Abstract
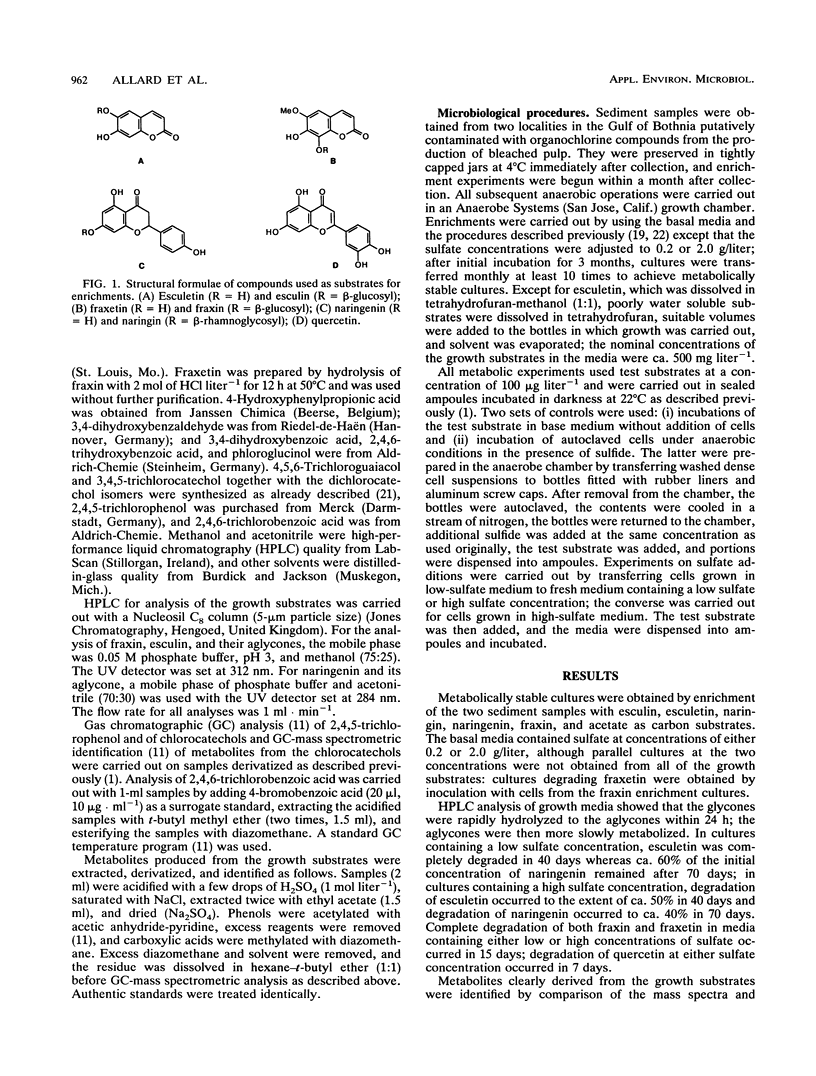
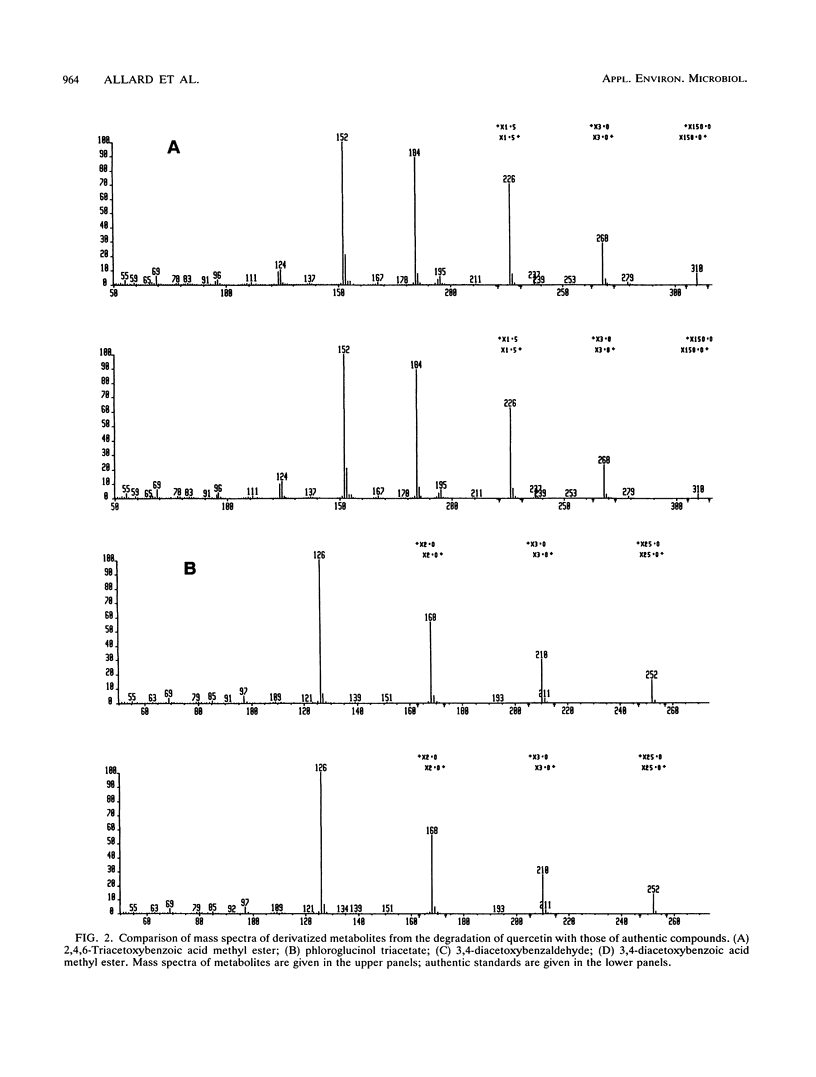
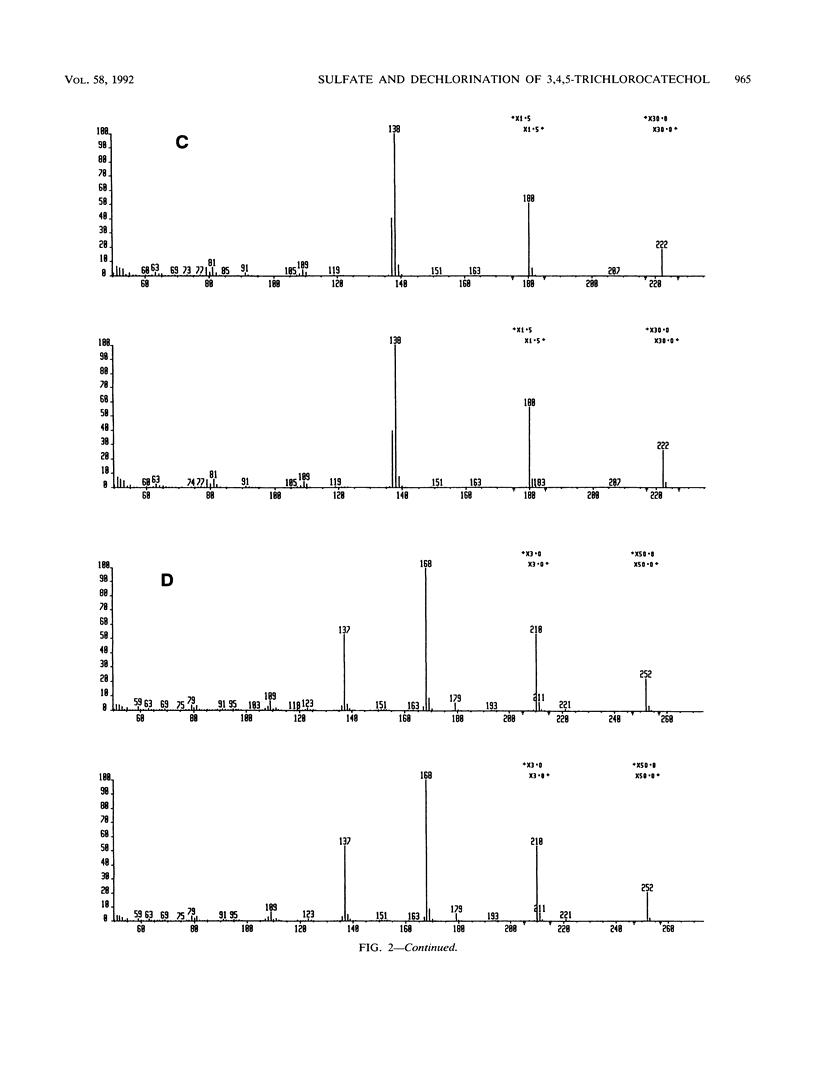
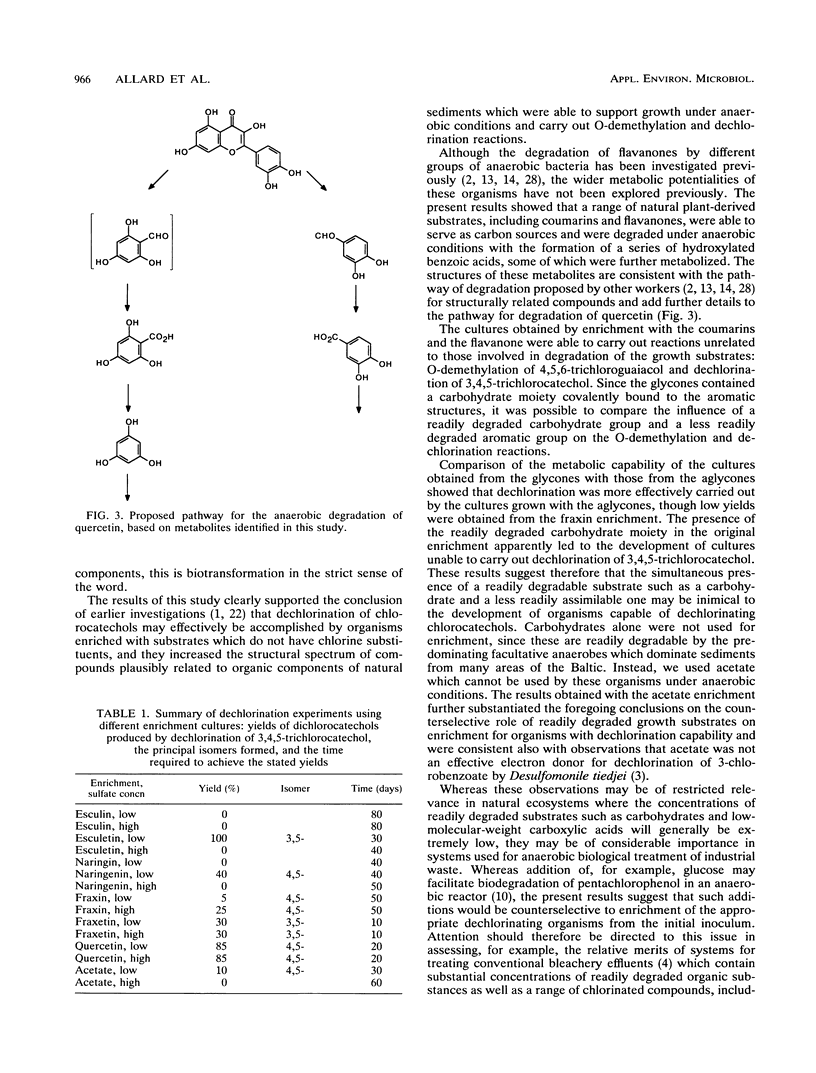
Metabolically stable anaerobic enrichment cultures have been obtained from sediment samples contaminated with chlorophenolic compounds. Enrichment was carried out with esculin, esculetin, naringin, naringenin, fraxin, quercetin, and acetate in media with two sulfate concentrations. These cultures were used to examine the O-demethylation of 4,5,6-trichloroguaiacol and the dechlorination of 3,4,5-trichlorocatechol. Whereas O-demethylation was observed in all cultures, the occurrence of dechlorination was significantly more restricted. The presence of the carbohydrate moiety in the cultures enriched with the glycones repressed development of populations which were able to carry out dechlorination. Although sulfate at a concentration of 2 g/liter in the primary enrichments blocked the development of populations able to bring about dechlorination, addition of sulfate at this concentration did not inhibit dechlorination in cultures possessing this capability. Different dichlorocatechol isomers were produced under the various conditions, so that in view of the established resistance of some of these to further dechlorination, the ultimate fate of 3,4,5-trichlorocatechol in the natural environment remains partly unresolved. No enrichment culture containing a low sulfate concentration was able to dechlorinate either 2,4,5-trichlorophenol or 2,4,6-trichlorobenzoate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard A. S., Hynning P. A., Lindgren C., Remberger M., Neilson A. H. Dechlorination of chlorocatechols by stable enrichment cultures of anaerobic bacteria. Appl Environ Microbiol. 1991 Jan;57(1):77–84. doi: 10.1128/aem.57.1.77-84.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Krishnamurty H. G., Jones G. A., Simpson F. J. Identification of products produced by the anaerobic degradation of naringin by Butyrivibrio sp. C3. Can J Microbiol. 1971 Jan;17(1):129–131. doi: 10.1139/m71-022. [DOI] [PubMed] [Google Scholar]
- DeWeerd K. A., Concannon F., Suflita J. M. Relationship between hydrogen consumption, dehalogenation, and the reduction of sulfur oxyanions by Desulfomonile tiedjei. Appl Environ Microbiol. 1991 Jul;57(7):1929–1934. doi: 10.1128/aem.57.7.1929-1934.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Price W. A., Pritchard P. H. Anaerobic Degradation of Chloroaromatic Compounds in Aquatic Sediments under a Variety of Enrichment Conditions. Appl Environ Microbiol. 1989 Jun;55(6):1466–1471. doi: 10.1128/aem.55.6.1466-1471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Suflita J. M. Anaerobic biodegradation of 2,4,5-trichlorophenoxyacetic Acid in samples from a methanogenic aquifer: stimulation by short-chain organic acids and alcohols. Appl Environ Microbiol. 1990 Jun;56(6):1825–1832. doi: 10.1128/aem.56.6.1825-1832.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Suflita J. M. Extrapolation of biodegradation results to groundwater aquifers: reductive dehalogenation of aromatic compounds. Appl Environ Microbiol. 1986 Oct;52(4):681–688. doi: 10.1128/aem.52.4.681-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom M. M., Young L. Y. Chlorophenol degradation coupled to sulfate reduction. Appl Environ Microbiol. 1990 Nov;56(11):3255–3260. doi: 10.1128/aem.56.11.3255-3260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohring G. W., Zhang X. M., Wiegel J. Anaerobic dechlorination of 2,4-dichlorophenol in freshwater sediments in the presence of sulfate. Appl Environ Microbiol. 1989 Oct;55(10):2735–2737. doi: 10.1128/aem.55.10.2735-2737.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurty H. G., Cheng K. J., Jones G. A., Simpson F. J., Watkin J. E. Identification of products produced by the anaerobic degradation of rutin and related flavonoids by Butyrivibrio sp. C3. Can J Microbiol. 1970 Aug;16(8):759–767. doi: 10.1139/m70-129. [DOI] [PubMed] [Google Scholar]
- Kuhn E. P., Townsend G. T., Suflita J. M. Effect of sulfate and organic carbon supplements on reductive dehalogenation of chloroanilines in anaerobic aquifer slurries. Appl Environ Microbiol. 1990 Sep;56(9):2630–2637. doi: 10.1128/aem.56.9.2630-2637.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S., Hendriksen H. V., Ahring B. K. Potential for thermophilic (50 degrees C) anaerobic dechlorination of pentachlorophenol in different ecosystems. Appl Environ Microbiol. 1991 Jul;57(7):2085–2090. doi: 10.1128/aem.57.7.2085-2090.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Allard A. S., Hynning P. A., Remberger M., Landner L. Bacterial methylation of chlorinated phenols and guaiacols: formation of veratroles from guaiacols and high-molecular-weight chlorinated lignin. Appl Environ Microbiol. 1983 Mar;45(3):774–783. doi: 10.1128/aem.45.3.774-783.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Allard A. S., Hynning P. A., Remberger M. Transformations of halogenated aromatic aldehydes by metabolically stable anaerobic enrichment cultures. Appl Environ Microbiol. 1988 Sep;54(9):2226–2236. doi: 10.1128/aem.54.9.2226-2236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Allard A. S., Lindgren C., Remberger M. Transformations of chloroguaiacols, chloroveratroles, and chlorocatechols by stable consortia of anaerobic bacteria. Appl Environ Microbiol. 1987 Oct;53(10):2511–2519. doi: 10.1128/aem.53.10.2511-2519.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H. The biodegradation of halogenated organic compounds. J Appl Bacteriol. 1990 Oct;69(4):445–470. doi: 10.1111/j.1365-2672.1990.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Remberger M., Allard A. S., Neilson A. H. Biotransformations of chloroguaiacols, chlorocatechols, and chloroveratroles in sediments. Appl Environ Microbiol. 1986 Mar;51(3):552–558. doi: 10.1128/aem.51.3.552-558.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dort H. M., Bedard D. L. Reductive ortho and meta Dechlorination of a Polychlorinated Biphenyl Congener by Anaerobic Microorganisms. Appl Environ Microbiol. 1991 May;57(5):1576–1578. doi: 10.1128/aem.57.5.1576-1578.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J., Moore L. H., Dowell V. R., Jr, Bokkenheuser V. D. C-ring cleavage of flavonoids by human intestinal bacteria. Appl Environ Microbiol. 1989 May;55(5):1203–1208. doi: 10.1128/aem.55.5.1203-1208.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


