Abstract
Mutation of an invariant glutamate residue found within the catalytic domain of guanylyl cyclases resulted in a dramatic 14-fold increase in the activity of the guanylyl cyclase-A receptor. Even in the presence of Mn2+/Triton X-100, a treatment previously thought to yield hormone-independent and maximum cyclase activity, the mutant enzyme remained 7-fold more active; to our knowledge, this is the first example of a protein modification or of an added agent that significantly increases cyclase activity in the presence of Mn2+/Triton X-100. Intracellular concentrations of cGMP in cells expressing the mutant (E974A) cyclase were only marginally elevated by the addition of atrial natriuretic peptide, and in broken-cell preparations, the mutant enzyme also was relatively insensitive to ligand/regulatory nucleotide. The marked increase in cyclase activity was not due to a relief of protein kinase domain inhibition, since the point mutation caused 7- to 13-fold elevations in guanylyl cyclase-A activity when the protein kinase homology domain was deleted. The E974A mutation also altered the kinetics from positive cooperative to linear with respect to MnGTP, suggesting disruption of subunit–subunit interactions. Thus, a single point mutation within the catalytic domain of a guanylyl cyclase results in a constitutively hyperactive enzyme that is independent of protein kinase domain regulation.
Guanylyl cyclases are classified into two broad families: the soluble forms activated by NO (nitric oxide) and the membrane-bound forms stimulated by peptide hormones. To date the soluble forms have been shown to exist as heterodimers and the membrane forms as homomers (1–5).
Guanylyl cyclase-A (GC-A), the apparent receptor for atrial natriuretic peptide (ANP) and B-type natriuretic peptide, requires both the peptide ligand and an adenine nucleotide (ATP) for effective activation of the cyclase (6). Although the mechanisms by which the cyclases are activated upon ligand binding are not known, it appears that the guanylyl cyclase subunits are associated prior to ligand binding for either the membrane or soluble forms; thus, ligand-induced aggregation does not appear to explain the activation, although one report on the receptor for heat-stable enterotoxin has suggested that a “functional” dimerization occurs in response to ligand binding (7). With GC-A, ATP is thought to bind to the protein kinase homology domain, and deletion of this region yields a constitutively active guanylyl cyclase that is no longer regulated by ANP and ATP (8); point mutations within the domain can also eliminate ANP/ATP regulation of the cyclase but activity remains low (9, 10). These results have led to suggestions that the protein kinase homology domain acts as a repressor of cyclase activity in the absence of peptide ligand and ATP and that catalytic domain activation, therefore, is a result of ANP/ATP release of the inhibition (8).
It was rationalized that if the protein kinase homology domain could serve as a repressor of the cyclase catalytic domain, then point mutations might be found that could mimic the hormone-activated state of the cyclase. We therefore used GC-A as a model to search for point mutations that would yield a hyperactive cyclase now relatively insensitive to ligand. Mutation of Glu-974, which is highly conserved in all guanylyl and adenylyl cyclases, resulted in a marked increase in enzyme activity and a loss of ANP/ATP sensitivity. However, the hyperactivity was also evident in Mn2+/Triton X-100 and in the absence of the protein kinase homology domain. Thus, the mutant form of GC-A is constitutively hyperactive, independent of protein kinase domain regulation, and the mutation relieves subunit–subunit cooperativity.
MATERIALS AND METHODS
Mutagenesis of Glu-974 in HCAT and GC-A.
HCAT and HCAT E974A mutants were constructed and cloned in pCMV5 as described (11). Full-length GC-A was cloned into the expression vector pCMV3 as described (12). For construction of the GC-A E974A mutant, a HindIII–XhoI fragment was excised from the HCAT E974A/pCMV5-construct and inserted into the corresponding position of full-length GC-A/pCMV3.
Expression of Constructs in COS-7 Cells.
COS-7 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, penicillin (100 units/ml), streptomycin (100 μg/ml), and amphotericin B (0.25 μg/ml). Cells were transiently transfected according to the DEAE-dextran method. The amount of plasmids coding for pCMV5, GC-A, HCAT, and corresponding E974 mutants used for transfection varied between 0.1 and 5 μg per 100-mm plate to obtain comparable levels of wild-type and mutant HCAT or GC-A. Cells were harvested 48 h after transfection; cells were washed twice with 5 ml of ice-cold phosphate-buffered saline and scraped into 0.5–1 ml of ice-cold homogenization buffer [10% glycerol/50 mM Hepes, pH 7.4/100 mM NaCl/1 mM EDTA/leupeptin (10 μg/ml)/pepstatin (1 μg/ml)/aprotinin (10 μg/ml)/2 mM dithiothreitol (DTT)]; DTT was omitted to avoid a potential ANP degradation in the GC-A assay. Cells were homogenized by sonication and centrifuged for 15 min at 2°C and 16,000 × g. For the HCAT and HCAT E974A mutant, the supernatant fluid was collected; for GC-A and GC-A E974A, the supernatant fluid was discarded and the membrane pellet washed twice with 500 μl of homogenization buffer. The membranes were resuspended in 1 ml homogenization buffer and suspended by sonication.
SDS/PAGE and Western Blot Analysis.
Laemmli sample buffer (5×) was added to 20 μl of cytosolic fractions (for HCAT and HCAT E974A) or membrane fractions (for GC-A and GC-A E974A) of COS-7 cells transfected with the respective plasmids, and proteins were electrophoretically separated on 8% or 10% SDS/PAGE gels. After electrophoretic transfer of proteins to nitrocellulose or poly(vinylidene difluoride) membranes nonspecific protein-binding sites of the membranes were blocked by incubation with TBST (Tris-buffered saline/Tween: 10 mM Tris·HCl, pH 7.5/150 mM NaCl/0.1% Tween 20) containing 5% nonfat dry milk at room temperature for 1 h or at 4°C overnight. Membranes were washed with TBST and incubated for 1 h at room temperature in a 1:1000 dilution of an antibody directed against a carboxyl-terminal peptide of GC-A. Membranes were washed three times with TBST and then incubated with a 1:10,000 dilution of a horseradish peroxidase-conjugated anti-rabbit antibody (Bio-Rad) in TBST. After washing the membranes three times with TBST, protein bands were detected using the enhanced chemiluminescence detection method (ECL).
Assay of Guanylyl Cyclase Activity.
Assays were performed in a total volume of 100 μl containing 25 mM Hepes (pH 7.4), 50 mM NaCl, 0.25 mM 3-isobutyl-1-methylxanthine, 0.1% BSA, 5 mM creatine phosphate, creatine phosphokinase (10 units per 100-μl assay), 10 mM NaN3, 1 mM DTT (for HCAT and HCAT E974A), 30–1000 μM GTP, [α-32P]GTP (NEN; 0.5 μCi per 100-μl assay, 3000 Ci/mmol, 10 mCi/ml; 1 Ci = 37 Gbq), 5 mM MnCl2 or MgCl2, and where indicated 1 μM ANP/1 mM ATP or 1% Triton X-100. Tubes with assay mixture were prewarmed for 40 sec at 37°C prior to the start of the reaction by addition of 20 μl of cytosolic fraction or membrane fraction obtained as described above. Reactions were stopped with zinc acetate/sodium carbonate after a 7- to 15-min incubation. Formed [α-32P]cGMP was determined as described previously and was linear with respect to time and protein concentration (13). For determination of cGMP formation by radioimmunassay, radioactively labeled GTP was omitted in the assay mixture and cGMP was quantitated as in ref. 13. For cGMP accumulation in COS-7 cells, 100-mm plates of cells transfected with the respective plasmid were washed twice with 2 ml of DMEM (see above, without additives) and incubated with DMEM containing 0.25 mM 3-isobutyl-1-methylxanthine at 37°C. After 10 min, this medium was exchanged with DMEM containing 0.25 mM 3-isobutyl-1-methylxanthine with or without 1 μM ANP and incubated for additional 10 min. The reaction was stopped by addition of 2 ml of 1 M perchloric acid. Formed cGMP was determined as described in ref. 13.
RESULTS AND DISCUSSION
Dominant negative mutations of soluble and membrane forms of GC-A that cause a loss of function have been described recently. Mutation of an aspartate in the catalytic domain of GC-A (D893A) and mutation of two aspartates in the catalytic domain of the α1 subunit of guanylyl cyclase (D513A and D529A) resulted in the formation of dominant negative proteins (11, 14). Other loss of function mutations of GC-A have been those where the protein kinase domain has been mutated at various residues (10, 15) or where the protein kinase homology domain has been deleted to render an enzyme unresponsive to ANP/ATP (8). Likewise, NO-insensitive point mutants and deletion mutants acting as dominant negative proteins have been described for the β1 subunit of soluble cyclase (16, 17). A splice variant of α2 also has been reported to act in a dominant negative manner (24).
Early work of Chinkers and Garbers (8) suggested that deletion of the protein kinase homology domain not only resulted in loss of function (no response to ANP/ATP) but also in elevated cyclase activity. It was suggested, since cyclases exist as preformed oligomers, that relief of kinase domain inhibition by the binding of ATP represented one mechanism of activation of GC-A (8).
We rationalized that it might be possible to define point mutations within the cyclase catalytic domain that would either result in relief of protein kinase domain repression or in the direct formation of an “active-state” conformation. Scanning mutagenesis of various charged amino acids (data not shown) resulted in the detection of one such mutation (E974A). Glu-974 is invariant in all cloned guanylyl cyclases and is altered to an Asp in only a few eukaryotic adenylyl cyclases (Fig. 1).
Figure 1.
Glutamate corresponding to E974 of GC-A is conserved in guanylyl and adenylyl cyclases. The alignment of a sequence within the catalytic domain of GC-A is shown as an example of a membrane guanylyl cyclase isoform, the α1 and β1 subunits of the soluble isoform, and the first and second cytoplasmic domain (C1 and C2) of adenylyl cyclase IV. The corresponding amino acids are 957–977 (GC-A), 590–610 (α1), 537–557 (β1), 383–403 (adenylyl cyclase IV, C1), and 990-1010 (adenylyl cyclase IV, C2).
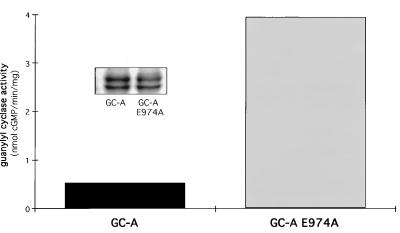
Maximal guanylyl cyclase activity is generally seen in the presence of Mn2+/Triton X-100 (18). Membrane preparations of COS-7 cells containing the E974A mutant of GC-A, however, exhibited about 7-fold higher activity than wild-type GC-A (Fig. 2). The differences in activity were not explained by a difference in cyclase expression (Fig. 2 Inset).
Figure 2.
Activity of GC-A and of GC-A E974A in membranes of transfected cells. Guanylyl cyclase activity was estimated in the presence of Mn2+ containing 1% Triton X-100. (Inset) Western blots demonstrating equivalent expression of GC-A and GC-A E974A.
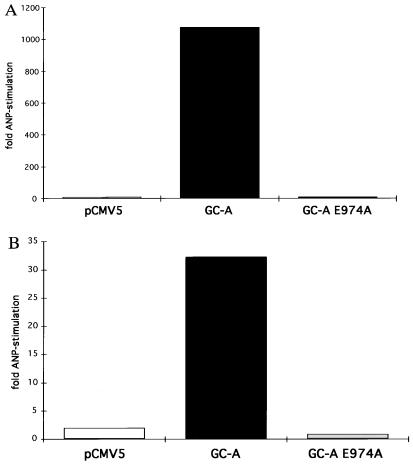
In intact COS-7 cells, cGMP concentrations were also higher in the cells expressing the mutant GC-A (about 14 times higher than in cells expressing wild-type GC-A). The stimulation of cGMP production (about 1073-fold) after ANP addition to cells expressing wild-type GC-A was almost abolished (6-fold) in cells expressing the E974A GC-A (Fig. 3A). Likewise, when plasma membranes of COS-7 cells were treated with ANP, wild-type enzyme was stimulated about 32-fold while no detectable stimulation was seen in the membranes expressing E974A (Fig. 3B).
Figure 3.
Effects of ANP on cGMP accumulation in cells or on guanylyl cyclase activity in broken-cell preparations. (A) ANP (1 μM) or no ANP was added to COS-7 cells transfected with pCMV5, GC-A, or GC-A E974A. (B) Membranes prepared from transfected COS-7 cells were assayed for guanylyl cyclase activity in the presence of Mg2+ and in the presence or absence of ANP/ATP.
Therefore, a single point mutation within a receptor guanylyl cyclase is capable of markedly activating cyclase activity, even in the presence of Mn2+/Triton X-100. Furthermore, the mutation essentially eliminated stimulation by the ligand (ANP) and the regulatory nucleotide (ATP). To our knowledge, such a constitutively hyperactive mutant enzyme has never been seen with cyclases.
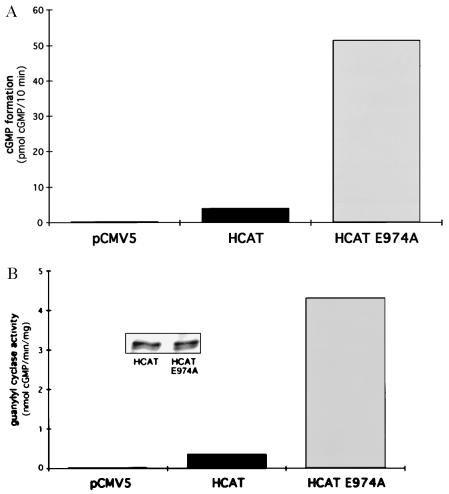
One possible explanation for the marked activation would be a relief of protein kinase domain inhibition, a model previously proposed (8). Therefore, E974 was then mutated in a truncated mutant of GC-A, where the extracellular ligand binding domain, the transmembrane region, and essentially all of the protein kinase homology domain were deleted. The now soluble protein (HCAT) continues to dimerize (data not shown) and retains enzyme activity (11). cGMP concentrations were 13-fold higher in COS-7 cells expressing HCAT E974A than HCAT (Fig. 4A). Also, HCAT E974A activity was 7- to 12-fold higher in the cytosolic fractions of COS-7 cells when compared with HCAT (Fig. 4B); this was not due to differences in protein expression (Fig. 4B Inset). Therefore, a derepression of protein kinase domain inhibition does not appear to account for the high activity of the E974A mutant.
Figure 4.
Effects of HCAT or of HCAT E974A on cellular cGMP concentrations or guanylyl cyclase activity. (A) COS-7 cells were transfected with pCMV5, HCAT, or HCAT E974A and cGMP concentrations were measured. (B) The soluble fraction of transfected COS-7 cells was collected after homogenization and the activity of guanylyl cyclase estimated. (Inset) Western blots demonstrating equivalent expression of HCAT and E974A HCAT.
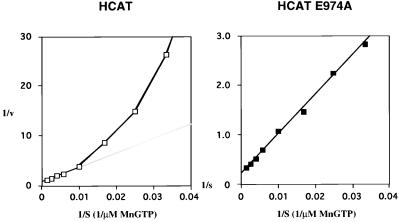
The membrane forms of guanylyl cyclase have long been known to display positive cooperative behavior as a function of MeGTP concentration (19–21). Cytosolic fractions from HCAT or HCAT E974A expressing cells were assayed for guanylyl cyclase activity with increasing concentrations of MnGTP in the presence of free Mn2+ and Triton X-100. Specific activity versus substrate concentrations are presented in a double reciprocal plot (Fig. 5). The apparent maximal velocity was about 30-fold higher for HCAT E974A than for HCAT (44.4 versus 1.3 nmol of cGMP per min per mg). The wild-type HCAT does not obey classic Michaelis–Menton kinetics (Hill coefficient, 1.4) as has been reported (22). In contrast, HCAT E974A displayed linear kinetics with a Hill coefficient of 1.0. The Km for HCAT E974A was determined from the Lineweaver–Burk plot as 363 μM MnGTP and the S0.5 value for HCAT was 341 μM MnGTP. These data suggest that E974 plays a crucial role in subunit–subunit interaction, since mutation of this residue causes loss of the allosteric interaction.
Figure 5.
Guanylyl cyclase activity as a function of MnGTP concentration. Guanylyl cyclase activity was estimated as described in the text and plotted in double reciprocal form.
A point mutation within the GC-A receptor (E974A), therefore, causes a dramatic increase in enzymatic activity. A relief of kinase homology domain inhibition appears not a cause of the hyperactivity, since GC-A E974A and HCAT E974A are both 7- to 14-fold higher in activity compared with wild-type GC-A or HCAT respectively. It therefore appears that the E974A mutation results in an activated state of the catalytic domain independent of the presence of the kinase homology domain.
To our knowledge, this is the first time a modification of a cyclase has resulted in an elevated activity in the presence of Mn2+/Triton X-100. Previously, the only modification that appeared to alter guanylyl cyclase activity under such assay conditions was the dephosphorylation of the sea urchin sperm enzyme (23). In that case, activity decreased to about 10% of that seen with the phosphorylated protein, and positive cooperativity as a function of MnGTP was lost.
Aside from the induction of an active-state conformation within the catalytic domain, other explanations for the activation are possible. It could be argued that the mutation has resulted in a decrease in improperly folded cyclases, thus accounting for an apparent increase in activity. However, the loss of responsiveness to ANP, the loss of cooperativity, and a constant state of phosphorylation (data not shown) argue strongly that an increase in the amount of active enzyme does not account for the apparent hyperactivity of the mutant.
It is also possible that the mutation results in the release of an inhibitor that is not the protein kinase homology domain but an associated molecule. If this is the case, then the inhibitor would also presumably bind to the catalytic region of the cyclase since HCAT is hyperactive. One would also postulate that ANP normally activates the cyclase through release of such an inhibitor. Furthermore, since cyclase activity is normally maximal in Mn2+/Triton X-100 and not further stimulated by ANP, it would seem likely that such an inhibitor would remain bound to the detergent-solubilized wild-type GC-A but no longer dissociate upon the binding of ANP, since detergent-solubilized GC-A E974A is still hyperactive relative to wild-type GC-A.
Acknowledgments
We thank Peter Yuen and Lynda Doolittle for characterization of antiserum A034. B.J.W. is supported by a fellowship from Deutsche Forschungsgesellschaft.
Footnotes
Abbreviations: ANP, atrial natriuretic peptide; GC-A, guanylyl cyclase-A receptor.
References
- 1.Kamisaki Y, Saheki S, Nakane M, Palmieri J A, Kuno T, Chang B Y, Waldman S A, Murad F. J Biol Chem. 1986;261:7236–7241. [PubMed] [Google Scholar]
- 2.Harteneck C, Koesling D, Soling A, Schultz G, Böhme E. FEBS Lett. 1990;272:221–223. doi: 10.1016/0014-5793(90)80489-6. [DOI] [PubMed] [Google Scholar]
- 3.Chinkers M, Wilson E M. J Biol Chem. 1992;267:18589–18597. [PubMed] [Google Scholar]
- 4.Iwata T, Uchida-Mizuno K, Katafuchi T, Ito T, Hagiwara H, Hirose S. J Biochem. 1991;110:35–39. doi: 10.1093/oxfordjournals.jbchem.a123539. [DOI] [PubMed] [Google Scholar]
- 5.Lowe D G. Biochemistry. 1992;31:10421–10425. doi: 10.1021/bi00158a001. [DOI] [PubMed] [Google Scholar]
- 6.Drewett J G, Garbers D L. Endocr Rev. 1994;15:135–162. doi: 10.1210/edrv-15-2-135. [DOI] [PubMed] [Google Scholar]
- 7.Vaandrager A B, Schulz S, DeJonge H R, Garbers D L. J Biol Chem. 1992;268:2174–2179. [PubMed] [Google Scholar]
- 8.Chinkers M, Garbers D L. Science. 1989;245:1392–1394. doi: 10.1126/science.2571188. [DOI] [PubMed] [Google Scholar]
- 9.Goraczniak R M, Duda T, Sharma R K. Biochem J. 1992;282:533–537. doi: 10.1042/bj2820533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koller K J, Lipari M T, Goeddel D V. J Biol Chem. 1993;268:5997–6003. [PubMed] [Google Scholar]
- 11.Thompson D K, Garbers D L. J Biol Chem. 1995;270:425–430. doi: 10.1074/jbc.270.1.425. [DOI] [PubMed] [Google Scholar]
- 12.Chinkers M, Garbers D L, Chang M, Lowe D G, Chin H, Goeddel D V, Schulz S. Nature (London) 1989;338:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- 13.Domino S E, Tubb D J, Garbers D L. Methods Enzymol. 1991;195:345–355. doi: 10.1016/0076-6879(91)95179-n. [DOI] [PubMed] [Google Scholar]
- 14.Yuen P S T, Doolittle L K, Garbers D L. J Biol Chem. 1994;269:791–793. [PubMed] [Google Scholar]
- 15.Duda T, Goraczniak R M, Sharma R K. Proc Natl Acad Sci USA. 1991;88:7882–7886. doi: 10.1073/pnas.88.17.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wedel B, Humbert P, Harteneck C, Foerster J, Malkewitz J, Bohme E, Schultz G, Koesling D. Proc Natl Acad Sci USA. 1994;91:2592–2596. doi: 10.1073/pnas.91.7.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wedel B, Harteneck C, Foerster J, Friebe A, Schultz G, Koesling D. J Biol Chem. 1995;270:24871–24875. doi: 10.1074/jbc.270.42.24871. [DOI] [PubMed] [Google Scholar]
- 18.Potter L R, Garbers D L. J Biol Chem. 1992;267:14531–14534. [PubMed] [Google Scholar]
- 19.Kimura H, Murad F. J Biol Chem. 1974;249:6910–6916. [PubMed] [Google Scholar]
- 20.Chrisman T D, Garbers D L, Parks M A, Hardman J G. J Biol Chem. 1975;250:374–381. [PubMed] [Google Scholar]
- 21.Garbers D L, Hardman J G, Rudolph F B. Biochemistry. 1974;13:4166–4171. [Google Scholar]
- 22.Thorpe D S, Niu S, Morkin E. Biochem Biophys Res Commun. 1996;218:670–673. doi: 10.1006/bbrc.1996.0120. [DOI] [PubMed] [Google Scholar]
- 23.Ramarao C S, Garbers D L. J Biol Chem. 1988;263:1524–1529. [PubMed] [Google Scholar]
- 24.Behrends S, Harteneck C, Schultz G, Koesling D. J Biol Chem. 1995;270:21109–21113. doi: 10.1074/jbc.270.36.21109. [DOI] [PubMed] [Google Scholar]