Abstract
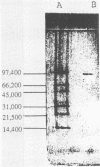
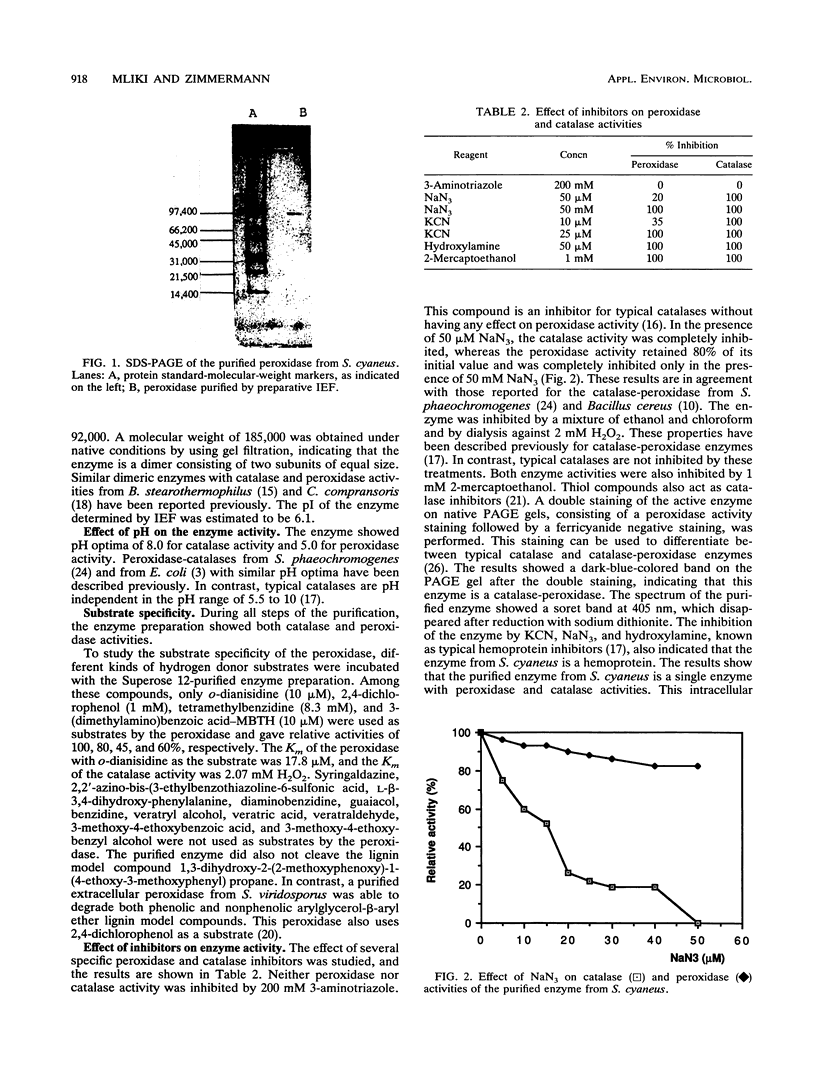
An intracellular peroxidase (EC 1.11.1.7) from Streptomyces cyaneus was purified to homogeneity. The enzyme had a molecular weight of 185,000 and was composed of two subunits of equal size. It had an isoelectric point of 6.1. The enzyme had a peroxidase activity toward o-dianisidine with a Km of 17.8 microM and a pH optimum of 5.0. It also showed catalase activity with a Km of 2.07 mM H2O2 and a pH optimum of 8.0. The purified enzyme did not catalyze C alpha-C beta bond cleavage of 1,3-dihydroxy-2-(2-methoxyphenoxy)-1-(4-ethoxy-3-methoxyphenyl) propane, a nonphenolic dimeric lignin model compound. The spectrum of the peroxidase showed a soret band at 405 nm, which disappeared after reduction with sodium dithionite, indicating that the enzyme is a hemoprotein. Testing the effects of various inhibitors on the enzyme activity showed that it is a bifunctional enzyme having catalase and peroxidase activities.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhi T. P., Korus R. A., Crawford D. L. Production of Major Extracellular Enzymes during Lignocellulose Degradation by Two Streptomycetes in Agitated Submerged Culture. Appl Environ Microbiol. 1989 May;55(5):1165–1168. doi: 10.1128/aem.55.5.1165-1168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Claiborne A., Fridovich I. Purification of the o-dianisidine peroxidase from Escherichia coli B. Physicochemical characterization and analysis of its dual catalatic and peroxidatic activities. J Biol Chem. 1979 May 25;254(10):4245–4252. [PubMed] [Google Scholar]
- Garfin D. E. Isoelectric focusing. Methods Enzymol. 1990;182:459–477. doi: 10.1016/0076-6879(90)82037-3. [DOI] [PubMed] [Google Scholar]
- Goldstein D. B. A method for assay of catalase with the oxygen cathode. Anal Biochem. 1968 Sep;24(3):431–437. doi: 10.1016/0003-2697(68)90148-6. [DOI] [PubMed] [Google Scholar]
- Hildebraunt A. G., Roots I. Reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent formation and breakdown of hydrogen peroxide during mixed function oxidation reactions in liver microsomes. Arch Biochem Biophys. 1975 Dec;171(2):385–397. doi: 10.1016/0003-9861(75)90047-8. [DOI] [PubMed] [Google Scholar]
- Hochman A., Shemesh A. Purification and characterization of a catalase-peroxidase from the photosynthetic bacterium Rhodopseudomonas capsulata. J Biol Chem. 1987 May 15;262(14):6871–6876. [PubMed] [Google Scholar]
- Knoch M., van Pée K. H., Vining L. C., Lingens F. Purification, properties and immunological detection of a bromoperoxidase-catalase from Streptomyces venezuelae and from a chloramphenicol-nonproducing mutant. J Gen Microbiol. 1989 Sep;135(9):2493–2502. doi: 10.1099/00221287-135-9-2493. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loprasert S., Negoro S., Okada H. Thermostable peroxidase from Bacillus stearothermophilus. J Gen Microbiol. 1988 Jul;134(7):1971–1976. doi: 10.1099/00221287-134-7-1971. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A., SCHEJTER A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J. 1960 Feb;74:339–348. doi: 10.1042/bj0740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti M. B., Pometto A. L., 3rd, Nuti M. P., Crawford D. L. Lignin-solubilizing ability of actinomycetes isolated from termite (Termitidae) gut. Appl Environ Microbiol. 1990 Jul;56(7):2213–2218. doi: 10.1128/aem.56.7.2213-2218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra M., Crawford D. L., Hertel G. Characterization of an extracellular lignin peroxidase of the lignocellulolytic actinomycete Streptomyces viridosporus. Appl Environ Microbiol. 1988 Dec;54(12):3057–3063. doi: 10.1128/aem.54.12.3057-3063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Miyahara T., Hachimori A., Samejima T. The interactions of thiol compounds with porcine erythrocyte catalase. J Biochem. 1980 Feb;87(2):429–439. doi: 10.1093/oxfordjournals.jbchem.a132763. [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Diaz G. A. A double staining method for differentiating between two classes of mycobacterial catalase in polyacrylamide electrophoresis gels. Anal Biochem. 1986 Aug 15;157(1):89–92. doi: 10.1016/0003-2697(86)90200-9. [DOI] [PubMed] [Google Scholar]
- Zeiner R., Van Pée K. H., Lingens F. Purification and partial characterization of multiple bromoperoxidases from Streptomyces griseus. J Gen Microbiol. 1988 Dec;134(12):3141–3149. doi: 10.1099/00221287-134-12-3141. [DOI] [PubMed] [Google Scholar]
- van Pée K. H., Lingens F. Purification and molecular and catalytic properties of bromoperoxidase from Streptomyces phaeochromogenes. J Gen Microbiol. 1985 Aug;131(8):1911–1916. doi: 10.1099/00221287-131-8-1911. [DOI] [PubMed] [Google Scholar]