Abstract
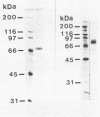
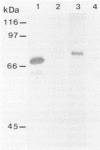
An exopolygalacturonase (exoPG) and an exopolymethylgalacturonase (exoPMG) produced by Sclerotinia sclerotiorum have been purified by ammonium sulfate precipitation, gel filtration, and ion exchange chromatography. The exoPG and the exoPMG were purified 66- and 50-fold, respectively, by using a series of separation procedures that included ammonium sulfate precipitation and gel chromatography. Molecular masses of the native proteins were 68 kDa for exoPG and 140 kDa for exoPMG. The pH optima of the enzymes were about pH 5, and their optimum temperature was 45°C. Activities of both enzymes were inhibited by Hg2+, Zn2+, Cu2+, and p-chloromercuribenzoate. ExoPMG activity, in contrast to exoPG activity, was stimulated by Mn2+ and Co2+. ExoPMG hydrolyzed only citrus pectin, while exoPG degraded sodium polygalacturonate and, to a lesser extent, citrus pectin. The exo mode of action of the enzymes was revealed by thin-layer chromatography of substrate hydrolysates. Antibodies raised against each purified protein exhibited no cross-reaction, thus confirming the biochemical specificities of the enzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Keon J. P., Waksman G. Common amino acid domain among endopolygalacturonases of ascomycete fungi. Appl Environ Microbiol. 1990 Aug;56(8):2522–2528. doi: 10.1128/aem.56.8.2522-2528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester H. C., Visser J. Purification and characterization of polygalacturonases produced by the hyphal fungus Aspergillus niger. Biotechnol Appl Biochem. 1990 Apr;12(2):150–160. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MANDELS M., REESE E. T. Induction of cellulase in fungi by cellobiose. J Bacteriol. 1960 Jun;79:816–826. doi: 10.1128/jb.79.6.816-826.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Riou C., Freyssinet G., Fevre M. Production of Cell Wall-Degrading Enzymes by the Phytopathogenic Fungus Sclerotinia sclerotiorum. Appl Environ Microbiol. 1991 May;57(5):1478–1484. doi: 10.1128/aem.57.5.1478-1484.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Craig J. S., Panaccione D. G., Cervone F., Walton J. D. Endopolygalacturonase is not required for pathogenicity of Cochliobolus carbonum on maize. Plant Cell. 1990 Dec;2(12):1191–1200. doi: 10.1105/tpc.2.12.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]