Abstract
The activity of rat liver phenylalanine hydroxylase (PAH; phenylalanine 4-monooxygenase, EC 1.14.16.1) is regulated by interaction with its substrate, phenylalanine, and its coenzyme, BH4 [tetrahydrobiopterin (6R-dihydroxypropyl-l-erythro-5,6,7,8-tetrahydropterin)]. The structural changes accompanying these interactions have been studied by radiation target analysis. PAH purified from rat liver was incubated with 2 mM phenylalanine to achieve complete activation of the enzyme. Frozen samples were irradiated with various doses of high energy electrons; samples were subsequently thawed, and several surviving properties of the enzyme were determined. Each parameter decreased as a single exponential function of radiation dose. Radiation target analysis of enzymatic activity yielded a dimeric target size. Similar radiation effects on subunit monomers and on tetrameric structure were observed. Together with results from unactivated enzyme, these data show that phenylalanine increases the interactions between the subunits in a dimer and weakens the interactions between dimers in a tetramer. These alterations prevent the natural cofactor, a tetrahydrobiopterin, from exerting a negative effect on activity.
Keywords: regulation, subunit interaction, tetramer, dimer
Phenylalanine hydroxylase (PAH; phenylalanine 4-monooxygenase, EC 1.14.16.1) catalyzes the hydroxylation of phenylalanine to tyrosine. PAH has an absolute requirement for a tetrahydropterin cofactor; the natural cofactor is tetrahydrobiopterin (6R-dihydroxypropyl-l-erythro-5,6,7,8-tetrahydropterin; BH4), but several synthetic tetrahydropterins also can serve as effective cofactors. PAH catalyzes the initial step in the catabolism of phenylalanine and is a highly regulated enzyme. Activation of this enzyme is accomplished by reversible phosphorylation (1) and/or by substrate activation (2–8). Activation of PAH is only readily observed when the enzyme is assayed with BH4. This natural cofactor also acts as a significant negative effector of PAH activity. Accordingly, the regulation of PAH may be described in large part by opposing effects mediated by its substrate and its cofactor.
PAH purified from rat liver is free of both BH4 and phenylalanine and is predominantly tetrameric, consisting of 52-kDa subunits that are identical in primary structure (9). However, the purified enzyme has relatively low activity (10–12). PAH tertiary structure plays a crucial role in the regulation of enzyme activity. For example, phenylalanine hydroxylation by PAH is highly cooperative with respect to substrate phenylalanine concentration (10–12). Substrate-level activation of PAH has been proposed to involve cooperativity among all four subunits of the tetramer (13). However, little information relating PAH function to the tertiary structure of the enzyme has been ascertained to date. We previously examined the relationship of PAH structure to the regulation of activity of the purified enzyme using radiation inactivation target analyses (14), a technique that has proven to be very useful in defining the structure–function relationships of a large number of other proteins (15, 16). We found that the activity of PAH requires a structure whose mass is twice that of the 52-kDa subunit, implying that the minimum functional unit of purified PAH is a dimer, as we have previously suggested (17). With BH4 as the cofactor, irradiation revealed the inhibitory role of a large structure at least four times the mass of the 52-kDa subunit.
We have now extended these studies to examine the role of phenylalanine in the activation of purified PAH. In this report we show that phenylalanine activation of PAH alters the tertiary structure of the enzyme such that the association of the 52-kDa subunits is substantially strengthened. Nonetheless, the minimum reactive unit of phenylalanine-activated PAH remains a dimer. Our data are consistent with our previously presented model for the organization of the catalytic and regulatory binding sites for phenylalanine on rat hepatic PAH (17).
MATERIALS AND METHODS
6-Methyl-5,6,7,8-tetrahydropterin (6MPH4) and BH4 were obtained from Dr. B. Schirks Laboratories (Jona, Switzerland). Frozen rat livers were obtained from Pel-Freez Biologicals. All other reagents used were of the highest quality commercially available.
PAH was purified from rat liver either by the method of Parniak (18) or the hybrid procedure of Kaufman (19). Both methods provided enzyme with identical specific activities and physical properties. Samples for irradiation were prepared in 50 mM potassium phosphate, pH 7.0/2 mM phenylalanine, incubated for 20 min at 25°C, then aliquoted in equal amounts into glass ampoules and immediately frozen on dry ice. Normally, the concentration of PAH averaged 5 mg/ml to yield meaningful radiation target sizes (14, 20) and to avert the need to include free-radical scavengers in the samples (21). Ampoules were sealed without thawing the samples. Irradiation was performed at −135°C as described (14, 22). Thereafter, samples were maintained at temperatures no higher than −80°C until assay. At this point, frozen samples were rapidly thawed, then placed on ice for HPLC and SDS/PAGE analyses and measurement of PAH activity.
Enzyme assays were carried out as described (23). In experiments measuring the variation of PAH activity with changes in assay phenylalanine concentration, allowance was made for the contribution of phenylalanine in the preincubated enzyme samples. Data were fit to the equation
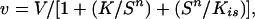 |
where V is the maximum velocity, K is the apparent Km for phenylalanine, n is the index of cooperativity, and Kis accounts for the substrate inhibition (24).
SDS/PAGE was performed with NOVEX (San Diego) minicell precast 10% polyacrylamide gels following the method of Laemmli (25). Gels were stained with FAST-STAIN (Zoion Research), dried, and analyzed by densitometry. HPLC size-exclusion analyses were performed at ambient temperature (18–21°C) essentially as described (17), using a mobile phase of 50 mM Tris·HCl, pH 7.2 (20°C)/0.1 M NaCl/2 mM phenylalanine.
RESULTS
Target Size of PAH as Determined by Enzyme Assay with Either 6MPH4 or BH4.
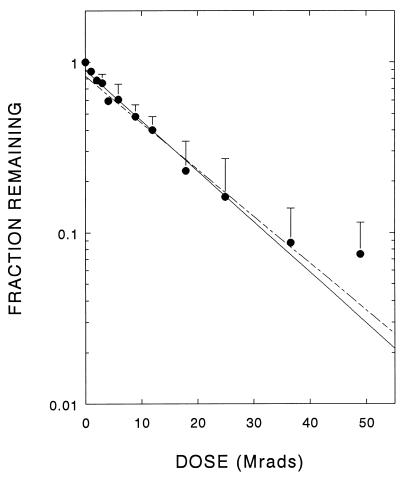
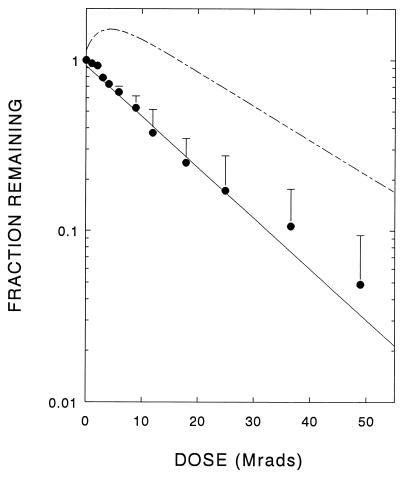
Following pretreatment with phenylalanine, the radiation-induced loss of 6MPH4-dependent PAH activity followed first-order kinetics (Fig. 1), yielding an enzyme target size of 119 kDa (Table 1). Similar results were previously found with the untreated enzyme (14). However, when assayed with the natural cofactor, BH4, the activity of phenylalanine-pretreated PAH also decayed as a simple first-order exponential, providing a target size of 120 kDa (Fig. 2 and Table 1), in direct contrast to results observed for untreated enzyme that showed complex inactivation profiles (14).
Figure 1.
Residual 6MPH4-dependent PAH activity after exposure of phenylalanine-preincubated enzyme to increasing doses of radiation. The data shown are the averages + SD from four independent experiments. The dashed line shows response obtained by irradiation of unactivated purified PAH (14).
Table 1.
Target sizes (in kDa) of purified PAH
| Target | PAH* | Pretreated with phenylalanine |
|---|---|---|
| Monomer SDS/PAGE | 59 ± 34 | 142 (n = 2) |
| Tetramer HPLC | 113 ± 10 | 144 (n = 2) |
| Activity | ||
| 6MPH4 | 132 ± 19 | 119 ± 39 |
| BH4 | 96 ± 17 | 120 ± 40 |
| 476 ± 206 |
Data are from ref. 14.
Figure 2.
Residual BH4-dependent PAH activity after exposure of phenylalanine-preincubated enzyme to increasing doses of radiation. The data shown are the averages + SD from four independent experiments. The dashed line shows response obtained by irradiation of unactivated purified PAH (14).
Target Size of PAH as Determined by HPLC.
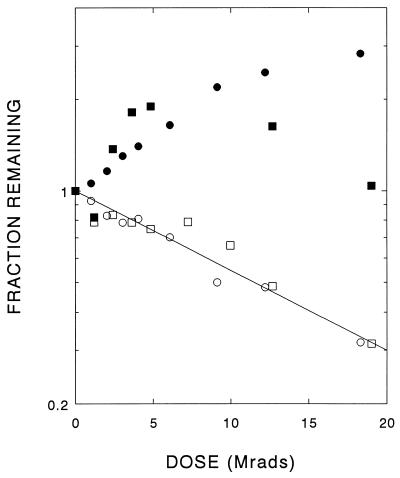
Chromatographic profiles from HPLC size-exclusion analyses of phenylalanine-treated PAH showed two unequal peaks corresponding to the tetramer and dimer forms of the enzyme; there was no evidence for free 52-kDa monomers. The apparent 220-kDa tetramer accounted for >90% of the PAH protein. After radiation exposure, the area under the main peak decreased exponentially with radiation dose, leading to a target size of ≈144 kDa (Table 1). The results are similar to those observed with the untreated enzyme (14). Thus, alteration of the tertiary structure of PAH due to radiation appears to proceed in increments of two (as opposed to four) 52-kDa subunits.
The dimer of untreated PAH decreased as a single exponential (Fig. 3), yielding a target size of 108 kDa. However, the phenylalanine-preactivated enzyme revealed a complex inactivation curve showing an initial increase in area of the dimer peak at low radiation doses (Fig. 3) before an eventual decline. These results imply that a single radiation hit in a phenylalanine-pretreated tetramer releases at least one structural dimer.
Figure 3.
Effect of radiation dose on PAH dimers, determined by size-exclusion chromatography. Loss of dimers of pure PAH as a function of radiation dose (open symbols) yields a target size of 108 kDa. In two paired experiments (circles and squares), phenylalanine-pretreated samples were also irradiated. The dimer peak increased after low radiation exposures (filled symbols).
SDS/PAGE Analysis of Phenylalanine-Pretreated PAH After Irradiation.
Samples of irradiated PAH were also analyzed by denaturing gel electrophoresis (SDS/PAGE). The 52,000 Mr monomer band was visualized and quantitated by Coomassie blue staining. The stain intensity was proportional to the amount of enzyme (data not shown). The surviving protein in the monomer band decreased after irradiation as a simple exponential function of the dose, and a target size of 142 kDa was determined (Table 1). This result differs from that previously determined for the untreated enzyme in which an average target size of 59 kDa was determined (14).
Effect of Irradiation on Kinetics of Phenylalanine Hydroxylation.
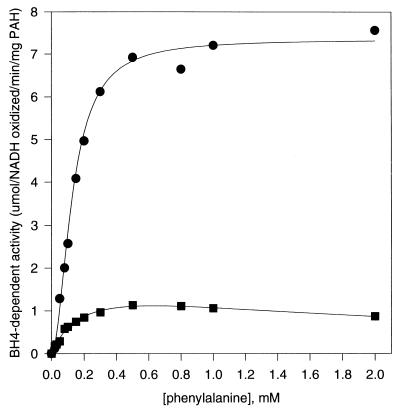
Preincubation of PAH with phenylalanine leads to a 10- to 20-fold increase in BH4-dependent enzyme activity (2–6). Unlike other activation phenomena, such as exposure to phospholipids (26, 27) or modification with N-ethylmaleimide (23), the BH4-dependent activity of PAH pretreated with phenylalanine retains a sigmoid response to variations in phenylalanine concentration (Fig. 4). Following irradiation with 12 Mrad, the activity of pretreated PAH was substantially reduced when assayed with BH4 (see Fig. 2). This result is in direct contrast with that determined for the untreated enzyme that showed an increase in activity following the same irradiation dose and under the same assay conditions. Irradiation of phenylalanine-pretreated PAH also eliminates its cooperative response to phenylalanine concentration when assayed with BH4. The same effect on cooperativity was observed for untreated PAH in our earlier study (14).
Figure 4.
Variation of BH4-dependent activity of phenylalanine-preincubated PAH as a function of assay phenylalanine concentration. The solid lines are the best-fit calculated curves based on the equation v = V/[1 + (K/Sn) + (Sn/Kis)], as described. •, Phenylalanine-preincubated PAH not exposed to radiation: V = 7.4, K = 0.02, n = 1.9, Kis > 20. ▪, Phenylalanine-preincubated PAH after exposure to 12 Mrad radiation dose: V = 1.8, K = 0.2, n = 1.0, Kis = 2.
DISCUSSION
At the catalytic site of PAH a tetrahydropterin cofactor helps the enzyme catalyze the conversion of phenylalanine and oxygen to tyrosine and (ultimately) water. In addition, PAH also contains a regulatory site for phenylalanine and a reported regulatory site for tetrahydrobiopterin (28). Thus the purified tetrameric enzyme can have up to four different activity states determined by the binding of these two regulatory factors: (i) an unactivated state, where neither its natural cofactor, BH4, nor its substrate, phenylalanine, are bound to their respective regulatory sites on the enzyme; (ii) a deactivated state, where BH4 is bound to its regulatory site on the enzyme and the level of enzyme activity is depressed (the level is relatively unaffected by the artificial cofactor, 6MPH4); (iii) an activated state, where phenylalanine is bound to its regulatory site on the enzyme and the level of activity in significantly enhanced; and (iv) a conceivable state where both the substrate and cofactor are bound to their respective regulatory binding sites (assuming that such binding is not mutually exclusive).
These states are associated with distinct structural conformations. For example, when PAH is activated by phenylalanine, not only is an increase in activity observed when BH4 is the cofactor (6, 29, 30), but the enzyme also undergoes a substantial conformational change (6, 30, 31). In addition, a high apparent Arrhenius activation energy for phenylalanine activation, ≈35 kcal/mol, has been reported (11).
In the present experiments, purified PAH was preincubated with 2 mM phenylalanine to achieve complete substrate-level activation of the enzyme (2–6). This concentration of phenylalanine was maintained throughout the subsequent irradiation manipulations to ensure the continued activation state of the enzyme.
The present results, together with those of a previous radiation study of the purified (but not activated) PAH, fit a simple structure-function model having several key features: (i) the purified enzyme has a tetrameric structure, but enzymatic activity requires only two monomers, possibly as a dimer; (ii) the tetramer is susceptible to being inactivated by BH4, whereas the free dimer is not (14); and (iii) pretreatment with phenylalanine reversibly alters the conformation of the tetramer: it increases the interactions between the two subunits of the dimer and apparently weakens the interactions between the dimers in the tetramer, thereby preventing BH4 from acting as a negative effector.
This model is consistent with the data disclosed for the artificially activated enzyme species of PAH such as N-ethylmaleimide-activated (23) and chymotrypsin-activated enzyme (26) in which the activated enzyme is a free dimer. It is also consistent with the activation of PAH by alkaline pH (32) in which the free dimeric form of the enzyme is produced (31), although the activation of the enzyme is still predominantly a phenylalanine-dependent process. Thus a dimeric state alone is not sufficient to activate the enzyme, but rather serves to facilitate the activation of the enzyme by its substrate. Perhaps this facilitation is due, in part, to preventing BH4 from hindering the activation of the enzyme.
The model is based partly on the target size determined from the loss of catalytic activity. With 6MPH4 as the cofactor, the radiation response for enzymatic activity remains essentially the same regardless of whether the enzyme is pretreated with phenylalanine or not. However, when BH4 is the cofactor, the data are strikingly different depending on whether the enzyme was pretreated with phenylalanine or not: when the pretreated enzyme is assayed with BH4, the activity of the enzyme declines in an exponential fashion with respect to increasing radiation dose (Fig. 2) rather than initially increasing and then decreasing, as was observed for the untreated enzyme. Thus, phenylalanine removes the inhibition (“negative effect”) of BH4 on activity which was previously shown to require a tetrameric (or larger) structure (14).
SDS/PAGE analysis of the radiation dose-response of untreated PAH showed that a single radiation hit destroyed a single monomeric subunit (14). Because it was found that the enzyme activity decreased as a dimer, it was concluded that the minimum active form of untreated PAH was a dimer of two competent monomers (14). In contrast, the SDS/PAGE analysis of the radiation dose-response of PAH that had been pretreated with phenylalanine indicates that a single radiation hit destroys a pair of monomers. This result indicates that phenylalanine modifies the structure of the monomers within the tetramer such that a monomer can now transfer energy to an adjacent monomer. Presumably, pretreatment with phenylalanine causes a closer interaction of a pair of monomers in a dimer. The loss of the BH4 negative effect suggests that the two dimers in the tetramer are now less closely coupled.
Untreated PAH binds 1.5 mol of phenylalanine per monomer, whereas the activated enzyme binds only 1 mol/monomer, which has been interpreted as 1 mol of phenylalanine binding per monomeric catalytic site, and 1 mol of phenylalanine binding per dimeric regulatory site (5, 23). It was proposed that the regulatory site is unique to the dimer—i.e., it is formed by the interaction of the two monomers in the dimer pair (17). The present results are consistent with these ideas because they demonstrate a closer relationship between the individual monomers of the dimer when phenylalanine is bound to the regulatory site.
HPLC gel filtration analysis of untreated PAH followed the same dose-response curve as observed for the loss of catalytic activity: the disappearance of the tetramer peak on HPLC led to a dimeric target size (14). However, no new free dimer was formed; rather, as the tetramer was destroyed all of the monomers appeared to be lost. Thus, although a single radiation hit of the untreated tetramer is sufficient to prevent BH4 from acting as a negative effector, both dimers must be hit to break up the tetramer. Once the tetramer was dissociated, the two remaining intact monomers neither remained as, nor came together to form a free dimer (14). HPLC gel filtration analysis of the phenylalanine-pretreated enzyme differs significantly. The dose-response curve still shows the tetramer disappearing with a target size consistent with a dimer; however, the radiation damage done to the phenylalanine-pretreated enzyme leads to the release of additional dimers, a result in direct contrast with that observed for the untreated enzyme but consistent with an increased monomer–monomer interaction due to phenylalanine binding.
In our experimental protocol, both the untreated and pretreated enzyme were in the frozen state during irradiation. Under these conditions, it is unlikely that any significant dissociation of the tetramer can take place. However, when the samples are brought to ambient temperature, the subunits can reequilibrate. The phenylalanine-pretreated enzyme would be more likely to retain stable dimers because it already has greater dimeric character than the untreated enzyme. On the other hand, the untreated enzyme apparently is more likely to either remain as a tetramer or to reform a stable tetramer.
Our previous pH activation studies (32) support this model. The enzyme is in a dimeric state when incubated at high pH, but assumes a tetrameric structure when brought to a neutral pH (31, 32). This reequilibration appears to take less than a minute at room temperature, which is significantly shorter than the time frame for the present HPLC analysis. These pH activation results suggest that any free dimers formed as a result of radiation inactivation of the untreated enzyme tetramer should readily reassociate, because this is where the equilibrium between the dimer and tetramer appears to lie. On the other hand, once the phenylalanine-pretreated enzyme is liberated from the tetramer by radiation inactivation, the free dimeric form might be expected to be stable apart from the tetramer.
The tetrameric structure of the enzyme β-galactosidase is retained after a single radiation hit, as assessed by size-exclusion chromatography (33); multiple radiation events are required to alter the tetramer elution pattern. These data imply that radiation-damaged subunits are still able to maintain the subunit interactions involved in a tetrameric association. The dimeric target size obtained for the tetrameric destruction of untreated PAH (14) is consistent with the results observed with β-galactosidase. For the phenylalanine-pretreated enzyme, two subunits are found to be damaged by a single ionization. Therefore the loss of the tetrameric structure still results from the destruction of two subunits, but in this special case it is caused by a single ionization.
Our results indicate that the tertiary structure of purified PAH plays a critical role in the regulation of the activation state of the enzyme in vitro. The structure undoubtedly also plays an important role in the regulation of this enzyme in vivo.
Acknowledgments
This research was supported in part by grants (to M.A.P.) from the Medical Research Council of Canada. M.A.P. is a Chercheur-Boursier Senior (Career Research Scholar) of the Fonds de la Recherche en Santé du Québec.
Footnotes
.
Abbreviations: BH4, tetrahydrobiopterin (6R-dihydroxypropyl-l-erythro-5,6,7,8-tetrahydropterin); 6MPH4, 6-methyl-5,6,7,8-tetrahydropterin; PAH, phenylalanine 4-monooxygenase.
References
- 1.Donlon J, Kaufman S. J Biol Chem. 1978;253:6657–6659. [PubMed] [Google Scholar]
- 2.Nielsen K H. Eur J Biochem. 1969;7:360–369. doi: 10.1111/j.1432-1033.1969.tb19617.x. [DOI] [PubMed] [Google Scholar]
- 3.Shiman R, Gray D W. J Biol Chem. 1980;255:4793–4800. [PubMed] [Google Scholar]
- 4.Shiman R, Mortimore G E, Schworer C M, Gray D W. J Biol Chem. 1982;257:11213–11216. [PubMed] [Google Scholar]
- 5.Phillips R S, Parniak M A, Kaufman S. J Biol Chem. 1984;259:271–277. [PubMed] [Google Scholar]
- 6.Phillips R S, Parniak M A, Kaufman S. Biochemistry. 1984;23:3836–3842. doi: 10.1021/bi00312a007. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman S. J Biol Chem. 1970;249:4751–4759. [PubMed] [Google Scholar]
- 8.Tourian A. Biochim Biophys Acta. 1971;242:345–354. doi: 10.1016/0005-2744(71)90226-9. [DOI] [PubMed] [Google Scholar]
- 9.Dahl H H M, Mercer J F B. J Biol Chem. 1986;261:4148–4153. [PubMed] [Google Scholar]
- 10.Kaufman S, Fisher D B. In: Molecular Mechanisms of Oxygen Activation. Hayaishi O, editor. New York: Academic; 1974. pp. 285–369. [Google Scholar]
- 11.Shiman R. In: Folates and Pterins. Blakely R L, Benkovic S J, editors. Vol. 2. New York: Wiley; 1985. pp. 179–249. [Google Scholar]
- 12.Kaufman S. Adv Enzymol. 1993;67:77–264. doi: 10.1002/9780470123133.ch2. [DOI] [PubMed] [Google Scholar]
- 13.Shiman R, Jones S H, Gray D W. J Biol Chem. 1990;265:11633–11642. [PubMed] [Google Scholar]
- 14.Davis M D, Parniak M A, Kaufman S, Kempner E. Arch Biochem Biophys. 1996;325:235–241. doi: 10.1006/abbi.1996.0029. [DOI] [PubMed] [Google Scholar]
- 15.Kempner E S. Adv Enzymol. 1988;61:107–147. doi: 10.1002/9780470123072.ch3. [DOI] [PubMed] [Google Scholar]
- 16.Kempner E S. Trends Biochem Sci. 1993;18:236–239. doi: 10.1016/0968-0004(93)90169-n. [DOI] [PubMed] [Google Scholar]
- 17.Parniak M A. In: Unconjugated Pterins and Related Biogenic Amines. Curtius H-Ch, Blau N., editors. Berlin: de Gruyter; 1987. pp. 327–337. [Google Scholar]
- 18.Parniak M A. In: Chemistry and Biology of Pteridines. Curtius H-Ch, Ghisla S, Blau N., editors. Berlin: de Gruyter; 1989. pp. 672–677. [Google Scholar]
- 19.Kaufman S. Methods Enzymol. 1987;142:3–17. doi: 10.1016/s0076-6879(87)42003-x. [DOI] [PubMed] [Google Scholar]
- 20.Kempner E S, Miller J H. Anal Biochem. 1994;216:451–455. doi: 10.1006/abio.1994.1067. [DOI] [PubMed] [Google Scholar]
- 21.Eichler D C, Solomonson L P, Barber M J, McCreery M J, Ness G C. J Biol Chem. 1987;262:9433–9436. [PubMed] [Google Scholar]
- 22.Harmon J T, Nielsen T B, Kempner E S. Methods Enzymol. 1985;117:65–94. doi: 10.1016/s0076-6879(85)17008-4. [DOI] [PubMed] [Google Scholar]
- 23.Parniak M A, Kaufman S. J Biol Chem. 1981;256:6876–6882. [PubMed] [Google Scholar]
- 24.Segel I. Enzyme Kinetics. New York: Wiley; 1975. [Google Scholar]
- 25.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Fisher D B, Kaufman S. J Biol Chem. 1973;248:4345–4353. [PubMed] [Google Scholar]
- 27.Abita J P, Parniak M A, Kaufman S. J Biol Chem. 1984;259:14560–14566. [PubMed] [Google Scholar]
- 28.Shiman R, Xia T, Hill M A, Gray D W. J Biol Chem. 1994;269:24647–24656. [PubMed] [Google Scholar]
- 29.Ayling J E, Helfand G D. In: Chemistry and Biology of Pteridines. Pfleiderer W, editor. Berlin: de Gruyter; 1975. pp. 305–319. [Google Scholar]
- 30.Shiman R, Gray D W, Pater A. J Biol Chem. 1979;254:11300–11306. [PubMed] [Google Scholar]
- 31.Kappock T J, Harkins P C, Friedenberg S, Caradonna J P. J Biol Chem. 1995;270:30532–30544. doi: 10.1074/jbc.270.51.30532. [DOI] [PubMed] [Google Scholar]
- 32.Parniak M A, Davis M D, Kaufman S. J Biol Chem. 1988;263:1223–1230. [PubMed] [Google Scholar]
- 33.Potier M, Thauvette L, Michaud L, Giroux S, Beauregard G. Biochemistry. 1991;30:8151–8157. doi: 10.1021/bi00247a009. [DOI] [PubMed] [Google Scholar]