Biochimica and Biophysica Acta. 1983;725:71–76. doi: 10.1016/0005-2728(83)90225-6. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. American Journal of Anatomy. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Bollen M, Keppens S, Stalmans W. Specific features of glycogen metabolism in the liver. Biochemical Journal. 1998;336:19–31. doi: 10.1042/bj3360019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes SB, Jackson NC, Papachristodoulou D, Umpleby AM, Sonksen PH. Effect of corticosterone on protein degradation in isolated rat soleus and extensor digitorum longus muscles. Journal of Endocrinology. 1996;148:501–507. doi: 10.1677/joe.0.1480501. [DOI] [PubMed] [Google Scholar]
- Brand MD, Chien LF, Ainscow EK, Rolfe DF, Porter RK. The causes and functions of mitochondrial proton leak. Biochimica and Biophysica Acta. 1994;1187:132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Brand MD, Hafner RP, Brown GC. Control of respiration in non-phosphorylating mitochondria is shared between the proton leak and the respiratory chain. Biochemical Journal. 1988;255:535–539. [PMC free article] [PubMed] [Google Scholar]
- Brillon DJ, Zheng B, Campbell RG, Matthews DE. Effect of cortisol on energy expenditure and amino acid metabolism in humans. American Journal of Physiology. 1995;268:E501–513. doi: 10.1152/ajpendo.1995.268.3.E501. [DOI] [PubMed] [Google Scholar]
- Cairns CB, Walther J, Harken AH, Banerjee A. Mitochondrial oxidative phosphorylation thermodynamic efficiencies reflect physiological organ roles. American Journal of Physiology. 1998;274:R1376–1383. doi: 10.1152/ajpregu.1998.274.5.R1376. [DOI] [PubMed] [Google Scholar]
- Capaccio JA, Galassi TM, Hickson RC. Unaltered aerobic power and endurance following glucocorticoid-induced muscle atrophy. Medicine Sciences Sports Exercise. 1985;17:380–384. [PubMed] [Google Scholar]
- Capitanio N, Capitanio G, De Nitto E, Villani G, Papa S. H+/e- stoichiometry of mitochondrial cytochrome complexes reconstituted in liposomes. Rate-dependent changes of the stoichiometry in the cytochrome c oxidase vesicles. FEBS Letters. 1991;288:179–182. doi: 10.1016/0014-5793(91)81029-8. [DOI] [PubMed] [Google Scholar]
- Chima CS, Barco K, Dewitt ML, Maeda M, Teran JC, Mullen KD. Relationship of nutritional status to length of stay, hospital costs, and discharge status of patients hospitalized in the medicine service. Journal of the American Dietetic Association. 1997;97:975–978. doi: 10.1016/S0002-8223(97)00235-6. quiz 979–980. [DOI] [PubMed] [Google Scholar]
- Franco-Colin M, Tellez-Lopez AM, Quevedo-Corona L, Racotta R. Effects of long-term high-sucrose and dexamethasone on fat depots, liver fat, and lipid fuel fluxes through the retroperitoneal adipose tissue and splanchnic area in rats. Metabolism. 2000;49:1289–1294. doi: 10.1053/meta.2000.9522. [DOI] [PubMed] [Google Scholar]
- Goglia F, Liverini G, Lanni A, lossa S, Barletta A. Light mitochondria and cellular thermogenesis. Biochemical and Biophysical Research Communications. 1988;151:1241–1249. doi: 10.1016/s0006-291x(88)80499-6. [DOI] [PubMed] [Google Scholar]
- Hausberger FX, Hausberger BC. Effect of insulin and cortisone on weight gain, protein and fat content of rats. American Journal of Physiology. 1958;103:455–460. doi: 10.1152/ajplegacy.1958.193.3.455. [DOI] [PubMed] [Google Scholar]
- Hill JO, Latiff A, DiGirolamo M. Effects of variable caloric restriction on utilization of ingested energy in rats. American Journal of Physiology. 1985;248:R549–559. doi: 10.1152/ajpregu.1985.248.5.R549. [DOI] [PubMed] [Google Scholar]
- Jani MS, Telang SD, Katyare SS. Effect of corticosterone treatment on energy metabolism in rat liver mitochondria. Journal of Steroid Biochemistry and Molecular Biology. 1991;38:587–591. doi: 10.1016/0960-0760(91)90317-x. [DOI] [PubMed] [Google Scholar]
- Kaur N, Sharma N, Gupta AK. Effects of dexamethasone on lipid metabolism in rat organs. Indian Journal of Biochemistry and Biophysics. 1989;26:371–376. [PubMed] [Google Scholar]
- Kedem O, Caplan SR. Degree of coupling and its relation to efficiency of energy conversion. Transactions Faraday Society. 1965;21:1897–1911. [Google Scholar]
- Kerppola W. Uncoupling of the oxidative phosphorylation with cortisone in liver mitochondria. Endocrinology. 1960;67:252–263. doi: 10.1210/endo-67-2-252. [DOI] [PubMed] [Google Scholar]
- Kimura S, Rasmussen H. Adrenal glucocorticoids, adenine nucleotide translocation, and mitochondrial calcium accumulation. Journal of Biological Chemistry. 1977;252:1217–1225. [PubMed] [Google Scholar]
- Kochakian CD, Robertson E. Adrenal steroids and body composition. Journal of Biological Chemistry. 1951;190:495–503. [PubMed] [Google Scholar]
- Koski CL, Rifenberick DH, Max SR. Oxidative metabolism of skeletal muscle in steroid atrophy. Archives of Neurology. 1974;31:407–410. doi: 10.1001/archneur.1974.00490420073008. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl S, Talos C, Fischer S, Reichen J. Toxicity of bile acids on the electron transport chain of isolated rat liver mitochondria. Hepatology. 1994;19:471–479. doi: 10.1002/hep.1840190228. [DOI] [PubMed] [Google Scholar]
- Landi F, Onder G, Gambassi G, Pedone C, Carbonin P, Bernabei R. Body mass index and mortality among hospitalized patients. Archives of Internal Medicine. 2000;160:2641–2644. doi: 10.1001/archinte.160.17.2641. [DOI] [PubMed] [Google Scholar]
- Lanni A, Moreno M, Lombardi A, Goglia F. Biochemical and functional differences in rat liver mitochondrial subpopulations obtained at different gravitational forces. International Journal of Biochemistry and Cell Biology. 1996;28:337–343. doi: 10.1016/1357-2725(95)00137-9. [DOI] [PubMed] [Google Scholar]
- Lesourd B, Mazzari L. Immune responses during recovery from protein energy malnutrition. Clinical Nutrition. 1997;16:37–46. doi: 10.1016/s0261-5614(97)80047-7. [DOI] [PubMed] [Google Scholar]
- Malgat M, Durrieu G, Mazat JP. Enzymatic and polarographic measurements of the respiratory chain complexes. In: Lestienne P, editor. Mitochondrial deases. Paris: Springer Verlag; 1999. pp. 357–377. [Google Scholar]
- Marone JR, Falduto MT, Essig DA, Hickson RC. Effects of glucocorticoids and endurance training on cytochrome oxidase expression in skeletal muscle. Journal of Applied Physiology. 1994;77:1685–1690. doi: 10.1152/jappl.1994.77.4.1685. [DOI] [PubMed] [Google Scholar]
- Max SR, Mill J, Mearow K, Konagaya M, Konagaya Y, Thomas JW, Banner C, Vitkovic L. Dexamethasone regulates glutamine synthetase expression in rat skeletal muscles. American Journal of Physiology. 1988;255:E397–402. doi: 10.1152/ajpendo.1988.255.3.E397. [DOI] [PubMed] [Google Scholar]
- Mazzuccheli MV, Confalonieri C, Schlechter P. The nervous system and lipid metabolism of adipose tissue. 2. Influence of denervation on the lipogenetic activity of adrenocortical hormones. Metabolism. 1960;10:330–334. [PubMed] [Google Scholar]
- Michaels JE, Cardell RR. Localization of glycogen phosphorylase activity in liver of fasted normal and adrenalectomized rats and in fasted adrenalectomized rats after injection of dexamethasone. Anatomical Record. 1997;248:406–412. doi: 10.1002/(SICI)1097-0185(199707)248:3<406::AID-AR13>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Minet-Quinard R, Moinard C, Villie F, Walrand S, Vasson MP, Chopineau J, Cynober L. Kinetic impairment of nitrogen and muscle glutamine metabolisms in old glucocorticoid-treated rats. American Journal of Physiology. 1999;276:E558–564. doi: 10.1152/ajpendo.1999.276.3.E558. [DOI] [PubMed] [Google Scholar]
- Minet-Quinard R, Moinard C, Walrand S, Villie F, Normand B, Vasson MP, Chopineau J, Cynober L. Induction of a catabolic state in rats by dexamethasone: dose or time dependency? Journal of Parenteral and Enteral Nutrition. 2000;24:30–36. doi: 10.1177/014860710002400130. [DOI] [PubMed] [Google Scholar]
- Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. American Journal of Physiology. 1999;276:C1132–1138. doi: 10.1152/ajpcell.1999.276.5.C1132. [DOI] [PubMed] [Google Scholar]
- Moriscot A, Rabelo R, Bianco AC. Corticosterone inhibits uncoupling protein gene expression in brown adipose tissue. American Journal of Physiology. 1993;265:E81–87. doi: 10.1152/ajpendo.1993.265.1.E81. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Bedu M, Caillaud D, Beaufrere B, Beaujon G, Vasson M, Coudert J, Ritz P. Increased resting energy expenditure is related to plasma TNF-alpha concentration in stable COPD patients. Clinical Nutrition. 1999;18:269–274. doi: 10.1016/s0261-5614(98)80023-x. [DOI] [PubMed] [Google Scholar]
- Nogueira V, Rigoulet M, Piquet MA, Devin A, Fontaine E, Leverve XM. Mitochondrial respiratory chain adjustment to cellular energy demand. Journal of Biological Chemistry. 2001;276:46104–46110. doi: 10.1074/jbc.M107425200. [DOI] [PubMed] [Google Scholar]
- Odedra BR, Bates PC, Millward DJ. Time course of the effect of catabolic doses of corticosterone on protein turnover in rat skeletal muscle and liver. Biochemical Journal. 1983;214:617–627. doi: 10.1042/bj2140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios E, Pinon-Lopez MJ, Racotta IS, Racotta R. Effect of lipectomy and long-term dexamethasone on visceral fat and metabolic variables in rats. Metabolism. 1995;44:1631–1638. doi: 10.1016/0026-0495(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Papa S, Capitanio N, Capitanio G, De Nitto E, Minuto M. The cytochrome chain of mitochondria exhibits variable H+/e- stoichiometry. FEBS Letters. 1991;288:183–186. doi: 10.1016/0014-5793(91)81030-c. [DOI] [PubMed] [Google Scholar]
- Piquet MA, Nogueira V, Devin A, Sibille B, Filippi C, Fontaine E, Roulet M, Rigoulet M, Leverve XM. Chronic ethanol ingestion increases efficiency of oxidative phosphorylation in rat liver mitochondria. FEBS Letters. 2000;468:239–242. doi: 10.1016/s0014-5793(00)01225-4. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radical Biology and Medicine. 2000;29:946–968. doi: 10.1016/s0891-5849(00)00417-2. [DOI] [PubMed] [Google Scholar]
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiological Reviews. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Roussel D, Lhenry F, Ecochard L, Sempore B, Rouanet JL, Favier R. Differential effects of endurance training and creatine depletion on regional mitochondrial adaptations in rat skeletal muscle. Biochemical Journal. 2000;350(Pt 2):547–553. [PMC free article] [PubMed] [Google Scholar]
- Savary I, Debras E, Dardevet D, Rambourdin F, Vasson MP, Obled C, Grizard J. Evidence for an alteration of plasma and liver proteins response to dexamethasone in aging rats. Mechanisms of Ageing and Development. 2001;122:105–120. doi: 10.1016/s0047-6374(00)00224-4. [DOI] [PubMed] [Google Scholar]
- Strack AM, Bradbury MJ, Dallman MF. Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. American Journal of Physiology. 1995;268:R183–191. doi: 10.1152/ajpregu.1995.268.1.R183. [DOI] [PubMed] [Google Scholar]
- Stucki JW. The optimal efficiency and the economic degrees of coupling of oxidative phosphorylation. European Journal of Biochemistry. 1980;109:269–283. doi: 10.1111/j.1432-1033.1980.tb04792.x. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. American Journal of Physiology. 1996;271:E317–325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- Tiao G, Fagan J, Roegner V, Lieberman M, Wang JJ, Fischer JE, Hasselgren PO. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. Journal of Clinical Investigation. 1996;97:339–348. doi: 10.1172/JCI118421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama K, Himms-Hagen J. Enhanced acute response to corticosterone in genetically obese (ob/ob) mice. American Journal of Physiology. 1989;257:E133–138. doi: 10.1152/ajpendo.1989.257.2.E133. [DOI] [PubMed] [Google Scholar]
- Tucker HN, Miguel SG. Cost containment through nutrition intervention. Nutrition Reviews. 1996;54:111–121. doi: 10.1111/j.1753-4887.1996.tb03885.x. [DOI] [PubMed] [Google Scholar]
- Vignos PJ, Greene R. Oxidative respiration of skeletal muscle in experimental corticosteroid myopathy. Journal of Laboratory Clinical Medicine. 1973;81:365–378. [PubMed] [Google Scholar]
- Wakat DK, Haynes RC., Jr Glucocorticoid-stimulated utilization of substrates in hepatic mitochondria. Archives of Biochemistry and Biophysics. 1977;184:561–571. doi: 10.1016/0003-9861(77)90466-0. [DOI] [PubMed] [Google Scholar]
- Wallace JI, Schwartz RS, LaCroix AZ, Uhlmann RF, Pearlman RA. Involuntary weight loss in older outpatients: incidence and clinical significance. Journal of the American Geriatrics Society. 1995;43:329–337. doi: 10.1111/j.1532-5415.1995.tb05803.x. [DOI] [PubMed] [Google Scholar]
- Weber CA, Kletzien RF. Hormonal and nutritional factors influencing glycogen deposition in primary cultures of rat liver parenchymal cells. Journal of Cellular Physiology. 1982;110:300–303. doi: 10.1002/jcp.1041100313. [DOI] [PubMed] [Google Scholar]
- Weber K, Bruck P, Mikes Z, Kupper JH, Klingenspor M, Wiesner RJ. Glucocorticoid hormone stimulates mitochondrial biogenesis specifically in skeletal muscle. Endocrinology. 2002;143:177–184. doi: 10.1210/endo.143.1.8600. [DOI] [PubMed] [Google Scholar]
- Woodward CJ, Emery PW. Energy balance in rats given chronic hormone treatment. 2. Effects of corticosterone. British Journal of Nutrition. 1989;61:445–452. doi: 10.1079/bjn19890134. [DOI] [PubMed] [Google Scholar]