Abstract
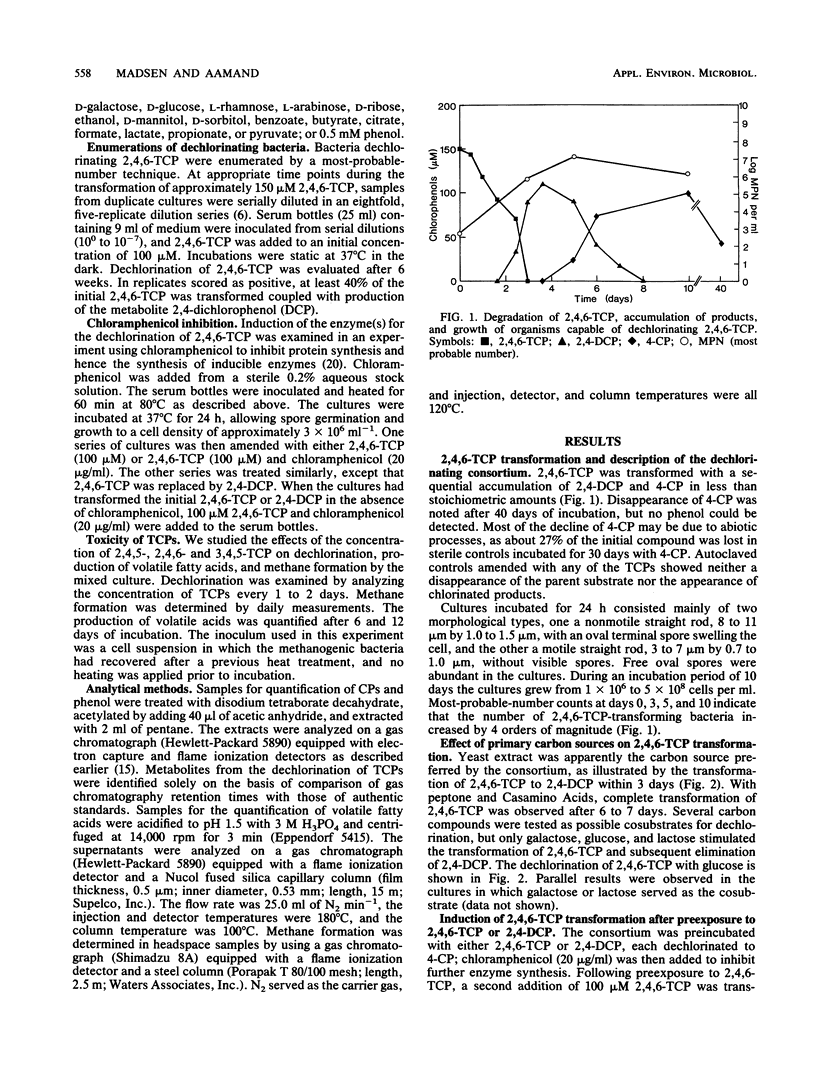
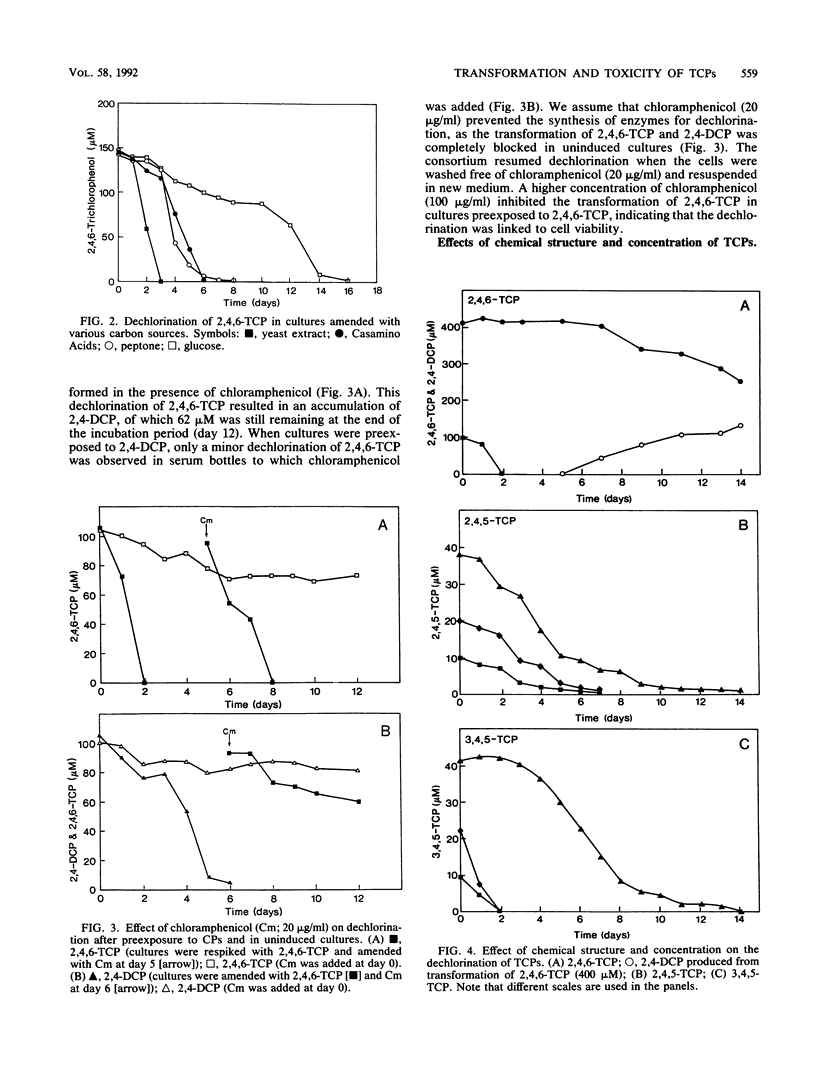
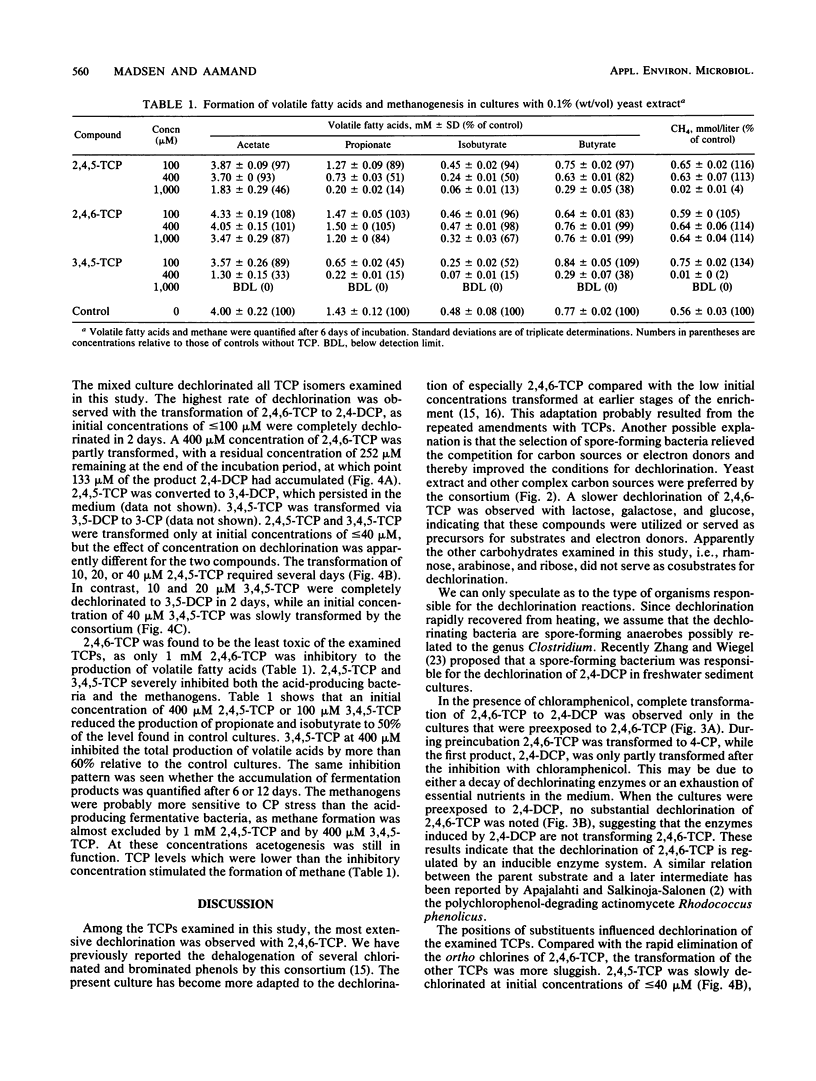
The transformation and toxicity of trichlorophenols (TCPs) were studied with a methanogenic enrichment culture derived from sewage sludge. Transformation of TCPs rapidly resumed after heating of the culture at *) degrees C for 1 h, suggesting that the dechlorinating bacteria are spore-forming anaerobes. 2,4,6-TCP was rapidly dechlorinated via 2,4-dichlorophenol to 4-chlorophenol. During the transformation of 2,4,6-TCP, the most probable number of dechlorinating bacteria increased by 4 orders of magnitude. The most extensive dechlorination was observed in media with complex carbon sources such as yeast extract, peptone, and Casamino Acids, but glucose, galactose, and lactose were also used by the consortium. Experiments using chloramphenicol indicated that the reductive dechlorination of 2,4,6-TCP was regulated by an inducible enzyme system. The highest initial concentration at which dechlorination of 2,4,6-TCP was observed was 400 microM. 2,4,5-TCP and 3,4,5-TCP were dechlorinated to, respectively, 3,4-dichlorophenol and 3-chlorophenol at initial concentrations of less than or equal to 40 microM. Toxicity for the acid-producing and methanogenic bacteria in the consortium was a function of chemical structure, as the inhibition of these activities increased from 2,4,6-TCP, via 2,4,5-TCP, to 3,4,5,-TCP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apajalahti J. H., Salkinoja-Salonen M. S. Dechlorination and para-hydroxylation of polychlorinated phenols by Rhodococcus chlorophenolicus. J Bacteriol. 1987 Feb;169(2):675–681. doi: 10.1128/jb.169.2.675-681.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay N., Daniels L. Production of ethane, ethylene, and acetylene from halogenated hydrocarbons by methanogenic bacteria. Appl Environ Microbiol. 1987 Jul;53(7):1604–1610. doi: 10.1128/aem.53.7.1604-1610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd S. A., Shelton D. R. Anaerobic biodegradation of chlorophenols in fresh and acclimated sludge. Appl Environ Microbiol. 1984 Feb;47(2):272–277. doi: 10.1128/aem.47.2.272-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone D. G., Reese D. D., Kiene R. P. Effects of metals on methanogenesis, sulfate reduction, carbon dioxide evolution, and microbial biomass in anoxic salt marsh sediments. Appl Environ Microbiol. 1983 May;45(5):1586–1591. doi: 10.1128/aem.45.5.1586-1591.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfing J., Tiedje J. M. Influence of substituents on reductive dehalogenation of 3-chlorobenzoate analogs. Appl Environ Microbiol. 1991 Mar;57(3):820–824. doi: 10.1128/aem.57.3.820-824.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Suflita J. M. Extrapolation of biodegradation results to groundwater aquifers: reductive dehalogenation of aromatic compounds. Appl Environ Microbiol. 1986 Oct;52(4):681–688. doi: 10.1128/aem.52.4.681-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom M. M., Young L. Y. Chlorophenol degradation coupled to sulfate reduction. Appl Environ Microbiol. 1990 Nov;56(11):3255–3260. doi: 10.1128/aem.56.11.3255-3260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkfield T. G., Suflita J. M., Tiedje J. M. Characterization of the acclimation period before anaerobic dehalogenation of halobenzoates. Appl Environ Microbiol. 1989 Nov;55(11):2773–2778. doi: 10.1128/aem.55.11.2773-2778.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Thomson K., Kaiser K. L. Quantitative structure-toxicity relationship of halogenated phenols on bacteria. Bull Environ Contam Toxicol. 1982 Aug;29(2):130–136. doi: 10.1007/BF01606140. [DOI] [PubMed] [Google Scholar]
- Madsen T., Aamand J. Effects of sulfuroxy anions on degradation of pentachlorophenol by a methanogenic enrichment culture. Appl Environ Microbiol. 1991 Sep;57(9):2453–2458. doi: 10.1128/aem.57.9.2453-2458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikesell M. D., Boyd S. A. Complete reductive dechlorination and mineralization of pentachlorophenol by anaerobic microorganisms. Appl Environ Microbiol. 1986 Oct;52(4):861–865. doi: 10.1128/aem.52.4.861-865.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikesell M. D., Boyd S. A. Dechlorination of chloroform by methanosarcina strains. Appl Environ Microbiol. 1990 Apr;56(4):1198–1201. doi: 10.1128/aem.56.4.1198-1201.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel G., Renner G., Schwarz K. Effects of pentachlorophenol and some of its known and possible metabolites on different species of bacteria. Appl Environ Microbiol. 1987 Nov;53(11):2689–2692. doi: 10.1128/aem.53.11.2689-2692.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiert J. G., Pignatello J. J., Crawford R. L. Degradation of chlorinated phenols by a pentachlorophenol-degrading bacterium. Appl Environ Microbiol. 1987 May;53(5):907–910. doi: 10.1128/aem.53.5.907-910.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wiegel J. Sequential anaerobic degradation of 2,4-dichlorophenol in freshwater sediments. Appl Environ Microbiol. 1990 Apr;56(4):1119–1127. doi: 10.1128/aem.56.4.1119-1127.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


