Abstract
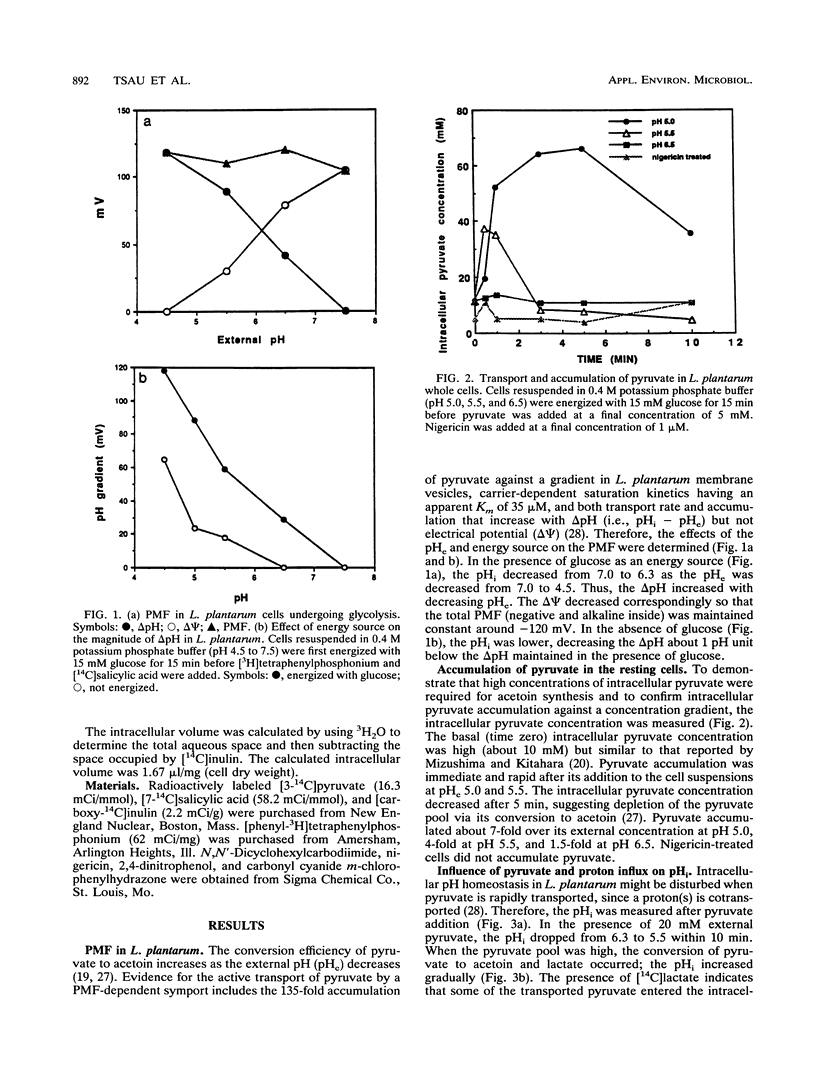
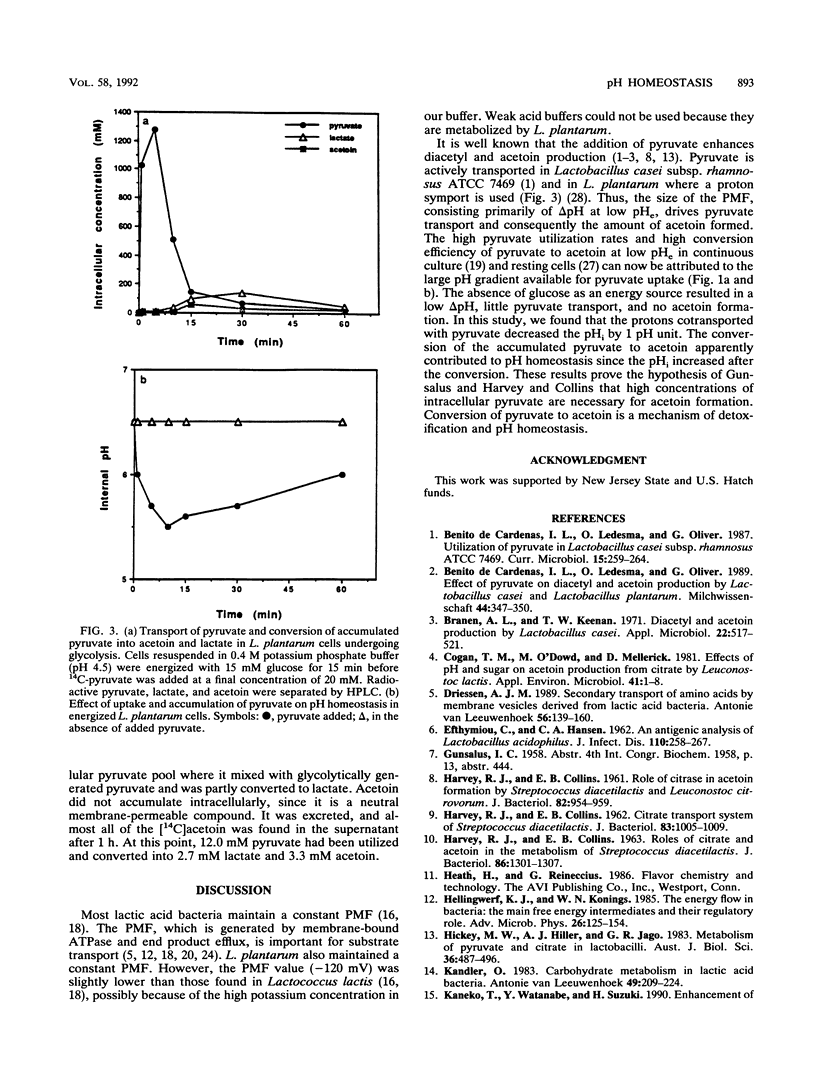
Pyruvate is the substrate for diacetyl and acetoin synthesis by lactobacilli. Exogenous pyruvate stimulates acetoin production when glucose is present as an energy source. In Lactobacillus plantarum ATCC 8014, the energy derived from glucose via glycolysis generated a constant proton motive force of about -120 mV. At a low external pH, energized cells rapidly transported and accumulated pyruvate but did not do so when they were deenergized by nigericin. When large amounts of pyruvate were transported and subsequently accumulated internally, the cotransported protons rapidly lowered the internal pH. The conversion of pyruvate to acetoin instead of acidic end products contributed to the maintenance of pH homeostasis. This is the first report showing that the conversion of pyruvate to acetoin serves as a mechanism of pH homeostasis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branen A. L., Keenan T. W. Diacetyl and acetoin production by Lactobacillus casei. Appl Microbiol. 1971 Oct;22(4):517–521. doi: 10.1128/am.22.4.517-521.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan T. M., O'dowd M., Mellerick D. Effects of pH and Sugar on Acetoin Production from Citrate by Leuconostoc lactis. Appl Environ Microbiol. 1981 Jan;41(1):1–8. doi: 10.1128/aem.41.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J. Secondary transport of amino acids by membrane vesicles derived from lactic acid bacteria. Antonie Van Leeuwenhoek. 1989 Aug;56(2):139–160. doi: 10.1007/BF00399978. [DOI] [PubMed] [Google Scholar]
- EFTHYMIOU C., HANSEN P. A. An antigenic analysis of Lactobacillus acidophilus. J Infect Dis. 1962 May-Jun;110:258–267. doi: 10.1093/infdis/110.3.258. [DOI] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. Citrate transport system of Streptococcus diacetilactis. J Bacteriol. 1962 May;83:1005–1009. doi: 10.1128/jb.83.5.1005-1009.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. ROLES OF CITRATE AND ACETOIN IN THE METABOLISM OF STREPTOCOCCUS DIACETILACTIS. J Bacteriol. 1963 Dec;86:1301–1307. doi: 10.1128/jb.86.6.1301-1307.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. Role of citritase in acetoin formation by Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1961 Dec;82:954–959. doi: 10.1128/jb.82.6.954-959.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellingwerf K. J., Konings W. N. The energy flow in bacteria: the main free energy intermediates and their regulatory role. Adv Microb Physiol. 1985;26:125–154. doi: 10.1016/s0065-2911(08)60396-3. [DOI] [PubMed] [Google Scholar]
- Hickey M. W., Hillier A. J., Jago G. R. Metabolism of pyruvate and citrate in lactobacilli. Aust J Biol Sci. 1983;36(5-6):487–496. doi: 10.1071/bi9830487. [DOI] [PubMed] [Google Scholar]
- Kandler O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek. 1983 Sep;49(3):209–224. doi: 10.1007/BF00399499. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Otto R. Energy transduction and solute transport in streptococci. Antonie Van Leeuwenhoek. 1983 Sep;49(3):247–257. doi: 10.1007/BF00399501. [DOI] [PubMed] [Google Scholar]
- MIZUSHIMA S., KITAHARA K. QUANTITATIVE STUDIES ON GLYCOLYTIC ENZYMES IN LACTOBACILLUS PLANTARUM. II. INTRACELLULAR CONCENTRATIONS OF GLYCOLYTIC INTERMEDIATES IN GLUCOSE-METABOLIZING WASHED CELLS. J Bacteriol. 1964 Jun;87:1429–1435. doi: 10.1128/jb.87.6.1429-1435.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montville T. J., Hsu A. H., Meyer M. E. High-Efficiency Conversion of Pyruvate to Acetoin by Lactobacillus plantarum during pH-Controlled and Fed-Batch Fermentations. Appl Environ Microbiol. 1987 Aug;53(8):1798–1802. doi: 10.1128/aem.53.8.1798-1802.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Speckman R. A., Collins E. B. Diacetyl biosynthesis in Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1968 Jan;95(1):174–180. doi: 10.1128/jb.95.1.174-180.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C. P., Tsau J. L., Montville T. J. Bioenergetic consequences of catabolic shifts by Lactobacillus plantarum in response to shifts in environmental oxygen and pH in chemostat cultures. J Bacteriol. 1991 Jul;173(14):4411–4416. doi: 10.1128/jb.173.14.4411-4416.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


