Abstract
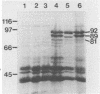
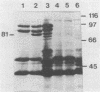
In Pseudomonas sp. strain M114, the outer membrane receptor for ferric pseudobactin M114 was shown to transport ferric pseudobactins B10 and A225, in addition to its own. The gene encoding this receptor, which was previously cloned on pCUP3, was localized by Tn5 mutagenesis to a region comprising >1.6 kb of M114 DNA. A mutant (strain M114R1) lacking this receptor was then created by a marker exchange technique. Characterization of this mutant by using purified pseudobactin M114 in radiolabeled ferric iron uptake studies confirmed that it was completely unable to utilize this siderophore for acquisition of iron. In addition, it lacked an outer membrane protein band of 89 kDa when subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. As a result, growth of the mutant was severely restricted under low-iron conditions. However, this phenotype was reversed in the presence of another fluorescent siderophore (pseudobactin MT3A) from Pseudomonas sp. strain MT3A, suggesting the presence of a second receptor in strain M114. Furthermore, wild-type Pseudomonas sp. strain B24 was not able to utilize ferric pseudobactin MT3A, and this phenotype was not reversed upon expression of the M114 receptor encoded on pCUP3. However, a cosmid clone (pMS1047) that enabled strain B24 to utilize ferric pseudobactin MT3A was isolated from an M114 gene bank. Radiolabel transport assays with purified pseudobactin MT3A confirmed this event. Plasmid pMS1047 was shown to encode an outer membrane protein of 81 kDa in strain B24 under iron-limiting conditions; this protein corresponds to a similar protein in strain M114.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter W., Marugg J. D., de Weger L. A., Tommassen J., Weisbeek P. J. The ferric-pseudobactin receptor PupA of Pseudomonas putida WCS358: homology to TonB-dependent Escherichia coli receptors and specificity of the protein. Mol Microbiol. 1991 Mar;5(3):647–655. doi: 10.1111/j.1365-2958.1991.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Buyer J. S., Wright J. M., Leong J. Structure of pseudobactin A214, a siderophore from a bean-deleterious Pseudomonas. Biochemistry. 1986 Sep 23;25(19):5492–5499. doi: 10.1021/bi00367a022. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin D., Ditta G., Helinski D. R. Clustering of nitrogen fixation (nif) genes in Rhizobium meliloti. J Bacteriol. 1982 Jan;149(1):221–228. doi: 10.1128/jb.149.1.221-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Magazin M. D., Moores J. C., Leong J. Cloning of the gene coding for the outer membrane receptor protein for ferric pseudobactin, a siderophore from a plant growth-promoting Pseudomonas strain. J Biol Chem. 1986 Jan 15;261(2):795–799. [PubMed] [Google Scholar]
- Marugg J. D., Nielander H. B., Horrevoets A. J., van Megen I., van Genderen I., Weisbeek P. J. Genetic organization and transcriptional analysis of a major gene cluster involved in siderophore biosynthesis in Pseudomonas putida WCS358. J Bacteriol. 1988 Apr;170(4):1812–1819. doi: 10.1128/jb.170.4.1812-1819.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. D., de Weger L. A., Nielander H. B., Oorthuizen M., Recourt K., Lugtenberg B., van der Hofstad G. A., Weisbeek P. J. Cloning and characterization of a gene encoding an outer membrane protein required for siderophore-mediated uptake of Fe3+ in Pseudomonas putida WCS358. J Bacteriol. 1989 May;171(5):2819–2826. doi: 10.1128/jb.171.5.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores J. C., Magazin M., Ditta G. S., Leong J. Cloning of genes involved in the biosynthesis of pseudobactin, a high-affinity iron transport agent of a plant growth-promoting Pseudomonas strain. J Bacteriol. 1984 Jan;157(1):53–58. doi: 10.1128/jb.157.1.53-58.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- O'Sullivan D. J., Morris J., O'Gara F. Identification of an additional ferric-siderophore uptake gene clustered with receptor, biosynthesis, and fur-like regulatory genes in fluorescent Pseudomonas sp. strain M114. Appl Environ Microbiol. 1990 Jul;56(7):2056–2064. doi: 10.1128/aem.56.7.2056-2064.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan D. J., O'Gara F. Regulation of iron assimilation: nucleotide sequence analysis of an iron-regulated promoter from a fluorescent pseudomonad. Mol Gen Genet. 1991 Aug;228(1-2):1–8. doi: 10.1007/BF00282440. [DOI] [PubMed] [Google Scholar]
- Philson S. B., Llinás M. Siderochromes from Pseudomonas fluorescens. II. Structural homology as revealed by NMR spectroscopy. J Biol Chem. 1982 Jul 25;257(14):8086–8090. [PubMed] [Google Scholar]
- Postle K. TonB and the gram-negative dilemma. Mol Microbiol. 1990 Dec;4(12):2019–2025. doi: 10.1111/j.1365-2958.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Teintze M., Hossain M. B., Barnes C. L., Leong J., van der Helm D. Structure of ferric pseudobactin, a siderophore from a plant growth promoting Pseudomonas. Biochemistry. 1981 Oct 27;20(22):6446–6457. doi: 10.1021/bi00525a025. [DOI] [PubMed] [Google Scholar]
- Yang C. C., Leong J. Structure of pseudobactin 7SR1, a siderophore from a plant-deleterious Pseudomonas. Biochemistry. 1984 Jul 17;23(15):3534–3540. doi: 10.1021/bi00310a023. [DOI] [PubMed] [Google Scholar]
- de Weger L. A., van Arendonk J. J., Recourt K., van der Hofstad G. A., Weisbeek P. J., Lugtenberg B. Siderophore-mediated uptake of Fe3+ by the plant growth-stimulating Pseudomonas putida strain WCS358 and by other rhizosphere microorganisms. J Bacteriol. 1988 Oct;170(10):4693–4698. doi: 10.1128/jb.170.10.4693-4698.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]