Abstract
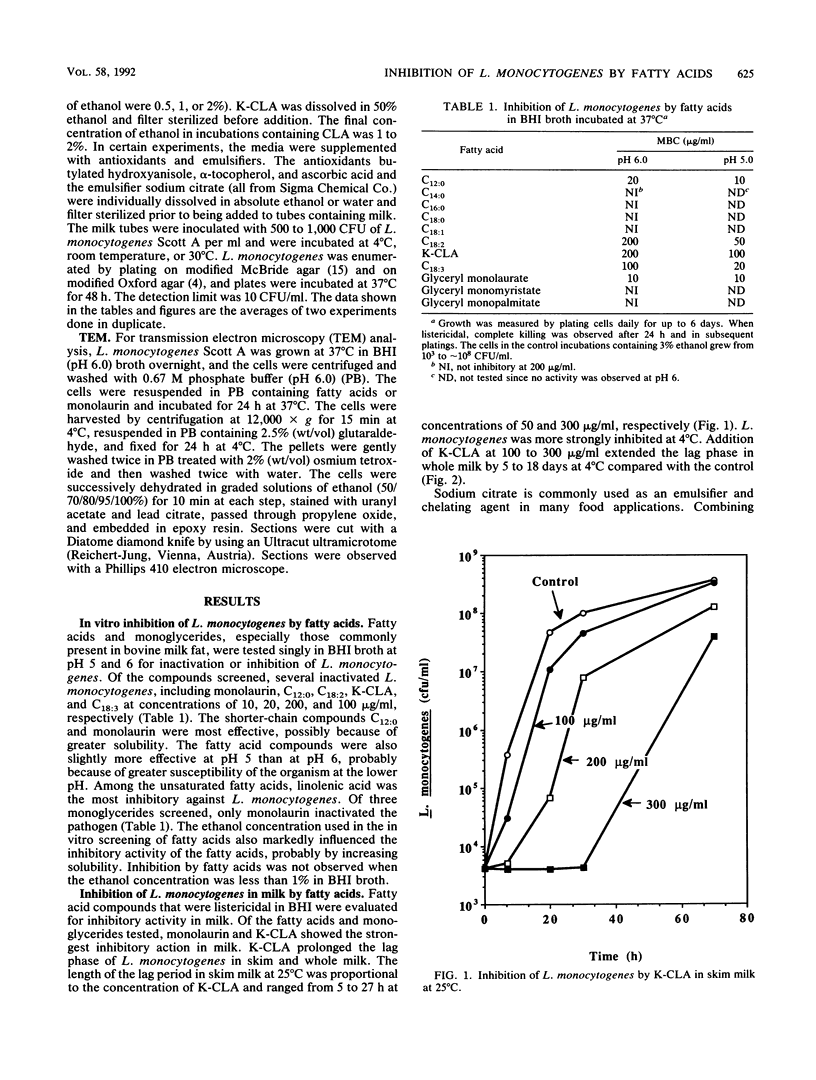
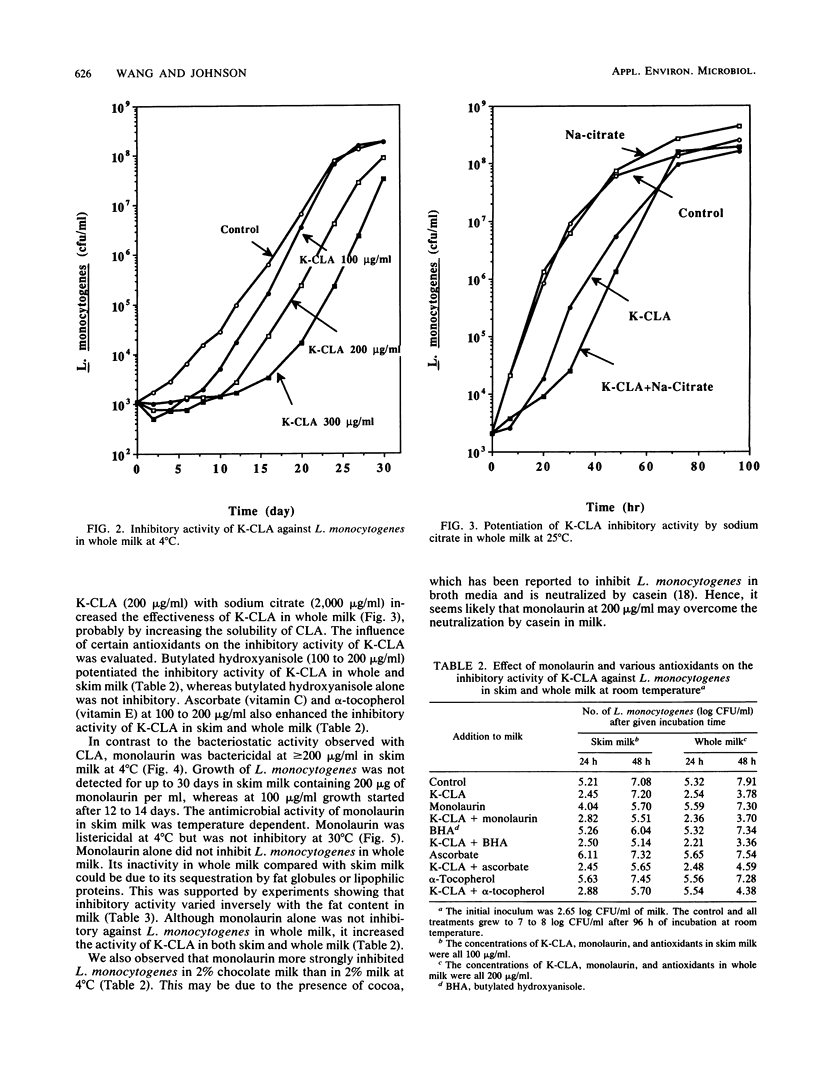
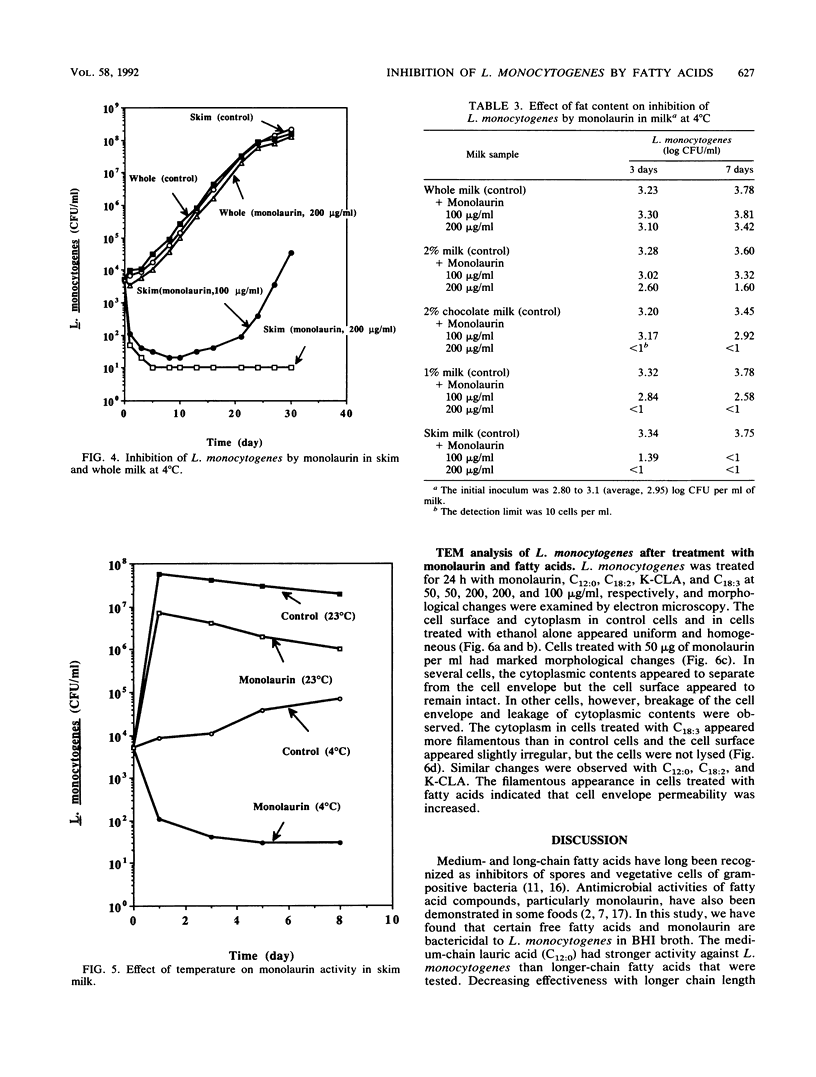
Fatty acids and monoglycerides were evaluated in brain heart infusion broth and in milk for antimicrobial activity against the Scott A strain of Listeria monocytogenes. C12:0, C18:3, and glyceryl monolaurate (monolaurin) had the strongest activity in brain heart infusion broth and were bactericidal at 10 to 20 micrograms/ml, whereas potassium (K)-conjugated linoleic acids and C18:2 were bactericidal at 50 to 200 micrograms/ml. C14:0, C16:0, C18:0, C18:1, glyceryl monomyristate, and glyceryl monopalmitate were not inhibitory at 200 micrograms/ml. The bactericidal activity in brain heart infusion broth was higher at pH 5 than at pH 6. In whole milk and skim milk, K-conjugated linoleic acid was bacteriostatic and prolonged the lag phase especially at 4 degrees C. Monolaurin inactivated L. monocytogenes in skim milk at 4 degrees C, but was less inhibitory at 23 degrees C. Monolaurin did not inhibit L. monocytogenes in whole milk because of the higher fat content. Other fatty acids tested were not effective in whole or skim milk. Our results suggest that K-conjugated linoleic acids or monolaurin could be used as an inhibitory agent against L. monocytogenes in dairy foods.
Full text
PDF
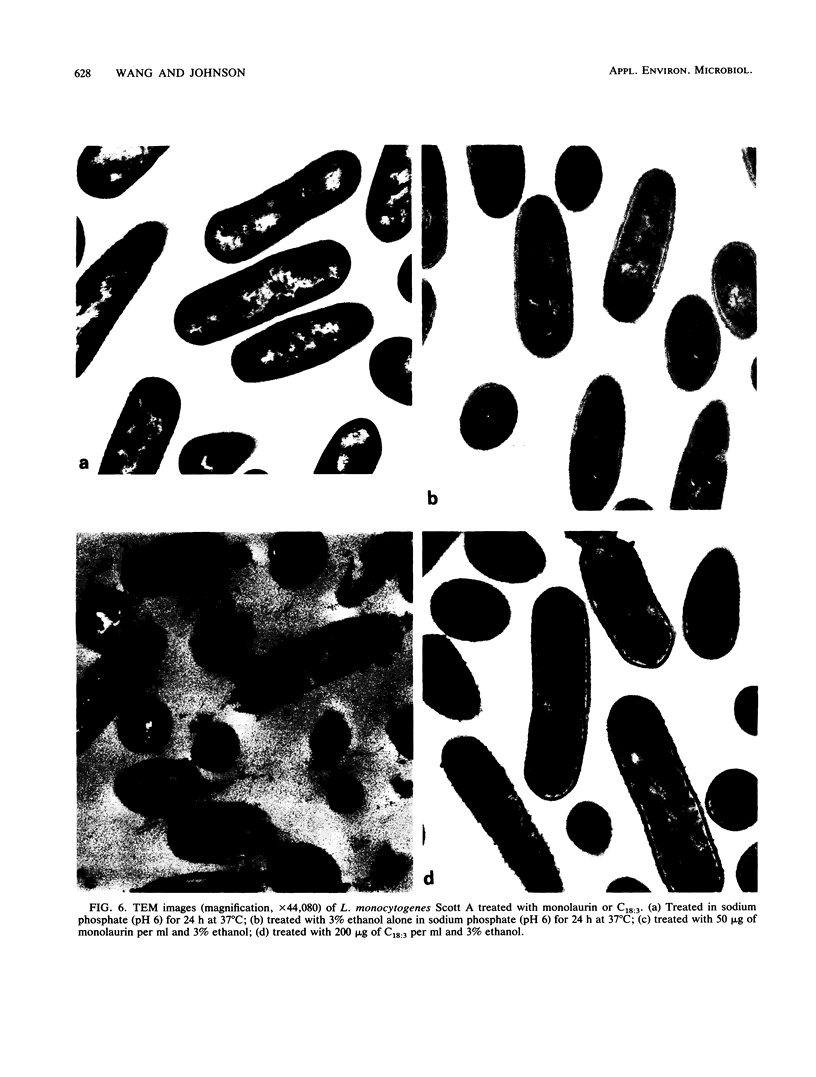

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Sheu C. W., Galliers E. Function of lipophilic acids as antimicrobial food additives. Nature. 1973 Feb 2;241(5388):321–325. doi: 10.1038/241321a0. [DOI] [PubMed] [Google Scholar]
- GRECZ N., WAGENAAR R. O., DACK G. M. Relation of fatty acids to the inhibition of Clostridium botulinum in aged surface ripened cheese. Appl Microbiol. 1959 Jul;7(4):228–234. doi: 10.1128/am.7.4.228-234.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway D. L., Dyke K. G. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. J Gen Microbiol. 1979 Nov;115(1):233–245. doi: 10.1099/00221287-115-1-233. [DOI] [PubMed] [Google Scholar]
- Hogan J. S., Pankey J. W., Duthie A. H. Growth inhibition of mastitis pathogens by long-chain fatty acids. J Dairy Sci. 1987 May;70(5):927–934. doi: 10.3168/jds.S0022-0302(87)80096-6. [DOI] [PubMed] [Google Scholar]
- Ismail G., Sawyer W. D., Wegener W. S. Effect of hydrogen peroxidase and superoxide radical on viability of Neisseria gonorrhoeae and related bacteria. Proc Soc Exp Biol Med. 1977 Jun;155(2):264–269. doi: 10.3181/00379727-155-39786. [DOI] [PubMed] [Google Scholar]
- Knapp H. R., Melly M. A. Bactericidal effects of polyunsaturated fatty acids. J Infect Dis. 1986 Jul;154(1):84–94. doi: 10.1093/infdis/154.1.84. [DOI] [PubMed] [Google Scholar]
- NIEMAN C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954 Jun;18(2):147–163. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viegas C. A., Sá-Correia I. Activation of plasma membrane ATPase of Saccharomyces cerevisiae by octanoic acid. J Gen Microbiol. 1991 Mar;137(3):645–651. doi: 10.1099/00221287-137-3-645. [DOI] [PubMed] [Google Scholar]