Abstract
Objective
To assess the cost effectiveness and cost effectiveness acceptability of symptom control delivered by shared care (SCSC) and aggressive treatment delivered in hospital (ATH) for established rheumatoid arthritis (RA).
Methods
Economic data were collected within the British Rheumatoid Outcome Study Group randomised controlled trial of SCSC and ATH. A broad perspective was used (UK National Health Service, social support services and patients). Cost per quality adjusted life year (QALY) gained, net benefit statistics and cost effectiveness acceptability curves were estimated. Costs and outcomes were discounted at 3.5%. Sensitivity analysis tested the robustness of the results to analytical assumptions.
Results
The mean (SD) cost per person was £4540 (4700) in the SCSC group and £4440 (4900) in the ATH group. The mean (SD) QALYs per person for 3 years were 1.67 (0.56) in the SCSC group and 1.60 (0.60) in the ATH group. If decision makers are prepared to pay ⩾£2000 to gain 1 QALY, SCSC is likely to be cost effective in 60–90% of cases.
Conclusions
The primary economic analysis and sensitivity analyses indicate that SCSC is likely to be more cost effective than ATH in 60–90% of cases. This result seems to be robust to assumptions required by the analysis. This study is one of a limited number of randomised controlled trials to collect detailed resource use and health status data and estimate the costs and QALYs of treatment for established RA. This trial is one of the largest RA studies to use the EuroQol.
Treatment for rheumatoid arthritis (RA) aims to decrease and control pain and stiffness, reduce or prevent cumulative joint damage, maximise physical function, and improve quality of life. In the UK, primary and secondary care usually share responsibility for prescribing and monitoring disease‐modifying antirheumatic drugs (DMARDs). RA management is interactive, with regular assessment of disease activity and progression.
Established disease is defined as disease persisting at 5 years from symptom onset.1 Consistent DMARD use can reduce disability by 30%.2,3 Many patients stop DMARD treatment because of side effects or inefficacy.4,5 Patients with established RA are more likely to discontinue treatment than patients with early RA.6 For maximum benefit, DMARD treatment must start early, be aggressive, and keep laboratory tests and clinical signs normal.6,7,8,9,10,11,12,13,14
Relatively few RA trials have included an economic component; evidence of value for money of aggressive versus symptomatic treatment in established RA is lacking. A key question is whether economic differences exist between management targeting symptom (pain and stiffness) control or more aggressive management targeting control of symptoms, laboratory and clinical indices of joint inflammation. A study (British Rheumatoid Outcome Study Group (BROSG)) in patients with stable established RA15,16 showed no benefit for aggressive treatment delivered predominantly in hospital (ATH) compared with symptom control delivered predominantly by shared care (SCSC). The trial included an economic evaluation to assess the cost effectiveness acceptability (CEAA) of SCSC and ATH for established RA, which is reported here.
Methods
Clinical trial
The trial was a five‐centre, randomised, controlled, observer‐blinded study of SCSC versus ATH in patients with established RA in England conducted between 1997 and 2002. Full details of the design and results are reported elsewhere15,16 The main inclusion criteria were that patients were able and willing to provide informed consent; >18 years old; were diagnosed with RA using the 1987 American College of Rheumatology criteria17; and were current outpatient attenders for at least 12 months. Patients were also required to have had a disease duration >5 years; no change in drug or dosage for at least 6 months (DMARDs or steroids); been taking ⩽7.5 mg prednisolone or equivalent daily. Patients were assessed every 4 months for 36 months. Sample size was calculated to detect a difference of 0.25 in Health Assessment Questionnaire (HAQ)18,19 scores at 90% power and 5% significance. Allowing for 20% loss to follow‐up, 480 patients were required; 466 patients were recruited.
Patients offered SCSC were managed in primary care by general practitioners (GPs), with annual hospital review to control joint pain, stiffness and related symptoms using analgesics, non‐steroidal anti‐inflammatory drugs, intra‐articular steroid injections (maximum of one per month), DMARDs and low‐dose steroids (⩽7.5 mg daily). The GP contacted the rheumatologist if a change in DMARD or initiation of steroids was indicated, for advice or for specialist assessment. DMARD treatment was monitored using standard guidelines for the trial centres. Non‐pharmacological treatments were allowed. The patient was encouraged to visit the GP with any new or deteriorating symptoms. Patients receiving SCSC were not discharged from secondary care.
Patients in the ATH arm were managed in the hospital clinic for symptom control and to suppress clinical and laboratory evidence of inflammation. This included minimising the number of inflamed joints and keeping the C reactive protein below twice the upper limit of normal. Ciclosporin, parenteral steroids, medium‐dose oral steroids (up to 10 mg daily) and cyclophosphamide were allowed in addition to the treatments in the SCSC arm. If GPs monitored DMARD treatment before the trial, they continued to do so during the trial. The patient attended the hospital clinic every 4 months or more often if clinically indicated. Ethical approval was obtained from local research ethics committees (South Cheshire, Mid Staffordshire, North Staffordshire, Cornwall and King's College Hospital, London).
Economic evaluation
Economic evaluation used the perspectives of the UK National Health Service, social support services and patients, the main providers and beneficiaries of care, thus approximating a societal perspective. The evaluation aimed to inform policy and treatment decisions in district general hospitals and primary care in the UK and asked: What is the likelihood that SCSC is cost effective compared with ATH?
Cost effectiveness analysis was used to calculate incremental cost effectiveness ratios (ICERs), using quality adjusted life years (QALYs) as the effect measure. Previously, economists suggested that if there were no statistically significant differences in health outcomes, economic evaluations could be reduced to cost minimisation analyses. In many cases where there are no statistically significant differences in effectiveness or costs, analysis of the data indicates that some cases are less effective and more costly and that some are more effective and less costly, making cost minimisation analysis inappropriate. CEAA is a superior approach20,21,22,23 allowing estimation of the likelihood that the net cost per QALY of an intervention is above or below acceptable cost/QALY threshold values, and estimation of uncertainty when the cost and effect measures are combined into an ICER.21,22,23
Cost and QALY data were collected at each 4‐month assessment. Costs and utility were estimated for the 3 years of scheduled follow‐up and were discounted at 3.5% (UK Treasury37).
Quality adjusted life years
Health was measured by the EuroQol (EQ‐5D), a validated and widely used generic health status index24 covering mobility, self‐care, usual activities, pain, discomfort and anxiety/depression. These health profiles were converted to utility values using published population UK utility tariffs for the EQ‐5D derived using time trade‐off methods.25 The EQ‐5D has been shown to discriminate between health states in RA.31,32 QALYs were estimated for each patient as utility multiplied by survival for each assessment period.
Direct costs
All patients were issued a diary at the start of the study to record all healthcare and related service use, and no attempt was made to distinguish RA‐specific consumption. National average unit costs (£s sterling, 2001) were obtained from published national databases,38,39,40 to approximate the relative opportunity costs of care. Unit costs were adjusted to a single price year (2001) using a hospital and community health price index.26 Direct costs included hospital inpatient, outpatient and domiciliary visits, primary care visits, visits to other healthcare professionals, prescribed medications, aids and appliances. The mean (SD) cost of events was estimated from the trial data multiplied by national average unit cost data.
Imputation of missing information
Utility values could not be estimated if patients had missing observations on one or more domains of the EQ‐5D within an assessment. The missing utility value was imputed by linear interpolation (value of previous period plus value of next period divided by 2) if and only if observations to the left and right of the missing item were available and the patient completed scheduled follow‐up. This approach assumed that utility at each assessment was linearly related to previous and future assessments. QALYs were estimated as: QALY = Σ((Ui+Ui+1)/2)×(ti+1+ti); U = utility value, t = number of days between assessments.
If patients completed scheduled follow‐up but had missing observations on frequency or intensity of service use within an assessment period, multiple imputation (propensity score method) was used to estimate the cost for each cost category.27,28,29 Patients had to have one completed assessment at the start and one at the end of follow‐up to be included in the multiple imputation. Patients without a final assessment were included in the censored case analysis. It was assumed that missing observations within an assessment period were missing at random and that missing observations would be relatively infrequent. Fixed covariates were included (study period and treatment group). Data on medicines were based on patient self‐report. Over 50% of reports for medicines were incomplete, making multiple imputation inappropriate. The minimum cost of medicines for each patient was estimated. The net ingredient cost per prescription for each preparation was estimated from English prescription data (http://www.dh.gov.uk/PublicationsAndStatistics/Statistics/StatisticalWorkAreas/StatisticalHealthCare/StatisticalHealthCareArticlelfs/en?CONTENT_ID = 4015555&chk = 1wM7HI). The duration of drug treatment was used to estimate the number of prescriptions and total preparation cost for each patient. If no data on duration were available, it was assumed that only one prescription was given. Dispensing costs were not included; it was assumed that the costs to administer drug treatment were included in the costs of consultations of reported healthcare.
Patients with one or more missing utility or cost observations at the end of scheduled follow‐up were treated as censored cases. Survival analysis was used to impute censored data (Cox regression, using patient status (alive, dead or withdrawn) and treatment allocation). The QALYs for censored cases were estimated as: QALYC = Σ((Ui+Ui+1)/2) ((Si+Si+1)/2) (ti+1− ti); U = utility value, S = probability of survival, t = days between assessments. The costs for censored cases were estimated as COSTC = ΣCi×Si; Ci = cost of assessment period I, Si = probability of survival of assessment period I.
Primary analysis
The primary analysis estimated mean (SD) costs, QALYs and the ICER. The primary measure was the ICER. Accordingly, differences between allocation groups for utility, QALYs or costs were not tested for statistical significance. Bootstrapping was used to derive net benefit statistics and cost effectiveness acceptability curves. A £0–30 000 range of cost/QALY ceiling threshold values (increments of £1000) was used to estimate the mean net benefit and the probability that SCSC was cost effective, instead of statistical tests of differences between groups to quantify uncertainty. The maximum threshold value of £30 000 is within the £25 000–35 000 willingness to pay to gain 1 QALY implied by UK policy decisions.41 Each imputed cost dataset was bootstrapped and the bootstrapped datasets averaged to estimate costs for the analysis.
Sensitivity analyses
Sensitivity analysis tested alternative approaches to imputing missing data; varying the discount rate between 0 and 6%; and the impact of the costs of drug treatment and trial protocol‐driven visits (additional 4‐monthly home visits by a hospital‐based clinical nurse specialist for SCSC, and additional 4‐monthly visits to the rheumatology clinic for ATH). Most patients did not report these visits and they were not included in the primary analysis. The effect of baseline differences in utility was tested by adjusting both QALYs and costs (general linear main effects regression model, with treatment and baseline utility values as covariates). The adjusted data were bootstrapped for the CEAA.
The sensitivity analyses used the same analytic approach as the primary analyses.
Results
The two groups were similar in mean utilities, the distribution of utilities at each assessment and outlier values (table 1).
Table 1 Observed EuroQol utility values and days of follow‐up, by year in trial.
| Assessment period | SCSC | ATH | ||
|---|---|---|---|---|
| n | Mean (SD; 95% CI; range) utility | n | Mean (SD) utility (95% CI; range) | |
| Baseline | 228 | 0.60 (0.21; 0.58 to 0.64; −0.18 to 1) | 232 | 0.57 (0.23; 0.54 to 0.60; −0.18 to 1) |
| 12 Months | 217 | 0.57 (0.25; 0.54 to 0.61; −0.18 to 1) | 226 | 0.54 (0.27; 0.51 to 0.59; −0.28 to 1) |
| 24 Months | 207 | 0.56 (0.25; 0.53 to 0.60; −0.24 to 1) | 211 | 0.54 (0.27; 0.51 to 0.58; −0.24 to 1) |
| 36 Months | 195 | 0.57 (0.24; 0.51 to 0.58; −0.13 to 1) | 199 | 0.54 (0.27; 0.50 to 0.58; −0.18 to 1) |
ATH, aggressive treatment delivered in hospital; SCSC, symptom control delivered by shared care.
The mean duration of follow‐up was 3 years for both groups. The use of services and costs did not differ for most categories of service use (table 2).
Table 2 Average resource use unit costs and costs.
| SCSC | ATH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of resource use | n | Mean quantity* | SD | Mean unit cost | SD | n | Mean quantity* | SD | Mean unit cost | SD |
| Inpatient admissions (number) | 91 | 2 | 1 | – | – | 90 | 2 | 1 | – | – |
| Inpatient length of stay (days) | 91 | 14 | 20 | 279 | 72 | 90 | 10 | 21 | 304 | 130 |
| Outpatient visits | 220 | 14 | 14 | 80 | 7 | 230 | 18 | 15 | 81 | 6 |
| Primary care visits | 221 | 21 | 18 | 26 | 13 | 225 | 19 | 19 | 24 | 13 |
| Other healthcare professionals | 175 | 9 | 9 | 15 | 11 | 184 | 9 | 11 | 14 | 6 |
| Drug treatment | 225 | 11 | 6 | 14 | 4 | 233 | 11 | 5 | 14 | 5 |
| Aids and adaptations | 124 | 3 | 2 | 55 | 163 | 122 | 4 | 2 | 55 | 100 |
| n | Mean cost (undiscounted)† | SD | n | Mean cost (undiscounted) † | SD | |||||
| Inpatient admissions | 224 | 1575 | 4198 | 229 | 1261 | 4486 | ||||
| Outpatient visits | 226 | 997 | 1148 | 231 | 1369 | 1203 | ||||
| Primary care visits | 226 | 502 | 431 | 231 | 395 | 337 | ||||
| Other healthcare professionals | 225 | 98 | 158 | 230 | 90 | 165 | ||||
| Drug treatment | 226 | 1475 | 1170 | 231 | 1403 | 966 | ||||
| Aids and adaptations | 224 | 68 | 204 | 230 | 76 | 248 | ||||
ATH, aggressive treatment delivered in hospital; SCSC, symptom control delivered by shared care.
*Per patient reporting use of services.
†Per patient enrolled in the clinical trial (includes imputation of missing observations and excludes censored cases).
Over 3 years, the mean (SD) cost was £4540 (4700) for SCSC versus £4440 (£4900) for ATH. The corresponding mean (SD) QALYs were 1.67 (0.56) for SCSC and 1.60 (0.60) for ATH. Table 3 summarises the incremental costs, QALYs and ICERs for the primary and sensitivity analyses.
Table 3 Discounted costs and QALYs at 3 years, £s, 2001.
| Analysis | Incremental QALY's mean (95% CI) | Incremental costs mean (95% CI) | ICER |
|---|---|---|---|
| SCSC−ATH | SCSC−ATH | SCSC−ATH | |
| Primary analysis | |||
| Imputed data for censored cases and missing observations, discount rate 3.5% | 0.07 (−0.04 to 0.18) | 106 (−768 to 979) | 1517 |
| Sensitivity analyses | |||
| Adjustment of QALY's and costs for baseline utility values | 0.014 (−0.07 to 0.099) | 259 (−600 to 1117) | 18 500 |
| No imputation of missing observations or censored data | 0.09 (−0.03 to 0.21) | 111 (−785 to 1007) | 1261 |
| 0% Discount rate QALYs and costs | 0.07 (−0.04 to 0.18) | 124 (−809 to 1057) | 1737 |
| 6% Discount rate QALYs and costs | 0.05 (−0.04 to 0.17) | 94 (−742 to 929) | 1376 |
| Costs of drug treatment excluded | 0.07 (−0.04 to 0.18) | 41 (−782 to 865) | 596 |
| Full costs of protocol visits included | 0.07 (−0.04 to 0.18) | 440 (−433 to 1313) | 6328 |
| Full costs of protocol visits included and costs of drug treatment excluded | 0.07 (−0.04 to 0.18) | 409 (−397 to 1215) | 5885 |
ATH, aggressive treatment delivered in hospital; SCSC, symptom control delivered by shared care.
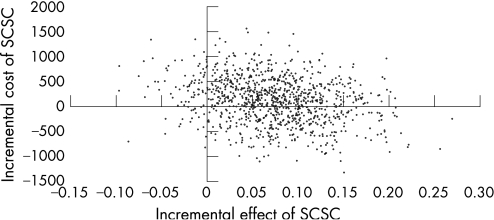
The cost effectiveness plane (fig 1) gives the distribution of bootstrapped pairs of incremental costs and QALYs (primary analysis). The dispersion of costs and effects indicates that in most cases SCSC was more effective and cost saving or cost additive. CEAA is required, to explore the probability that SCSC is cost effective given varying threshold values of willingness‐to‐pay for a QALY.21,22,23
Figure 1 Cost effectiveness plane of the incremental cost and quality adjusted life year (QALY) of symptom control delivered by shared care (SCSC) versus aggressive treatment delivered in hospital (ATH).
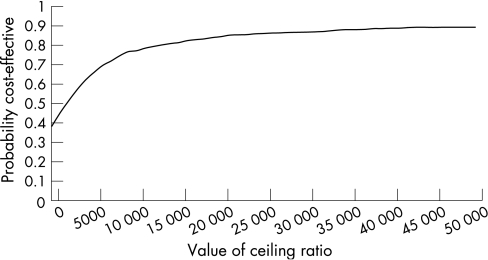
The CEAA curve in fig 2 indicates that, if decision makers are prepared to pay £2000 or more to gain one QALY, SCSC is cost effective in 50% of bootstrapped estimates of incremental costs and QALYs. This suggests that SCSC is likely to be cost effective compared with ATH. If decision makers are prepared to pay £13 000 or more to gain one QALY, SCSC is likely to be cost effective in over 80% of cases.
Figure 2 Cost effectiveness acceptability curve for SCSC versus ATH.
Table 4 reports the estimated net benefit statistics for SCSC. The net benefit statistic was calculated as the bootstrapped incremental QALYs gained by SCSC (0.07) multiplied by the amount a hypothetical decision maker is willing to pay (WTP) (£30 000) to gain 1 QALY, minus the bootstrapped incremental cost. The cost effectiveness plane, net benefit statistic and the CEAA, all indicate that SCSC is likely to be cost effective compared with ATH.
Table 4 Summary of net benefit and likelihood that SCSC is cost effective.
| Analysis | Mean net benefit of SCSC at £30 000/QALY WTP threshold* | Probability that SCSC is cost effective at £30 000/QALY WTP threshold* |
|---|---|---|
| Primary analysis | ||
| Imputed data for censored cases and missing observations, discount rate 3.5% | 2059 (−1199 to 5752) | 0.87 |
| Sensitivity analyses | ||
| Adjustment of QALYs and costs for baseline utility values | 445 (−4111 to 4799) | 0.58 |
| No imputation of missing observations or censored data | 2240 (−2748 to 7287) | 0.82 |
| 0% Discount rate QALYs and costs | 2098 (−1308 to 5497) | 0.88 |
| 6% Discount rate QALYs and costs | 2109(−1440 to 5744) | 0.88 |
| Costs exclude costs of drug treatment | 2154 (−1255 to 5523) | 0.91 |
| Full costs of protocol visits included | 1578 (−2084 to 5096) | 0.81 |
| Full costs of protocol visits included and costs of drug treatment excluded | 1683 (−1580 to 5046) | 0.84 |
QALY, quality adjusted life year; SCSC, symptom control delivered by shared care; WTP, willingness to pay.
The sensitivity analyses indicate that the cost effectiveness of SCSC is not affected by the assumptions tested (tables 3 and 4). However, the probability that SCSC is cost effective is reduced if costs and QALYs are adjusted for differences in baseline utility.
Discussion
Using data from a prospectively defined sample of patients in the BROSG trial, this analysis indicates that SCSC is likely to be more cost effective than ATH. A high follow‐up rate for data collected in an randomised control trial framework suggests that this result is internally valid. However, assumptions underpinning the analysis may affect the robustness of the results. First, patients completed a large number of record forms and assessments, and some only recorded positive resource use rather than zero use. Use of a service was assumed to be zero if there was no information about both frequency and intensity of use, and the use of other services within an assessment period was recorded.
Second, data about the use of medicines were incomplete, which might have resulted in underestimation of the total costs. However, data for DMARDs (the main drug category) were available for over 90% of patients.
Third, there were missing observations and censored cases for the economic data. However, hospital and DMARD data (the main cost components) were complete for over 90% of participants. There was a monotone pattern of missing observations with no major anomalies, supporting the use of multiple imputation. Descriptive analysis suggested a linear relationship between utility values at each assessment, supporting linear interpolation to impute missing utility observations. Sensitivity analysis indicated that the imputation methods and survival analysis did not affect the conclusions of the primary analysis.
To estimate QALYs, we assumed that the EQ‐5D and utility tariff discriminated between groups and captured the relevant aspects of health related quality of life for RA. Utility values correlated with the HAQ, the primary outcome measure in the trial. The clinical analysis indicated that health deteriorated over the 3 years on most measures used,15 mirrored by a decrease in EQ‐5D scores and utility. The EQ‐5D, HAQ and other clinical measures indicated small, non‐significant differences between groups at 36 months in favour of SCSC. This suggests that the EQ‐5D was sensitive to changes and consistent with most outcome measures used in the clinical analysis. Other studies, with similar utility values to this trial, indicate that EQ‐5D discriminates between different levels of RA.30,31
The costs in this trial are lower than those reported elsewhere. Adjusting for time frame and currency, these equate to between £2500 and £9000 per person per year.30,31,32,33,34 Ward et al reported a lower cost of $1702 per person per year in the USA (approximately £1100)35; and inpatient costs were 27–37% and drug costs 31% of the total cost. The distribution of costs by category of service differs widely, but inpatient costs in other studies were the main cost. Higher costs may be associated with higher disease severity or age.30,36 However, there is no evidence that the lower costs found here were achieved because the participants had less severe disease or were younger.30,32 This suggests that the costs reported here may be robust, but lower than found elsewhere.
The trial protocol and trial centres may be atypical of routine practice, affecting transferability of the results to other patients, and time frames. The trial centres represented urban, rural, academic centres and district general hospitals, so the results are likely to be generalisable. An audit conducted over the 3‐year period indicated that the results of the BROSG trial were generalisable to around one‐third of current rheumatology attenders.15 The 3‐year follow‐up of patients is relatively long for trial‐based clinical and economic analyses. However, it is possible that the small benefit in the OSRA activity score noted in the ATH arm15 could lead to a difference in physical function between the groups beyond 3 years. In this case, the probability that SCSC is cost effective may be decreased.
Protocol defined visits were excluded from the analysis and national average unit cost data used, to facilitate transferability of the results to settings outside the trial in England. If protocol defined visits were part of the intervention and included in the costs, SCSC would still be likely to be cost effective.
The analysis did not indicate a benefit for aggressive treatment as used in the ATH arm. This may be because there were fewer differences in the management of patients than expected. Regular review in the SCSC arm meant that the need for treatment change was identified early. Treatment was not changed as often as indicated by measures of disease activity in the ATH arm.15 Consultants and patients were unwilling to change treatment for minor changes in disease activity. Some ATH patients were stable, not requiring changes in treatment during the trial.15 The cost data indicated few differences between the groups by type of service. The main difference in costs was for inpatient admission and drug treatment, both of which were higher in the SCSC arm than the ATH arm.
Overall, the economic analysis appears robust, indicating that SCSC is likely to be more cost effective than ATH in 60–90% of cases. This study is one of a few randomised control trials to collect detailed resource use and health‐status data to estimate the costs and QALYs of treatment for established RA, and is one of the largest studies to use the EQ‐5D in RA.16
The study was conducted between 1997 and 2002. Current treatment of RA is more aggressive, aiming for remission or to keep disease activity scores as low as possible. This analysis shows that SCSC is more cost effective than ATH care, which does not achieve a low disease activity. For clinical care now, patients with long standing stable RA should be managed according to the SCSC protocol evaluated here.15 Further clinical trials are required to establish whether further suppression of disease activity is feasible in these patients, using aggressive combination DMARD treatment or biological agents.
Acknowledgements
We acknowledge the contribution of Drs Andrew Hassell (North Staffordshire Rheumatology Centre), Diarmuid Mulherin (Cannock Rheumatology Centre), Susan Knight (Macclesfield District General Hospital), and Martin Davis (Royal Cornwall Hospital). Dr C Erhardt recruited some patients from Bromley to the King's College cohort. We also thank the research nurses at each centre.
Abbreviations
ATH - aggressive treatment delivered in hospital
BROSG - British Rheumatoid Outcome Study Group
CEAA - cost effectiveness acceptability
DMARD - disease modifying antirheumatic drug
EQ‐5D - EuroQol
GP - general practitioner
HAQ - Health Assessment Questionnaire
ICER - incremental cost effectiveness ratio
QALY - quality adjusted life year
RA - rheumatoid arthritis
SCSC - symptom control delivered by shared care
Footnotes
Funding: This study was funded by the UK Department of Health through its Health Technology Assessment Programme. The study was funded following acceptance of a detailed peer‐reviewed proposal.
Competing interests: None declared.
The proposal was submitted in response to a commissioning brief requesting tenders published by the funding body. The funding body had no involvement in study design, data collection, analysis or interpretation of the results, writing of this paper, or in the decision to submit the paper for publication. The opinions and conclusions expressed here are those of the authors and do not necessarily reflect those of the UK National Health Service or the Department of Health.
Financial interests: The sponsors of the project, the NCCHTA, have an agreement with the BMJ to allow all NHS‐funded research to be published under a non‐exclusive licence, as it falls under Crown copyright and so an exclusive license cannot be assigned by the author to the journal.
References
- 1.van Gestel A M, Stucki G. Evaluation of established rheumatoid arthritis. Baillieres Best Pract Res Clin Rheumatol 199913629–644. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J J, Wells G, Verhoeven A C, Felson D T. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum 20004322–29. [DOI] [PubMed] [Google Scholar]
- 3.Fries J F, Williams C A, Morfeld D, Singh G, Sibley J. Reduction in long‐term disability in patients with rheumatoid arthritis by disease‐modifying antirheumatic drug‐based treatment strategies. Arthritis Rheum 199639616–622. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe F, Hawley D J, Cathey M A. Termination of slow acting antirheumatic therapy in rheumatoid arthritis: a 14‐year prospective evaluation of 1017 consecutive starts. J Rheumatol 199017994–1002. [PubMed] [Google Scholar]
- 5.Morand E F, McCloud P I, Littlejohn G O. Life table analysis of 879 treatment episodes with slow acting antirheumatic drugs in community rheumatology practice. J Rheumatol 199219704–708. [PubMed] [Google Scholar]
- 6.Munro R, Hampson R, McEntegart A, Thomson E A, Madhok R, Capell H. Improved functional outcome in patients with early rheumatoid arthritis treated with intramuscular gold: results of a five year prospective study. Ann Rheum Dis 19985788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Dell J R. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum 200246283–285. [DOI] [PubMed] [Google Scholar]
- 8.Egsmose C, Lund B, Borg G, Pettersson H, Berg E, Brodin U.et al Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year followup of a prospective double blind placebo controlled study. J Rheumatol 1995222208–2213. [PubMed] [Google Scholar]
- 9.Tsakonas E, Fitzgerald A A, Fitzcharles M A, Cividino A, Thorne J C, M'Seffar A.et al Consequences of delayed therapy with second‐line agents in rheumatoid arthritis: a 3 year followup on the hydroxychloroquine in early rheumatoid arthritis (HERA) study. J Rheumatol 200027623–629. [PubMed] [Google Scholar]
- 10.van der Heide A, Jacobs J W, Bijlsma J W, Heurkens A H, Booma‐Frankfort C, van der Veen M J.et al The effectiveness of early treatment with “second‐line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med 1996124699–707. [DOI] [PubMed] [Google Scholar]
- 11.Stenger A A, van Leeuwen M A, Houtman P M, Bruyn G A, Speerstra F, Barendsen B C.et al Early effective suppression of inflammation in rheumatoid arthritis reduces radiographic progression. Br J Rheumatol 1998371157–1163. [DOI] [PubMed] [Google Scholar]
- 12.Albers J M, Paimela L, Kurki P, Eberhardt K B, Emery P, ‘t Hof M A.et al Treatment strategy, disease activity, and outcome in four cohorts of patients with early rheumatoid arthritis. Ann Rheum Dis 200160453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Symmons D P, Jones M A, Scott D L, Prior P. Longterm mortality outcome in patients with rheumatoid arthritis: early presenters continue to do well. J Rheumatol 1998251072–1077. [PubMed] [Google Scholar]
- 14.Landewe R B, Boers M, Verhoeven A C, Westhovens R, van de Laar M A, Markusse H M.et al COBRA combination therapy in patients with early rheumatoid arthritis: long‐term structural benefits of a brief intervention. Arthritis Rheum 200246347–356. [DOI] [PubMed] [Google Scholar]
- 15.Symmons D, Tricker K, Harrison M, Roberts C, Davis M, Dawes P.et al Patients with stable long‐standing rheumatoid arthritis continue to deteriorate despite intensified treatment with traditional disease modifying anti‐rheumatic drugs‐‐results of the British Rheumatoid Outcome Study Group randomized controlled clinical trial. Rheumatology (Oxford) 200645558–565. [DOI] [PubMed] [Google Scholar]
- 16.Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott D L. The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost‐effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis. Health Technol Assess 20059iiiiv, ixx, 1–iiiiv, ixx,78. [DOI] [PubMed] [Google Scholar]
- 17.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 18.Fries J F, Spitz P, Kraines R G, Holman H R. Measurement of patient outcome in arthritis. Arthritis Rheum 198023137–145. [DOI] [PubMed] [Google Scholar]
- 19.Kirwan J R, Reeback J S. Stanford Health Assessment questionnaire modified to assess disability in British patients with rheumatoid arthritis. Br J Rheumatol 198625206–209. [DOI] [PubMed] [Google Scholar]
- 20.Briggs A H. A Bayesian approach to stochastic cost‐effectiveness analysis. Health Econ 19998257–261. [DOI] [PubMed] [Google Scholar]
- 21.Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost‐effectiveness acceptability curves. Health Econ 200110779–787. [DOI] [PubMed] [Google Scholar]
- 22.Pedram‐Sendi P, Briggs A H. Affordability and cost‐effectiveness: decision‐making on the cost‐effectiveness plane. Health Econ 200110675–680. [DOI] [PubMed] [Google Scholar]
- 23.Briggs A H, O'Brien B J. The death of cost minimisation analysis? Health Econ 200310179–184. [DOI] [PubMed] [Google Scholar]
- 24.EuroQol Group EuroQol ‐ new facility for the measurement of health related quality of life. Health Policy 200316199–208. [DOI] [PubMed] [Google Scholar]
- 25.Dolan P, Gudex C, Kind P, William A.A social tariff for EuroQuol: results from a UK general population survey. University of York, York: Centre for Health Economics Discussion Paper 138, 1995
- 26.Netten A, Curtis L.Unit costs of health and social care. Personal Social Services Research Unit, University of Kent at Canterbury 2002
- 27.Statistical Solutions SOLAS for missing data analysis Version 3.2. Cork, Ireland: Statistical Solutions Ltd, 2001
- 28.Rubin D B, Schenker N. Multiple imputation in health‐care data bases: an overview and some applications. Stat Med 199110585–598. [DOI] [PubMed] [Google Scholar]
- 29.Lavori P W, Dawson R, Shera D. A multiple imputation strategy for clinical trials with truncation of patient data. Stat Med 1995141913–1925. [DOI] [PubMed] [Google Scholar]
- 30.Kobelt G, Jonsson L, Lindgren P, Young A, Eberhardt K. Modeling the progression of rheumatoid arthritis: a two‐country model to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheumatism 2002462310–2319. [DOI] [PubMed] [Google Scholar]
- 31.Van Den Hout W B, Tijhuis G J, Hazes J M W, Breedveld F C, Vliet Vlieland T P M. Cost effectiveness and cost utility analysis of multidisciplinary care in patients with rheumatoid arthritis: A randomised comparison of clinical nurse specialist care, inpatient team care, and day patient team care. Ann Rheum Dis 200362308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper N J. Economic burden of rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 20003928–33. [DOI] [PubMed] [Google Scholar]
- 33.Gabriel S E, Wagner J L, Zinsmeister A R, Scott C G, Luthra H S. Is rheumatoid arthritis care more costly when provided by rheumatologists compared with generalists? Arthritis Rheum 2001441504–1514. [DOI] [PubMed] [Google Scholar]
- 34.Ruof J, Hulsemann J L, Mittendorf T, Handelmann S, Von Der Schulenburg J M, Zeidler H.et al Costs of rheumatoid arthritis in Germany: a micro‐costing approach based on healthcare payer's data sources. Ann Rheum Dis 200362544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward M M, Javitz H S, Yelin E H. The direct cost of rheumatoid arthritis. Value in Health 20003243–252. [DOI] [PubMed] [Google Scholar]
- 36.Lajas C, Abasolo L, Bellajdel B, Hernandez‐Garcia C, Carmona L, Vargas E.et al Costs and predictors of costs in rheumatoid arthritis: a prevalence‐based study. Arthritis Rheum 20034964–70. [DOI] [PubMed] [Google Scholar]
- 37.National Institute for Clinical Excellence Guide to the methods of technology appraisal NO515. London: National Institute for Clinical Excellence, 2004 [PubMed]
- 38.Netten A, Curtis L.Unit costs of health and social care. Canterbury: Personal Social Services Research Unit, University of Kent at Canterbury, 2002
- 39.Department of Health Reference costs. 2001. http://www.doh.gov.uk/nhsexec (acessed 5 Febraury 2007)
- 40.The Chartered Institute of Public F i n a n c, Accountancy ( C I P F A.The Health Service Financial Database and Comparative Tool 2001. Croydon: Institute of Public Finance Limited, 2002
- 41.Rawlins M D, Culyer A J. National Institute for Clinical Excellence and its value judgments. BMJ 2004329224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]