Abstract
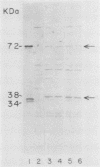
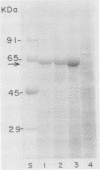
The mosquitocidal properties of Bacillus thuringiensis subsp. israelensis and B. thuringiensis subsp. morrisoni PG-14 are attributable to protein inclusions grouped together within a parasporal body. In both of these strains, the mosquitocidal activity resides in proteins with molecular masses of 27, 72, 128, and 135 kDa. In an attempt to determine the toxicity of each protein, the shuttle vector pHT3101 was used to express the cryIVD gene (encoding the 72-kDa CryIVD protein) from B. thuringiensis subsp. morrisoni in an acrystalliferous mutant of B. thuringiensis subsp. kurstaki. With this system, parasporal inclusions of the 72-kDa protein were obtained that were comparable in size, shape, and toxicity to those produced by parental B. thuringiensis subsp. morrisoni. The inclusions were bar shaped, measured 500 by 300 by 150 nm, and were easily visible with phase-contrast microscopy by 16 h of cell growth. A 50% lethal concentration of 64 ng/ml for these inclusions was determined in bioassays against fourth instars of Culex quinquefasciatus, which was similar to the 50% lethal concentration of 55 ng/ml obtained for the 72-kDa inclusion from B. thuringiensis subsp. israelensis. In contrast, expression of the cryIVD gene in Escherichia coli was very low and only detectable by immunoblot analysis. These results demonstrate that the pHT3101-B. thuringiensis expression system can be used to express the CryIVD protein in quantities and with properties comparable to that obtained with the natural host. This system may prove useful for the expression of other B. thuringiensis proteins and, in particular, for reconstitution experiments with inclusions produced by the mosquitocidal subspecies of B. thuringiensis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. F., Visick J. E., Whiteley H. R. A 20-kilodalton protein is required for efficient production of the Bacillus thuringiensis subsp. israelensis 27-kilodalton crystal protein in Escherichia coli. J Bacteriol. 1989 Jan;171(1):521–530. doi: 10.1128/jb.171.1.521-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgouin C., Delécluse A., Ribier J., Klier A., Rapoport G. A Bacillus thuringiensis subsp. israelensis gene encoding a 125-kilodalton larvicidal polypeptide is associated with inverted repeat sequences. J Bacteriol. 1988 Aug;170(8):3575–3583. doi: 10.1128/jb.170.8.3575-3583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcott C. N., Ellar D. J. Comparative toxicity of Bacillus thuringiensis var. israelensis crystal proteins in vivo and in vitro. J Gen Microbiol. 1988 Sep;134(9):2551–2558. doi: 10.1099/00221287-134-9-2551. [DOI] [PubMed] [Google Scholar]
- Delécluse A., Bourgouin C., Klier A., Rapoport G. Specificity of action on mosquito larvae of Bacillus thuringiensis israelensis toxins encoded by two different genes. Mol Gen Genet. 1988 Sep;214(1):42–47. doi: 10.1007/BF00340177. [DOI] [PubMed] [Google Scholar]
- Delécluse A., Charles J. F., Klier A., Rapoport G. Deletion by in vivo recombination shows that the 28-kilodalton cytolytic polypeptide from Bacillus thuringiensis subsp. israelensis is not essential for mosquitocidal activity. J Bacteriol. 1991 Jun;173(11):3374–3381. doi: 10.1128/jb.173.11.3374-3381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan W. P., Dankocsik C., Gilbert M. P. Molecular characterization of a gene encoding a 72-kilodalton mosquito-toxic crystal protein from Bacillus thuringiensis subsp. israelensis. J Bacteriol. 1988 Oct;170(10):4732–4738. doi: 10.1128/jb.170.10.4732-4738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earp D. J., Ellar D. J. Bacillus thuringiensis var. morrisoni strain PG14: nucleotide sequence of a gene encoding a 27kDa crystal protein. Nucleic Acids Res. 1987 Apr 24;15(8):3619–3619. doi: 10.1093/nar/15.8.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge A. Z., Shivarova N. I., Dean D. H. Location of the Bombyx mori specificity domain on a Bacillus thuringiensis delta-endotoxin protein. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4037–4041. doi: 10.1073/pnas.86.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. S., Hornung J. M., Ibarra J. E., Singh G. J., Federici B. A. Cytolytic activity and immunological similarity of the Bacillus thuringiensis subsp. israelensis and Bacillus thuringiensis subsp. morrisoni isolate PG-14 toxins. Appl Environ Microbiol. 1987 Jun;53(6):1251–1256. doi: 10.1128/aem.53.6.1251-1256.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider M. Z., Ellar D. J. Characterization of the toxicity and cytopathic specificity of a cloned Bacillus thuringiensis crystal protein using insect cell culture. Mol Microbiol. 1987 Jul;1(1):59–66. doi: 10.1111/j.1365-2958.1987.tb00527.x. [DOI] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., de Greve H., Seurinck J., Jansens S., Mahillon J., Ampe C., Vandekerckhove J., Vanderbruggen H., van Montagu M., Zabeau M. Structural and functional analysis of a cloned delta endotoxin of Bacillus thuringiensis berliner 1715. Eur J Biochem. 1986 Dec 1;161(2):273–280. doi: 10.1111/j.1432-1033.1986.tb10443.x. [DOI] [PubMed] [Google Scholar]
- Ibarra J. E., Federici B. A. Isolation of a relatively nontoxic 65-kilodalton protein inclusion from the parasporal body of Bacillus thuringiensis subsp. israelensis. J Bacteriol. 1986 Feb;165(2):527–533. doi: 10.1128/jb.165.2.527-533.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Eckblad W., Bulla L. A., Jr Diversity of protein inclusion bodies and identification of mosquitocidal protein in Bacillus thuringiensis subsp. israelensis. Biochem Biophys Res Commun. 1985 Jan 31;126(2):953–960. doi: 10.1016/0006-291x(85)90278-5. [DOI] [PubMed] [Google Scholar]
- Lereclus D., Arantès O., Chaufaux J., Lecadet M. Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol Lett. 1989 Jul 15;51(1):211–217. doi: 10.1016/0378-1097(89)90511-9. [DOI] [PubMed] [Google Scholar]
- McLean K. M., Whiteley H. R. Expression in Escherichia coli of a cloned crystal protein gene of Bacillus thuringiensis subsp. israelensis. J Bacteriol. 1987 Mar;169(3):1017–1023. doi: 10.1128/jb.169.3.1017-1023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurter W., Geiser M., Mathé D. Efficient transformation of Bacillus thuringiensis and B. cereus via electroporation: transformation of acrystalliferous strains with a cloned delta-endotoxin gene. Mol Gen Genet. 1989 Jul;218(1):177–181. doi: 10.1007/BF00330581. [DOI] [PubMed] [Google Scholar]
- Visick J. E., Whiteley H. R. Effect of a 20-kilodalton protein from Bacillus thuringiensis subsp. israelensis on production of the CytA protein by Escherichia coli. J Bacteriol. 1991 Mar;173(5):1748–1756. doi: 10.1128/jb.173.5.1748-1756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. S., Ellar D. J., Chilcott C. N. Single amino acid changes in the Bacillus thuringiensis var. israelensis delta-endotoxin affect the toxicity and expression of the protein. J Mol Biol. 1988 Aug 5;202(3):527–535. doi: 10.1016/0022-2836(88)90283-5. [DOI] [PubMed] [Google Scholar]
- Ward E. S., Ellar D. J. Cloning and expression of two homologous genes of Bacillus thuringiensis subsp. israelensis which encode 130-kilodalton mosquitocidal proteins. J Bacteriol. 1988 Feb;170(2):727–735. doi: 10.1128/jb.170.2.727-735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. S., Ridley A. R., Ellar D. J., Todd J. A. Bacillus thuringiensis var. israelensis delta-endotoxin. Cloning and expression of the toxin in sporogenic and asporogenic strains of Bacillus subtilis. J Mol Biol. 1986 Sep 5;191(1):13–22. doi: 10.1016/0022-2836(86)90418-3. [DOI] [PubMed] [Google Scholar]