Abstract
In response to a moderate dose of radiation, asynchronous mammalian cell populations rapidly and transiently down-regulate the rate of DNA synthesis to ≈50% of preirradiation values. We show here that only half of the reduction in overall replication rate can be accounted for by direct inhibition of initiation at origins in S-phase cells. The other half results from the operation of a newly defined cell cycle checkpoint that functions at the G1/S transition. This checkpoint senses damage incurred at any time during the last 2 hr of G1 and effectively prevents entry into the S period. The G1/S and S-phase checkpoints are both p53-independent and, unlike the p53-mediated G1 checkpoint, respond rapidly to radiation, suggesting that they may represent major damage-sensing mechanisms connecting the replication machinery with DNA repair pathways.
Mammalian cells operate several different mechanisms for coping with DNA damage. One of these is a p53-mediated checkpoint that functions at or near the restriction point in mid-G1 (1–6). This control either stalls cells in the G0/G1 compartment for an extended time interval to allow for repair (7) and/or shunts them toward an apoptotic death (8–10). A second checkpoint functions in G2 to prevent the fragmentation of incomplete or damaged templates by the shear forces of mitosis.
Neither of these pathways could protect cells that are either beyond the restriction point in G1 or in the S phase itself at the time of radiation from replicating through single-strand breaks. However, in response to a moderate dose of ionizing radiation, the overall rate of [3H]thymidine incorporation in an asynchronously growing mammalian cell culture is reduced to ≈50% of pre-irradiation values within 1.5–2 hr (11–15). It has been suggested that this rapid response functions by inhibiting subsequent initiation at origins of replication, while allowing forks already in progress to continue (12). Recently, this was shown directly by demonstrating the loss of replication bubbles from the amplified dihydrofolate reductase (DHFR) locus in the methotrexate-resistant Chinese hamster ovary (CHO) cell line, CHOC 400, using a two-dimensional (2D) gel replicon mapping technique (13). Since CHOC 400 cells have a mutated p53 gene (16) and lack the radiation-sensitive G1 checkpoint (13, 16), we have proposed the existence of a p53-independent S-phase damage-sensing pathway (13).
Interestingly, however, when CHOC 400 cells are collected at the beginning of the S period with either of the replication inhibitors, mimosine or aphidicolin, they are quite insensitive to a radiation challenge: the overall rate of [3H]thymidine incorporation is inhibited only slightly after drug removal, and 2D gel analysis shows that the majority of DHFR origins subsequently fires normally (ref. 13; unpublished observations). Thus, it is possible that mimosine- or aphidicolin-blocked cells have passed an important cell cycle checkpoint after which they are relatively refractile to DNA damage for some time period.
In the present report, we show that, indeed, there is a p53-independent damage-sensing checkpoint that functions at or near the G1/S boundary. The operation of this control prevents late G1 cells from entering the S period by directly or indirectly inhibiting initiation at the earliest-firing origins, with the consequence that the entire S period is effectively repositioned (delayed) along the cell cycle axis. This G1/S checkpoint appears to have a memory since cells that are as much as 2 hr away from entering the S period at the time of a radiation challenge are prevented from doing so.
MATERIALS AND METHODS
Cell Culture and Cell Synchrony.
CHOC 400 cells were maintained in MEM supplemented with nonessential amino acids and 10% HyClone II. To obtain synchronized S-phase cells, cultures were first arrested in G0 by isoleucine deprivation for 45 hr, followed by release into complete medium containing 200 μM mimosine for 14 hr (17). After drug removal, cells enter the S period ≈30 min later.
Radiation Treatment.
Radiation treatments were performed with a General Electric Maxima 250-III instrument operating at 200 kVp and 15 mA without a filter, or with a Gammacell 40 (energy source, Cs-137; Atomic Energy of Canada, Ontario). The dose rate was 100–250 cGy/min. Cells were irradiated in a container designed to mimic the conditions of the cell culture incubator (5% CO2 and 95% air at 37°C).
Determination of Replication Rates.
Cells growing in 24-well dishes were labeled with 0.5 μCi of [3H]thymidine (80 Ci/mmol; 1 Ci = 37 GBq; Amersham) per ml of culture medium for the intervals indicated in the figure legends, after which they were washed twice with PBS and fixed with citric acid; the amount of insoluble radioactivity was then determined at the end of the experiment as described (17).
Preparation of Replication Intermediates and 2D Gel Analysis.
Cells were harvested at the appropriate times and replication intermediates were purified exactly as described elsewhere (18). Briefly, nuclear matrix-halo structures were prepared by using lithium diiodosalicylate to extract histones, and matrix-affixed replication intermediates were isolated by digesting away loop (nonreplicating) DNA with EcoRI. The matrix-bound DNA replication intermediates were then purified by proteinase K treatment, followed by dialysis and ethanol precipitation; the resuspended DNA was fractionated further on benzoylated-naphthoylated DEAE cellulose (Sigma) to select for partially single-stranded DNA. Intermediates from an equal number of cells from each sample (5 × 107) were loaded and run on individual neutral/neutral 2D gels (18), which separate in the first dimension on the basis of molecular mass and in the second dimension by both mass and shape (19). After transfer to HyBond N+, digests were hybridized with a 32P-labeled 0.5 kb PvuII–XmnI probe specific for EcoRI fragment RF′, which contains ori-β (20).
p53 cDNA Isolation and Cell Transfection.
A 1.8-kb BamHI/EcoRI cDNA fragment containing the entire coding sequence of wild-type Chinese hamster p53 was cloned into pCDNA3 (Invitrogen) to generate the pCDNA-Chp53 plasmid (16). The plasmid was linearized at the single ScaI site and was transfected into CHOC 400 cells by electroporation (capacitance 980 μF at 200 volts) using a PG200 Progenitor II electroporator (Hoefer). Selection was carried out in 500 μg/ml G418 (BRL) for 2 days postelectroporation, and several healthy colonies were recloned and analyzed to select those with a pronounced G1 arrest phenotype (16).
Fluorescence-Activated Cell Sorter (FACS; Becton Dickinson) Analysis.
Cultures were washed once with cold PBS and were trypsinized and washed again in cold PBS. The cell pellet was immediately suspended in DNA staining solution containing 0.1% (wt/vol) sodium citrate, 0.3% (vol/vol) Nonidet P-40, 100 μg/ml RNase A (Sigma), and 150 μg/ml propidium iodide (Sigma). Samples were analyzed in the University of Virginia FACS facility.
RESULTS
Synchronized S-Phase Cells Are Less Sensitive to Radiation Than an Asynchronous Cell Population.
When an asynchronous population of CHOC 400 cells is irradiated with 10 Gy (which is the approximate dose at the inflection point between the steep and shallow components of the dose-response curve; refs. 13 and 21), the rate of DNA synthesis is almost immediately suppressed (refs. 11–13; Fig. 1B, log sample indicated with ▪). The replication rate is inhibited maximally to about 50% by 2 hr, followed by a slow recovery to pre-irradiation values 7–8 hr later (13).
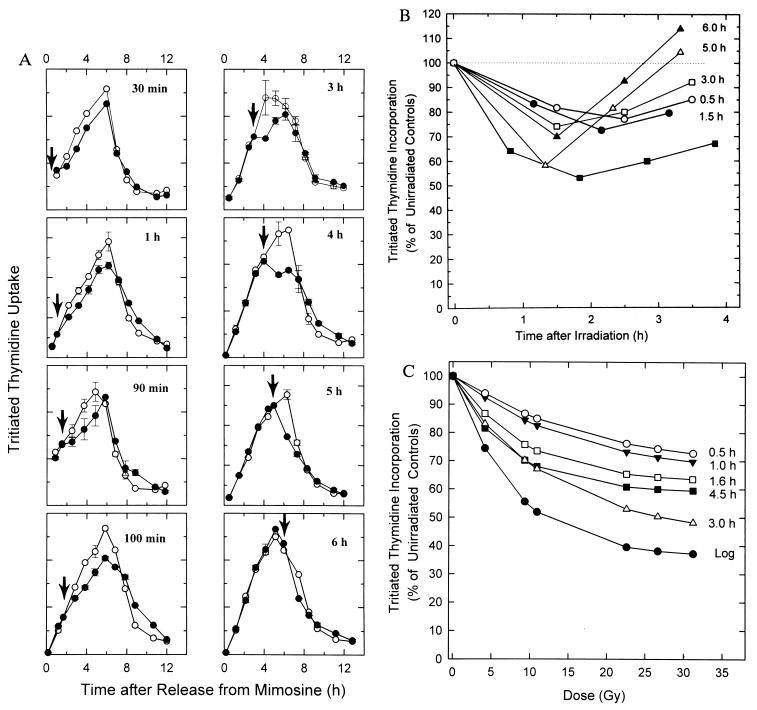
Figure 1.
Radiation effects on DNA synthesis in synchronized CHOC 400 cells. CHOC 400 cells growing in multiwell dishes were synchronized at the G1/S boundary and were released into the S period by removal of mimosine. (A) Replicate samples were mock-irradiated or gamma-irradiated (10 Gy) at different times in the S phase. Duplicate wells were labeled for 20 min with [3H]thymidine (0.5 μCi/ml, 80 Ci/mM; Amersham) at the indicated intervals thereafter. The averages of duplicate samples are plotted at the midpoint of the pulse period. (B) The data from A are expressed as percentage incorporation relative to a mock-irradiated control pulsed at the same time. Data are also shown for an asynchronous (log) cell population irradiated, pulsed, and sampled identically. (C) Synchronized CHOC 400 cells were irradiated with different doses at the indicated times in the S period. Duplicate wells were labeled for 20 min with [3H]thymidine at the following times, which correspond to the points of maximum depression (determined as in A): 0.5 hr, 2.0 hr later; 1.0 hr, 2.0 hr later; 1.6 hr, 2.0 hr later; 4.5 hr, 1.7 hr later; 3.0, 1.7 hr later; and 4.5 hr, 1.7 hr later. Asynchronous (log) cell cultures (▪) were irradiated at the same doses, pulsed with [3H]thymidine for 20 min, and harvested 1 hr later.
To study this acute response in more detail, we characterized the effects of the same radiation dose on DNA synthesis at different times in the S period. CHOC 400 cells were released from a G0 block and were then collected at the beginning of the S period with the replication inhibitor, mimosine; the drug was then removed, allowing synchronous entry into the S period after a lag of ≈30 min (17). Replicate cultures were irradiated at the indicated times (arrows) and the effect on the rate of [3H]thymidine incorporation was determined at selected intervals thereafter. The resulting time-course data is shown in Fig. 1A and is summarized in Fig. 1B.
Surprisingly, at no point in the S period did radiation treatment result in a 50% reduction in the subsequent rate of [3H]thymidine incorporation (Fig. 1A), even though 10 Gy delivered to an asynchronous culture inhibits the rate of DNA synthesis by ≈50% within 1.5–2 hr (Fig. 1B, ▪; refs. 11–13). With the exception of cells irradiated 5 hr after entry into the S period, in which inhibition reached ≈40% in this experiment, the average maximum inhibition was ≈25%. We have shown previously that when cells are irradiated immediately after removal of mimosine, DNA synthesis is not significantly inhibited when cells enter the S period 30 min later (13). Furthermore, cells synchronized at the G1/S boundary with aphidicolin, which begin replication immediately after drug removal, are similarly refractile to a radiation challenge (unpublished observations). Therefore, the inhibition of DNA replication in S-phase cells alone cannot account for the 50% reduction in the overall rate of DNA synthesis detected in an asynchronous population after a dose of 10 Gy (Fig. 1B, ▪).
To uncover any dose-dependent differences in response to radiation, CHOC 400 cells were irradiated with a range of doses either in asynchronous (log) populations, or at different times in the S period in synchronized cells. The rates of [3H]thymidine incorporation were determined at intervals thereafter. The percentage incorporation at the time of maximum depression was then plotted as a function of dose (Fig. 1C).
As anticipated, the dose-response curve for an asynchronous culture displays a steep, initial component that reaches ≈50% inhibition at ≈10 Gy, as well as a shallow, insensitive component at higher doses. These two components are thought to represent effects on initiation and elongation, respectively (21). However, at each of the times tested in the S period in synchronized cells, inhibition of replication rates at lower doses is much less dramatic than in the asynchronous population (Fig. 1C), and is particularly evident in samples irradiated 0.5 and 1.0 hr after removal of mimosine. These data suggest that this analysis excludes a subpopulation of cells in which the replication rate is inhibited much more severely.
Radiation Effects on the Early-Firing DHFR Origin.
To study radiation effects on initiation independently from effects on chain elongation and repair, we employed a 2D gel replicon mapping procedure (19) to analyze the well-defined ori-β initiation locus in the amplified DHFR domain of CHOC 400 cells before and after radiation treatment. This method allows the separation of restriction fragments containing replication bubbles (and therefore origins) from fragments replicated passively by a single fork emanating from an outside origin (Fig. 2D). Any fragment of interest can be analyzed in a transfer of such a gel by hybridization with an appropriate radioactive probe. ori-β and ori-γ are somewhat preferred initiation regions lying within a broad zone of potential initiation sites encompassing the entire region between the DHFR and 2BE2121 genes (refs. 20, 23, and 24; Fig. 2 Lower). Therefore, a restriction fragment containing either origin (e.g., fragment RF′) is sometimes replicated from an internal initiation site (thereby contributing to the bubble arc) and sometimes by a single fork that originated from another site in a neighboring fragment in the intergenic zone (contributing to the fork arc). As a consequence, in the first few hours of the S period when initiation occurs at one of the many potential sites in the intergenic region, fragment RF′ displays a composite pattern consisting of both a bubble arc and a single fork arc (Fig. 2D; refs. 20, 23, and 24).
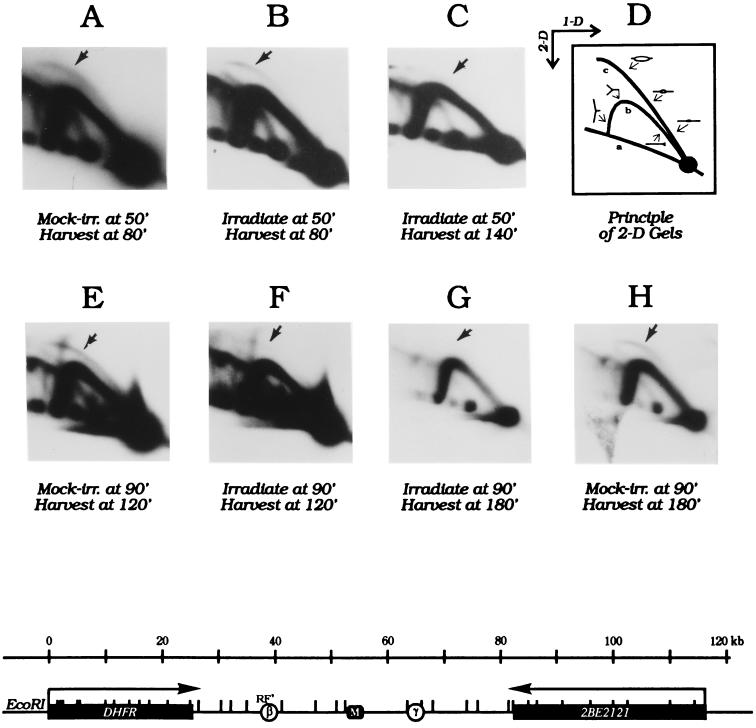
Figure 2.
Radiation effects on initiation in the DHFR ori-β locus. CHOC 400 cells were synchronized as in Fig. 1, and were mock-irradiated or irradiated with 9 Gy either 50 min (Upper) or 90 min (Lower) after release from mimosine. Replication intermediates were prepared 30 or 90 min later for analysis by the neutral/neutral 2D gel replicon mapping method (19). D is a cartoon of the patterns traced by restriction fragments containing either replication bubbles or single forks (see text). (Lower) A map of the 120 kb region surrounding the DHFR and 2BE2121 genes and the intergenic region, which contains ori-β and ori-γ, as well as a matrix attachment region (M; ref. 22).
We showed previously that when CHOC 400 cells are irradiated before or immediately after removal of mimosine, there is very little effect on initiation 80, 120, or 240 min later (13). Cells released from an aphidicolin block were similarly insensitive to radiation before or immediately after removal of the drug (unpublished observations). To analyze radiation effects on initiation at ori-β later in the S period, CHOC 400 cells were irradiated with 10 Gy either 50 or 90 min after release from mimosine, and cells were harvested 30 or 90 min later for 2D gel analysis. Transfers of the gels were then hybridized with a radioactive probe specific for fragment RF′, which contains ori-β (Fig. 2 Lower).
As shown in Fig. 2B, radiation treatment 50 min after entry into the S period had only a small effect on the pattern of replication intermediates in cells sampled 30 min later (i.e., 80 min after release from mimosine): neither the bubble arc nor the fork arc is greatly reduced in response to radiation (compare Fig. 2B to the mock-irradiated control sampled at the same time in Fig. 2A). However, 90 min after radiation treatment (140 min into the S period), the bubble arc has been reduced by more than 75% (Fig. 2C).
When cells are irradiated 90 min after entry into the S period, there is an obvious diminution in the number of replication bubbles after only 30 min (Fig. 2F). By 180 min (i.e., 90 min after irradiation), the bubble arc has almost disappeared (Fig. 2G), and comparison to the mock-irradiated control harvested 180 min after removal of mimosine (Fig. 2H) shows that initiation is still occurring at this time in the absence of DNA damage. This 2D gel analysis has been repeated four times on synchronized CHOC 400 cells with similar results in each experiment. Note that there is very little effect on the single fork arcs in any of the irradiated samples, indicating that effects on chain elongation are probably minimal (compare single fork arcs to mock-irradiated controls sampled at the same time in the S period).
Thus, the damage-sensing mechanism is quite insensitive and slow to respond in the early S period, but appears to be reactivated later in the S period. These data contrast with radiation effects on asynchronous CHOC 400 cells, in which the bubble arc in ori-β largely disappears within 30 min of irradiation (13).
Radiation Uncovers a New Checkpoint at the Beginning of the S Period.
Since neither the overall replication rates (Fig. 1) nor the behavior of the early-firing DHFR origin in synchronized cells (Fig. 2) can fully account for the extent of inhibition of DNA replication observed in asynchronous cell populations, it appears that a particularly sensitive component of the population is missing from our analysis of synchronized S-phase cells. This raised the possibility of a cell-cycle block that prevents entry of late G1 cells into the S period after a radiation challenge. In cells arrested at the beginning of the S period with either mimosine or aphidicolin, the earliest-firing origins effected by such a block would presumably have passed the critical activation window and would thus be immune to down-regulation.
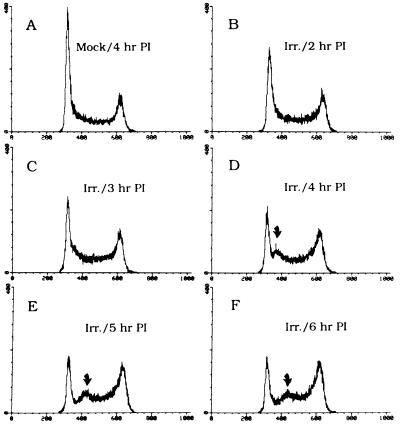
To address the possibility of a bona fide cell cycle arrest point at the beginning of S, we used FACS analysis to monitor the changing cell cycle distribution after a radiation challenge delivered to an asynchronous population. As shown in Fig. 3B, by 2 hr after a 10 Gy insult, the height of the G1 (2n) peak begins to diminish and it measurably broadens at its base. By 3 hr postirradiation, (Fig. 3C), a small shoulder is detected, which emerges as a discrete peak of synchronized cells by 4 hr postirradiation (Fig. 3D; compare with profile of mock-irradiated cells sampled at the same time in Fig. 3A). This synchronized population continues to progress through the S period, while the G1 peak continues to be depleted, presumably by cells that were in an unresponsive window of the G1 period at the time of irradiation. After 3–4 hr, cells with the 4n DNA content begin to accumulate at the G2/M checkpoint.
Figure 3.
FACS analysis of asynchronous CHOC 400 cells after a radiation challenge. Asynchronous CHOC 400 cells were irradiated with 10 Gy, and samples were taken 2, 3, 4, 5, and 6 hr later for FACS analysis as previously described, using propidium iodide (PI) to stain the DNA (17). A is the mock-irradiated control sampled 4 hr after irradiation of the other samples. Arrows indicate the peak of synchronized cells discussed in the text.
These data show that a population of CHOC 400 cells with the G1 DNA content is prevented from synthesizing DNA (i.e., entering the S period) for 2–3 hr after radiation treatment. Since the S period in these cells is 8–9 hr long, and since both initiation and elongation must be inhibited to prevent a net increase in DNA content, we believe that this population effectively accounts for the 25% inhibition of DNA synthesis that is not recapitulated in S-phase cells (as in Fig. 1A). Thus, there appears to be a damage-sensing checkpoint or pathway not only in the S phase itself, but also at or near the G1/S transition.
To investigate how this proposed G1/S-phase checkpoint is related to the p53-mediated G1 checkpoint, CHOC 400 cells were stably transfected with a wild-type p53 mini-gene, and several cell lines displaying the characteristic G1 arrest phenotype were isolated (H.L., unpublished observations). Asynchronous populations of one of these p53-positive cell lines, as well as control cells transfected with the vector alone, were then irradiated with 10 Gy, and cell cycle positions at various times thereafter were determined by FACS analysis (Fig. 4).
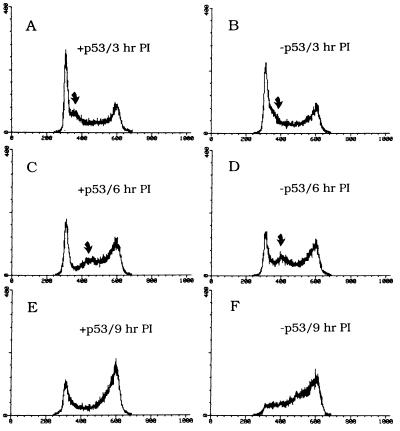
Figure 4.
Cells expressing wild-type p53 operate the p53-mediated G1 and the G1/S checkpoints. p53-negative CHOC 400 cells were stably transfected with a p53 minigene consisting of wild-type Chinese hamster p53 cDNA cloned into an expression vector (16). A second cell line was stably transfected with the vector only. Asynchronous cultures of each cell line were irradiated with 10 Gy, and samples were taken and prepared for FACS analysis 3 hr (A and B), 6 hr (C and D), or 9 hr (E and F) after irradiation.
As indicated in Fig. 4 B, D, and F, the p53-negative control cells completely vacate the G1 compartment by 9 hr after irradiation, and cells move toward and accumulate at the G2/M checkpoint; this includes the subpopulation synchronized transiently at the G1/S boundary by the radiation insult. However, in cells expressing wild-type p53, the G1 checkpoint is restored, as indicated by retention of a percentage of the population in G1 even after 9 hr (Fig. 4E). The subpopulation transiently synchronized at the G1/S boundary by radiation treatment is still observed, but is arrested for a somewhat shorter time than in the p53-negative control (compare Fig. 4 C and D). Since p53 may be involved in DNA repair (25–28), certain types of damage may be immediately repaired, thereby reducing the time that cells are arrested at the G1/S transition.
In addition to the three different CHOC 400 cell lines examined in Figs. 3 and 4, this synchronized late G1 subpopulation has been detected in two independent CHO cell lines (CHO-K1 and CHO Toronto). Several FACS analyses have been performed on each of the five cell lines after radiation treatment, and, in each case, the synchronized peak represents 30–40% of the total S phase population and 15–20% of the total cell population when measured 5–6 hr after irradiation (unpublished observations).
DISCUSSION
Our data indicate that a p53-independent damage-sensing checkpoint operates to prevent late G1 or very early S-phase cells from progressing through the S period for 2–3 hr after a radiation challenge. As shown here and in a previous study (13), cells that are in the S phase itself at the time of irradiation also respond acutely to DNA damage by down-regulating DNA synthesis at the level of individual origins. While the inhibition of overall replication rate rarely exceeds ≈25% when synchronized cells are irradiated at any time in the S period, the proposed G1/S checkpoint appears to completely suppress replication (i.e., prevent entry into the S period) for 2–3 hr. Therefore, we believe that the sum of the responses of the two populations can account for the 50% reduction in replication rate observed after irradiation of an unsynchronized cell population. Presumably, both of these pathways function to allow DNA damage to be repaired before it can be fixed into lethal consequences by conversion to double-strand breaks.
The kinetics of both G1/S and S-phase arrest suggest that cells can retain memory of damage incurred for ≈2 hr. By memory, we mean that actual down-regulation of DNA synthesis can occur at times considerably after the radiation challenge is received. For example, in a log population, all further initiations in the DHFR locus are prevented for 2 hr after irradiation (13), even though most DNA damage is thought to be repaired within 30–40 min of radiation treatment (29, 30). Furthermore, a substantial late G1 subpopulation in a log culture is synchronized at or near the G1/S boundary by radiation (Figs. 3 and 4) and cell cycle progression is delayed for 2–3 hr. There appears to be a short, relatively refractory period in early S phase when cells are insensitive to radiation damage, but at some point during the next 50 min, they regain their ability to sense and remember the damage, even though they are not able to respond to it until 120–140 min into the S period.
It is tempting to suggest that the insensitive, early S-phase component corresponds to the first bank of replicons to fire, which, once activated in some way, cannot be deactivated by the pathway that senses DNA damage. By this model, a subsequent bank of replicons normally firing 120–140 min later retains damage-sensing ability since it has not yet effected the critical activation step. This model requires, in addition, that the latter bank of replicons retains memory of the radiation insult for 2–3 hr. Because many cells in an asynchronous cell population respond immediately to irradiation by down-regulating DNA synthesis (Fig. 1), it is unlikely that the cell has to convert DNA damage to some secondary signal required to activate the damage-sensing mechanism.
These data cannot distinguish between a single S-phase-dependent damage-sensing pathway that functions at all individual origins regardless of when they fire in the S period, as opposed to two separate pathways, one of which functions at a G1/S control point and the other at individual origins. By either model, however, the net effect is far greater than when a radiation challenge is received at later times in S: entry into and passage through the S period by late G1 cells appears to be completely inhibited for about 2 hr and effectively repositions the S period in the cell cycle, a phenotype that has been used to define cell cycle checkpoints (31, 32); in contrast, samples irradiated in the S period itself continue to synthesize DNA at 50% of pre-irradiation values because chain elongation is not affected. The dose of radiation used in these studies (10 Gy) has been shown to produce one single-strand hit every 500–600 kb in mammalian DNA (33), and mammalian origins are spaced ≈100 kb apart (34). Thus, it seems unlikely that every origin in the cell could be directly down-regulated by a cis-acting structural hindrance to initiation. We consider it more likely that this regulatory mechanism(s) involves a trans-acting signal transduction pathway(s), and compelling evidence for this proposal has been obtained (35–37). In combination with data showing that the repair machinery is activated immediately after DNA damage (29, 30), we propose that this G1/S checkpoint may represent a major control mechanism connecting the replication machinery with damage-sensing and repair pathways.
This paper reports a possible G1/S checkpoint in a mammalian system that is independent of the p53-mediated G1 checkpoint. However, there are precedents for both types of checkpoints in Saccharomyces cerevisiae. One operates at START in mid-G1 and is apparently mediated by the RAD9 gene product (37, 38). A second checkpoint functions near the G1/S transition and acts between the cell cycle steps effected by the DBF4 and CDC7 gene products (36, 37). Interestingly, a defect in the G1/S checkpoint in yeast dramatically reduces cell survival rates after a radiation challenge (36, 37). It will be important to determine whether loss of the mammalian G1/S checkpoint is equally sensitizing to radiation-induced cell killing, since this pathway appears to be intact in most human tumors (21), and therefore may protect against radiation-based therapeutic strategies.
Acknowledgments
We are very grateful to Carlton White for his expert technical assistance, and to the other members of our laboratory for enthusiastic and valuable discussions. This work was supported by National Cancer Institute Grant CA52559 (to J.L.H). H.L. was supported in part by an American Cancer Society Institutional Research Grant (IRG149L). J.M.L. was supported in part by a Radiological Society of North America Scholar’s Award.
Footnotes
Abbreviations: 2D, two-dimensional; CHO, Chinese hamster ovary; DHFR, dihydrofolate reductase; FACS, fluorescence-activated cell sorter.
References
- 1.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 2.Kuerbitz S J, Plunkett B S, Walsh W V, Kastan M B. Proc Natl Acad Sci USA. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kastan M B, el Q. Zhan D W, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 4.Diller L, Kassel J, Nelson C E, Gryka M A, Litwak G, Gebhardt M, Bressac B, Ozturk M, Baker S J, Vogelstein B, Friend S H. Mol Cell Biol. 1990;10:5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercer W E, Shields M T, Amin M, Sauve G J, Appella E, Romano J W, Ullrich S J. Proc Natl Acad Sci USA. 1990;87:6166–6170. doi: 10.1073/pnas.87.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin D, Shields M T, Ullrich S J, Appella E, Mercer W E. Proc Natl Acad Sci USA. 1992;89:9210–9214. doi: 10.1073/pnas.89.19.9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizaka Y, Chernov M V, Burns C M, Stark G R. Proc Natl Acad Sci USA. 1995;92:3224–3228. doi: 10.1073/pnas.92.8.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan J J, Danish R, Gottlieb C A, Clarke M F. Mol Cell Biol. 1993;13:711–719. doi: 10.1128/mcb.13.1.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke A R, Purdie C A, Harrison D J, Morris R G, Bird C C, Hooper M L, Wyllie A H. Nature (London) 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 10.Lowe S W, Schmitt E M, Smith S W, Osborne B A, Jacks T. Nature (London) 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 11.Walters R A, Hildebrand C E. Biochem Biophys Res Commun. 1975;65:265–271. doi: 10.1016/s0006-291x(75)80088-x. [DOI] [PubMed] [Google Scholar]
- 12.Painter R B, Young B R. Radiat Res. 1975;64:648–656. [PubMed] [Google Scholar]
- 13.Larner J M, Lee H, Hamlin J L. Mol Cell Biol. 1994;14:1901–1908. doi: 10.1128/mcb.14.3.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makino F, Okada S. Radiat Res. 1975;62:37–51. [PubMed] [Google Scholar]
- 15.Watanabe I. Radiat Res. 1974;58:541–556. [PubMed] [Google Scholar]
- 16.Lee, H., Larner, J. M. & Hamlin, J. L. (1996) Gene, in press. [DOI] [PubMed]
- 17.Mosca P J, Dijkwel P A, Hamlin J L. Mol Cell Biol. 1992;12:4375–4383. doi: 10.1128/mcb.12.10.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dijkwel P A, Vaughn J P, Hamlin J L. Mol Cell Biol. 1991;11:3850–3859. doi: 10.1128/mcb.11.8.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewer B J, Fangman W L. Cell. 1987;51:463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- 20.Dijkwel P A, Hamlin J L. Mol Cell Biol. 1995;15:3023–3031. doi: 10.1128/mcb.15.6.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Painter R B. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49:771–781. doi: 10.1080/09553008514552981. [DOI] [PubMed] [Google Scholar]
- 22.Dijkwel P A, Hamlin J L. Mol Cell Biol. 1988;8:5398–5409. doi: 10.1128/mcb.8.12.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaughn J P, Dijkwel P A, Mullenders L H, Hamlin J L. Nucleic Acids Res. 1990;18:1965–1969. doi: 10.1093/nar/18.8.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dijkwel P A, Hamlin J L. Mol Cell Biol. 1992;12:3715–3722. doi: 10.1128/mcb.12.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakalkin G, Yakovleva T, Selivanova G, Magnusson K P, Szekely L, Kiseleva E, Klein G, Terenius L, Wiman K G. Proc Natl Acad Sci USA. 1994;91:413–417. doi: 10.1073/pnas.91.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X W, Forrester K, Yeh H, Feitelson M A, Gu J R, Harris C C. Proc Natl Acad Sci USA. 1994;91:2230–2234. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tornaletti S, Pfeifer G P. Science. 1994;263:1436–1438. doi: 10.1126/science.8128225. [DOI] [PubMed] [Google Scholar]
- 28.Smith M L, Chen I T, Zhan Q, Bae I, Chen C Y, Gilmer T M, Kastan M B, O’Connor P M, Fornace A J., Jr Science. 1994;266:1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 29.Hartwell L H, Weinert T A. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 30.Weinert T A. Radiat Res. 1992;132:141–143. [PubMed] [Google Scholar]
- 31.Zaider M. Radiat Res. 1993;134:1–8. [PubMed] [Google Scholar]
- 32.Wang Y, Huq M S, Cheng X, Iliakis G. Radiat Res. 1995;142:169–175. [PubMed] [Google Scholar]
- 33.Wang Y, Huq M S, Iliakis G. Radiat Res. 1996;145:408–418. [PubMed] [Google Scholar]
- 34.Cleaver J E, Rose R, Mitchell D L. Radiat Res. 1990;124:294–299. [PubMed] [Google Scholar]
- 35.Kemp L M, Sedgwick S G, Jeggo P A. Mutat Res. 1984;132:189–196. doi: 10.1016/0167-8817(84)90037-3. [DOI] [PubMed] [Google Scholar]
- 36.Olive P L, Banath J P, Durand R E. Radiat Res. 1990;122:86–94. [PubMed] [Google Scholar]
- 37.Siede W, Friedberg A S, Friedberg E C. Proc Natl Acad Sci USA. 1993;90:7985–7989. doi: 10.1073/pnas.90.17.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siede W, Friedberg A S, Dianova I, Friedberg E C. Genetics. 1994;138:271–281. doi: 10.1093/genetics/138.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]