Abstract
Background
Antineutrophil cytoplasmic antibodies (ANCA) are associated with small‐vessel vasculitis and have been implicated in its pathogenesis. The subclass distribution of ANCA IgG deviates from normal patterns, and it has been suggested that the IgG3 subclass may have pathogenic potential over the IgG1 subclass and may be more likely to be associated with active disease and renal involvement.
Objective
To deal with potential pathogenicity, chimeric antibodies were constructed of IgG1 and three subclasses with human IgG1 or three constant regions and a murine‐derived variable region that binds an epitope within the ANCA antigen proteinase 3 (PR3) that is recognised by human autoantibodies.
Methods
The antibodies were characterised for binding to PR3, including affinity and avidity, before being used as tools to explore their ability to activate human neutrophils for superoxide release, cytokine release, degranulation and ability to induce neutrophil adhesion under flow.
Results
Both subclass antibodies elicited similar neutrophil responses for superoxide release, degranulation and interleukin (IL) 8 production, although quantitative responses showed that the IgG1 subclass favoured degranulation and the IgG3 subclass favoured IL8 production. Both antibodies were able to convert neutrophils from selectin‐dependent rolling adhesion to integrin‐dependent stationary adhesion in a flow assay.
Conclusions
These findings indicate that humanised antibodies directed against a single epitope of PR3 can recapitulate the effects of polyclonal human ANCA, which recognises multiple PR3 epitopes. Further, PR3‐ANCA of both IgG1 and IgG3 subclasses can activate neutrophils, although the more potent IL8 response by IgG3 PR3‐ANCA may encourage further neutrophil recruitment and amplify injury.
Antineutrophil cytoplasmic antibodies (ANCA) against neutrophil enzyme targets, proteinase 3 (PR3) and myeloperoxidase (MPO), are strongly associated with systemic vasculitis. Although useful diagnostically, ANCA IgG are increasingly implicated in its pathogenesis, on the basis of clinical observations,1,2 analysis of the in vitro effects of ANCA IgG on neutrophil function3,4,5,6 and by effects in animal models. The demonstration that MPO‐ANCA IgG are sufficient to induce vasculitic lesions,7 as well as to induce microvascular lesions during intravital studies,8 is compelling evidence for the pathogenicity of these antibodies.
Several studies have investigated the relationship between ANCA IgG subclass titres and clinical disease, often with differing and conflicting conclusions. Taken together, ANCA autoantibodies have been detected within all four subclasses, but the frequency has varied between studies (table 1). The IgG1 and IgG3 subclasses have been most consistently reported as present. The IgG3 subclass has been particularly associated with clinical evidence of disease activity, including renal disease, and one study has suggested that it may have greater potential to activate neutrophils (table 1).
Table 1 Studies of antineutrophil cytoplasmic antibody IgG subclasses in systemic vasculitis.
| Author, year | cANCA subclasses | pANCA subclasses | Subclass association with clinical effect | Subclass association with neutrophil function |
|---|---|---|---|---|
| Brouwer et al,9 1991 | IgG1, IgG4* | IgG1, IgG4* | IgG3 associated with renal involvement | — |
| Esnault et al,10 1993 | — | IgG1, IgG3, IgG4 | IgG3 decreased during remission | IgG3 had highest relative functional affinity |
| Jayne et al,11 1991 | IgG3* | — | IgG3 associated with acute phase of disease | — |
| Segelmark et al,12 1993 | IgG1, IgG3, IgG4* | IgG1, IgG2, IgG4* | Higher IgG3 cANCA during acute disease | — |
| Mellbye et al,13 1994 | IgG1, IgG4* | IgG1, IgG4 (no IgG3) | — | All cANCA sera caused deposition of C3c on neutrophils |
| Mulder et al,14 1995 | IgG1, IgG3, IgG4 (IgG2 not done) | — | IgG3 ANCA were more potent at inducing a neutrophil respiratory burst | |
| Locke et al,15 1999 | — | IgG1, IgG4* | — | — |
| Harper et al,16 2001 | IgG1, IgG4* | IgG1, IgG4* | — | No particular subclass linked to neutrophil activation |
| Nowack et al,17 2001 | IgG1, IgG3, IgG4 (IgG2 not done) | IgG1, IgG3, IgG4 (IgG2 not done) | IgG3 cANCA associated with ANCA persistence, multiple organ involvement and grumbling disease activity | — |
| Holland et al,18 2004 | IgG1, IgG3, IgG4 (IgG2 not done) | — | — | IgG4 cANCA activated a neutrophil respiratory burst |
ANCA, antineutrophil cytoplasmic antibodies; cANCA, cytoplasmic ANCA; pANCA, perinuclear ANCA.
*ANCA were detected in all subclasses in these studies, but only the dominant subclasses are given.
Each of the four human IgG subclasses expresses a unique structural and functional profile that reflects their differing abilities to engage with Fc γ receptors (FcγR) and to activate the classic complement pathway (table 2). Neutrophils express FcγRIIa and FcγRIIIb constitutively and may upregulate FcγRI in response to interferon γ. Co‐ligation of antigen (PR3 or MPO) and FcγRIIa/FcγRIIIb on the neutrophil surface is suggested to be critical for ANCA IgG‐induced neutrophil activation, with recruitment of tyrosine kinase and G protein signal transduction pathways.19,20,21 Most functional studies with neutrophils have relied on either human polyclonal ANCA IgG, containing all IgG subclasses and of which PR3‐ or MPO‐specific antibodies constitute a small fraction, or mouse monoclonal IgG, whose Fc portions have binding characteristics for Fc receptors different from those of human antibodies. Analysing the properties of different ANCA IgG subclasses using human antibodies would require fractionation with potential for cross contamination, whereas use of mouse monoclonal antibodies may not mimic the human disease. Further, human polyclonal ANCA IgG, whether directed to PR3 or MPO, contains a spectrum of antibodies with differing fine recognition of antigenic epitopes, which could allow complex cross‐linking between antigen and FcγRIIa/FcγRIIIb on the neutrophil surface. For these reasons, mouse/human chimeric antibodies were generated of the same PR3 epitope specificity, bearing the same mouse V regions, and with human IgG1 and three subclass constant regions.
Table 2 Specificity of human IgG subclasses for Fc γ receptors.
| IgG1 | IgG2 | IgG3 | IgG4 | |
|---|---|---|---|---|
| FcγRI (CD64) | +++ | – | +++ | + |
| FcγRII (CD32) | ||||
| FcγRIIa‐H131 | ++ | ++ | +++ | – |
| FcγRIIa‐R131 | ++ | – | +++ | – |
| FcγRIIb | ++ | – | +++ | + |
| FcγRIII (CD16) | ||||
| FcγRIIIa | ++ | + | ++ | + |
| FcγRIIIb | ++ | + | ++ | – |
FcγR, Fc γ receptor; + refers to affinity of binding; – denotes that a subclass is reported not to bind.
Materials and methods
Extension PCR and cloning
RNA was isolated from a hybridoma expressing mouse anti‐PR3 antibody 4A5 (a gift from Professor J Wieslander, Lund, Sweden). cDNA was synthesised using a first‐strand cDNA synthesis kit (Amersham, Little Chalfont, UK).
Forward primers corresponding to consensus sequences in mouse κ, γ1 and γ3 IgG signal regions and reverse primers to sequences in the constant regions were designed by AERES Biomedical (London, UK). PCR was performed, products were cloned into sequencing vectors using a TA Cloning Kit (Invitrogen, Paisley, UK) and transformed into Escherichia coli according to the manufacturer's instructions (for conditions, see supplementary information available at http://ard.bmj.com/supplemental). Automated sequencing was carried out by the Genomics Laboratory, University of Birmingham, Birmingham, UK.
Subcloning and screening
Products were modified to contain restriction sites, Kozak sequences and splice donor sites by PCR, as appropriate for their particular vector (see supplementary information available at http://ard.bmj.com/supplemental). Primers also incorporated a leader sequence taken from a database of proteins of immunological interest (KABAT) that was predicted to be an appropriate match. PCR products were cloned and transformed as above. DNA from positive clones was sequenced to confirm the modifications.
Single clones of κ, γ1 and γ3 were chosen to subclone into vectors containing the appropriate constant regions of the IgG chain (supplied by AERES Biomedical). Restriction digests were performed and products ligated using T4 DNA ligase. Plasmids were transformed into maximum‐efficiency DH5α E coli. Clones were PCR screened with the primers used to modify the variable regions and sequenced as above. Single positive clones of κ, γ1 and γ3 were chosen to generate DNA.
Expression of chimeric antibody
Dihydrofolate reductase‐deficient Chinese hamster ovary cells (AERES Biomedical) were cultured in α minimum essential medium containing ribonucleosides and deoxyribonucleosides with 10% fetal calf serum. A total of 10 μg each of κ and γ1, or 10 μg each of κ and γ3 vectors was added to 1×107 Chinese hamster ovary cells. Cells were subjected to a 1900 V, 25 μFarad pulse, then transferred into medium without ribonucleosides or deoxyribonucleosides, with 0.5 mg/ml geneticin.
Single‐cell clones were produced. IgG production was monitored by haemagglutination as described previously.22 Approximately 50 clones of each were derived by selection, around 60% of these produced a detectable IgG. From these, approximately six of the highest producers were expanded, re‐tested and then the best ones were further expanded. Finally, the highest producing of these was expanded into a hollow fibre bioreactor.23 IgG1 was purified from culture supernatant using staphylococcal protein A‐Sepharose and IgG3 purified using streptococcal protein G‐Sepharose.
Chimeric mouse/human IgG1 and three antibodies with specificity for the 4‐hydroxyl‐3‐nitrophenylacetyl (NP) hapten, generated as described previously,24 were used as controls in the following experiments.
Verification of IgG production by ELISA, sodium dodecyl sulphate‐polyacrylamide gel electrophoresis and mass spectrometry
Cell culture supernatant was assessed for IgG by capture ELISA using goat anti‐human IgG Fc to capture (0.4 μg/ml), and goat anti‐human IgG κ horseradish peroxidase (1 μg/ml) to detect.
Non‐reduced and reduced (using 10 mM dithiothreitol) purified IgG was subjected to electrophoresis on a 4–12% Bis–Tris gel. Gels were stained with Coomassie blue.
Electrospray ionisation mass spectrometry was used to define the glycoform profiles of the chimeric IgG1 and IgG3 PR3‐ANCA. IgG was deglycosylated for 72 h at 37°C using peptide‐N‐glycosidase F (1 unit/mg IgG) in deglycosylation buffer (40 mM KH2PO4, 10 mM EDTA; pH 7.4). The purified IgG (1 mg/ml) was reduced with 10 mM dithiothreitol for 1 h. Electrospray ionisation mass spectrometry was performed using an LCT instrument (Micromass, Wythenshawe, Manchester, UK) with MassLynx data acquisition.
Assessment of specificity of antigen recognition
Surface plasmon resonance was performed using a BIAcore 3000 and CM5 biosensor chips. PR3 (Athens Research and Technology, Athens, Georgia, USA) was immobilised on the CM5 chip. The chimeric IgG1 PR3‐ANCA, IgG3 PR3‐ANCA and 4A5 antibodies were perfused (10 μl/min) separately over the PR3‐coated chip at increasing concentrations (0.625–20 nmol/l) and binding monitored as an increase in SPR signal (resonance units (RUs)). Phenylmethanesulphonylfluoride (0.17 μmol/l) was added to the IgG perfusate to minimise the proteinase activity of the PR3. A plot of the equilibrium level of response (RU) against the free concentration of antibody was fitted to a curve using non‐linear regression (Graphpad Prism) to derive the binding constant (KD).
To confirm recognition of native antigen, whole‐cell lysates of neutrophils (isolated as described previously25), azurophil granules from neutrophils and recombinant PR3 (20 ng/ml) were subjected to electrophoresis on a 12% gel and transferred to nitrocellulose membranes. Blots were blocked, then incubated with purified chimeric IgG1 or IgG3 PR3‐ANCA 1 μg/ml overnight at 4°C. Bands were visualised using sheep anti‐human IgG horse radish peroxidase (10 μg/ml).
Immunofluorescence was performed to confirm binding to PR3 expressed in human neutrophils. Ethanol‐fixed cytospins of neutrophils (binding site) were exposed to purified IgG (1.5 μg/ml) and bound antibodies detected using mouse anti‐human IgG‐fluorescein isothiocyanate.
Flow cytometry was used to verify binding to surface‐expressed antigen on human neutrophils. Neutrophils were incubated with 4 μg/ml chimeric IgG1 or IgG3 PR3‐ANCA. Bound antibody was detected using mouse anti‐human IgG‐fluorescein isothiocyanate and samples were fixed in 2% paraformaldehyde.
Assessment of functional activation
Neutrophils from normal donors taken with ethical permission and informed consent were used for functional assays, and to control for potential differences in function between donors, the chimeric antibodies were always tested in parallel using cells from the same donor. The ability of chimeric IgG1 or IgG3 PR3‐ANCA to elicit superoxide production was measured by the superoxide dismutase inhibitable reduction of ferricytochrome c as described previously.26 Neutrophils were primed with 2 ng/ml tumour necrosis factor (TNF)α and 5 μg/ml cytochalasin B for 15 min at 37°C, stimulated with 1 μM N‐formyl‐methionyl‐leucyl‐phenylalanine (fMLP), 200 μg/ml ANCA/normal IgG or varying concentrations of chimeric IgG1 or IgG3 PR3‐ANCA/control anti‐NP and superoxide release measured over 120 min. The concentrations of the IgG1 and IgG3 antibodies were adjusted to be equimolar.
Neutrophil degranulation was assessed by the release of serine proteases. Neutrophils at 2.5×106/ml were primed as above and incubated with 1 μM fMLP or varying concentrations of chimeric IgG1 or IgG3 PR3‐ANCA, or control chimeric anti‐NP IgG1 or IgG3, for 15 min. Supernatants were removed and incubated for 22 h at 30°C with 2 mM N‐methoxysuccinyl‐Ala‐Ala‐Pro‐Val‐P‐nitroanilide (Sigma, Poole, UK), a substrate for PR3 and elastase, in 1.25% dimethyl suphoxide, and 0.1% Tween 20, and the optical densities measured at 405 nm.
Cytokine production by neutrophils was determined using the release of IL8. Neutrophils were primed as above and incubated with varying concentrations of chimeric IgG1 or IgG3 PR3‐ANCA/control chimeric anti‐NP for 6 h. Supernatants were removed and analysed by IL8 ELISA (BD Biosciences, Cowley, UK).4
Flow assay
The flow‐based adhesion assay has been described previously.27 Microslides were coated with 1 μg/ml P selectin in 1% bovine serum albumin/phosphate‐buffered saline, and neutrophils flowed through at a wall shear stress of 0.1 Pa for 5 min. Chimeric IgG1/IgG3 PR3‐ANCA or control chimeric anti‐NP (60 μg/ml) were perfused over the neutrophils for 10 min. Stably adhered neutrophils were defined as those that had stopped and spread. The cells in six fields were characterised. Antibodies were used in excess (60 μg/ml) to obviate any small dilutional effects that may occur when the neutrophils and antibody first enter the flow chamber.
p21ras activation assay
Neutrophils were primed with 2 ng/ml TNFα, and then stimulated for 15 min with equimolar concentrations, 25 or 29 μg/ml, respectively, of chimeric IgG1 or IgG3 PR3‐ANCA/control chimeric anti‐NP at 37°C. Antibody concentrations were chosen as those that produced strong responses in the degranulation and IL8 assays. The p21ras activation assay was performed as described previously.28 Immobilised Ras‐binding domain of Raf‐1 was used to isolate activated p21ras, whereas whole‐cell lysates of the samples were used to confirm that equal amounts of the molecule were present for binding.
Statistical analysis
Statistical analysis of flow cytometric data was performed using independent‐samples t tests. To test for differences between functional abilities of the IgG1 and IgG3 PR3‐ANCA, a two‐way analysis of variance was performed using dose and type of antibody. Normal probability plots were used to confirm that distributions of the residuals were approximately normal. Two‐way analysis of variance was performed to compare how subclass/concentration affected association/dissociation rates and dissociation constant (KD) of antibodies to PR3 by surface plasmon resonance. A Wilcoxon signed ranks test was used to test whether the PR3‐ANCA chimeric antibodies induced significantly higher stable adhesion in the flow model than in controls.
Results
Cloning and expression
Chimeric mouse/human anti‐PR3 antibodies of IgG1 and IgG3 subclasses (chimeric IgG1 or IgG3 PR3‐ANCA) were generated, which retain the mouse PR3 binding variable regions spliced to human constant regions.
IgG production
Stable clones were produced and individual clones screened for production of IgG by haemagglutination and by ELISA using a capture antibody specific for the Fc region (data not shown). IgG was purified and a single band was observed of either approximately 150 or 135 kDa on the non‐reducing gel (data not shown), confirming a pure protein of the correct weight for IgG3 and IgG1, respectively. Under reducing conditions, two bands were observed for each subclass, the molecular weights of which concurred with those for the IgG1 heavy chain (50 kDa), the IgG3 heavy chain (60 kDa) and the κ light chain (25 kDa).
The glycoforms of IgG were assessed. Mass values for the light and deglycosylated IgG1 and IgG3 heavy chains were in broad agreement with those calculated from the amino acid composition of the proteins. The masses determined for the glycosylated heavy chain of IgG1 and IgG3 corresponded to a fucosylated “core” heptasaccharide (G0), and the mono‐ galactosylated (G1) and di‐galactosylated (G2) glycoforms in an approximate ratio of 2:3:1.
Subclass specificity, antigen recognition and FcR binding
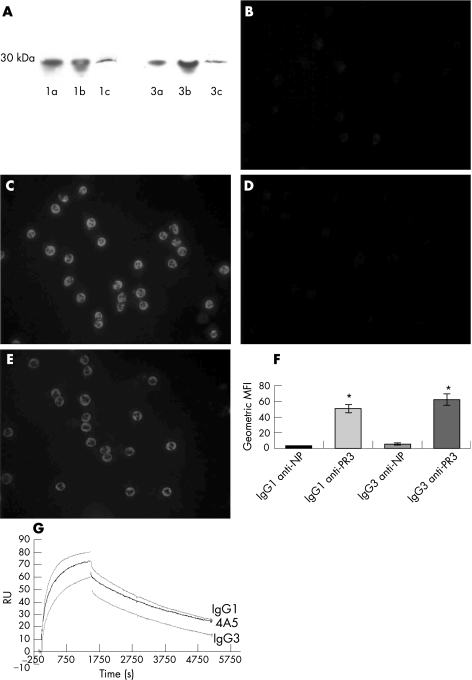
PR3‐specific ELISA was used to confirm the IgG1/IgG3 subclass of each chimeric PR3‐ANCA (data not shown). Western blotting, cytoplasmic staining of ethanol‐fixed neutrophils and fluorescence‐activated cell sorting of freshly isolated human neutrophils further established antigen recognition. Western blots of recombinant PR3, neutrophil azurophil granules and whole‐cell lysates using the chimeric antibodies as primary antibodies detected a single band of the correct molecular weight (29 kDa; fig 1A). Staining of ethanol‐fixed neutrophils showed the characteristic cytoplasmic staining pattern for both the chimeric IgG1 and IgG3 PR3‐ANCA typical of human polyclonal PR3‐ANCA, whereas the control IgG1 and IgG3 chimeric antibodies against the hapten molecule NP gave no staining (figs 1B–E). Binding to native, cell‐membrane‐associated PR3 was demonstrated by surface staining of TNFα‐primed neutrophils; chimeric IgG1 and IgG3 PR3‐ANCA bound significantly (p = 0.002 and 0.006, respectively) more cells than the control anti‐NP antibodies (fig 1F).
Figure 1 (A) Demonstration by western blot that the anti‐proteinase 3 (PR3) chimeric antibodies recognise recombinant PR3 and PR3 present in neutrophil whole‐cell lysate. 1, IgG1 anti‐PR3 ; 3, IgG3 anti‐PR3. (a) 180 ng recombinant PR3 loaded, (b) 360 ng recombinant PR3 and (c) whole‐cell lysate. PR3 has a molecular weight of 29 kDa. Molecular weight indicated alongside. (B–E) Indirect immunofluorescence of ethanol‐fixed neutrophils: (B) IgG1 anti‐4‐hydroxyl‐3‐nitrophenylacetyl (NP); (C) chimeric IgG1 PR3‐antineutrophil cytoplasmic antibodies (ANCA); (D) IgG3 anti‐NP; and (E) chimeric IgG3 PR3‐ANCA. The IgG1 and IgG3 PR3–ANCA bind to neutrophils with a cytoplasmic pattern (F) Binding of chimeric IgG1 and IgG3 PR3‐ANCA to human neutrophils by flow cytometry. IgG1 and IgG3 anti‐NP versus chimeric IgG1 and IgG3 PR3‐ANCA as geometric mean fluorescence intensity (MFI; n = 4). *p<0.05 by independent samples t test. (G) Surface plasmon resonance comparing binding of 4A5 (mouse monoclonal IgG anti‐PR3), chimeric IgG1 PR3–ANCA and IgG3 PR3–ANCA to immobilised PR3. The graph shows the binding association and dissociation curves of 4A5, IgG1 PR3–ANCA and IgG3 PR3–ANCA measured in resonance units (RU, see text), whereas the associated tables show the rates together with statistical analysis of the effect of IgG (n = 3).
Affinity/avidity was evaluated by surface plasmon resonance (measured in RU). This measures the binding of the antibody to its antigen (association rate) and the uncoupling (dissociation rate). From these are derived the dissociation constant (KD), which assesses the persistence of the interaction. Differences were observed between the parent mouse 4A5 monoclonal antibody and between chimeric ANCA IgG subclasses, the affinities being similar but with disparities in the dissociation rates (fig 1G).
Functional characterisation
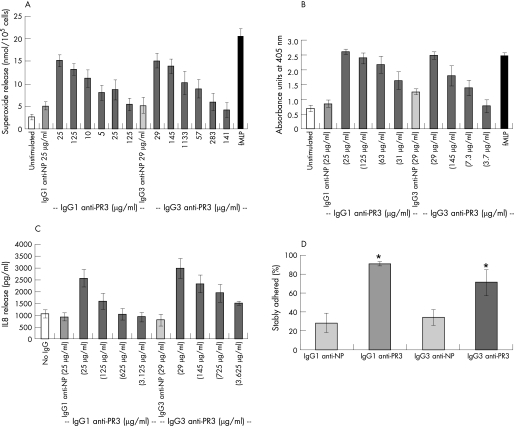
PR3‐ANCA derived from patients induce TNFα‐primed neutrophils to undergo a respiratory burst, releasing superoxide. The ability of the chimeric IgG1 and IgG3 PR3‐ANCA to induce this effect was tested. A dose‐dependent induction of superoxide release was observed, with significantly higher release of superoxide by the chimeric PR3‐ANCA than the chimeric anti‐NP controls (p = 0.001 and p = 0.002, respectively; fig 2A). When a two‐way analysis of variance was performed with the two factors being concentration and type of antibody, no difference was found between the chimeric IgG1 and IgG3 PR3‐ANCA. A comparison with human polyclonal PR3‐ANCA IgG was not made routinely since there is considerable variation in potency between samples of PR3‐ANCA IgG derived from different individuals. The release at the highest concentrations of chimeric IgG1 and IgG3 PR3‐ANCA tested was comparable with that seen with 1 μM fMLP.
Figure 2 (A) Superoxide production as measured by reduction of ferricytochrome c with units of superoxide expressed as nmol/105 cells (n = 5). Antibody concentrations were adjusted to equivalent molarities between IgG1 and IgG3 subclasses. N‐formyl‐methionyl‐leucyl‐phenylalanine (fMLP) is used as a positive control. Two‐way analysis of variance was performed. (B) Serine protease release as measured by conversion of N‐methoxysuccinyl‐Ala‐Ala‐Pro‐Val‐P‐nitroanilide (n = 5), data are expressed as absorbance units at 405 nm. Antibody concentrations were adjusted to equivalent molarities between IgG1 and IgG3 subclasses. fMLP is used as a positive control. Two‐way analysis of variance was performed, with chimeric IgG1 antineutrophil cytoplasmic antibodies (ANCA) being significantly different (p = 0.001). (C) Interleukin 8 (pg/ml) release as measured by ELISA (n = 6). Antibody concentrations were adjusted to equivalent molarities between IgG1 and IgG3 subclasses. Two‐way analysis of variance was performed, with chimeric IgG3 ANCA being significantly different (p = 0.006). (D) Percentage of neutrophils showing stable adhesion to P‐selectin under conditions of flow after treatment with either IgG1 or IgG3 anti‐NP, or chimeric IgG1 or IgG3 proteinase 3 (PR3)‐ANCA (n = 5). *p<0.05 by Wilcoxon signed ranks test.
Human neutrophils are able to release a variety of proteolytic enzymes via degranulation. To assess this event quantitatively, the release of serine proteases (PR3 and elastase) was measured via their ability to cleave a specific substrate. The chimeric IgG1 or IgG3 PR3‐ANCA was able to induce the release of serine proteases in a dose‐dependent manner, whereas the control chimeric IgG1 or IgG3 anti‐NP did not (p = 0.001; fig 2B). When a two‐way analysis of variance was performed, with the two factors being concentration and type of antibody, chimeric IgG1 PR3‐ANCA induced significantly greater degranulation than the chimeric IgG3 PR3‐ANCA (p = 0.001).
We have previously shown that PR3‐ANCA from patients can induce the production of IL8 by primed neutrophils.4 We used a cytometric bead array to screen for the release of cytokines by the chimeric IgG1 and IgG3 PR3‐ANCA, and demonstrated the release of IL8 only (cytokines tested for were IL1β, IL2, IL4, IL6, IL8, IL10, IL12, TNFα, interferon γ and granulocyte macrophage colony‐stimulating factor; data not shown). To examine this in more detail, a capture ELISA for IL8 was used. The chimeric IgG1 or IgG3 PR3–ANCA (but not the control chimeric IgG1 or IgG3 anti‐NP) was able to induce the release of IL8 in a dose‐dependent manner (p = 0.003 and 0.002, respectively; fig 2C). However, when a two‐way analysis of variance was performed, with the two factors being concentration and type of antibody, chimeric IgG3 PR3‐ANCA was shown to induce a significantly greater release of IL8 than the chimeric IgG1 PR3‐ANCA (p = 0.006). The dose was significant in each functional assay (superoxide, degranulation and IL8 release), but the difference in the effects of the two antibodies did not vary significantly with dose.
A flow‐based adhesion assay has previously been used to confirm the ability of patient‐derived PR3‐ANCA to induce stationary adhesion.27 The chimeric IgG1 and IgG3 PR3‐ANCA were also able to significantly induce stable adhesion (p = 0.028 and 0.046, respectively), but the control chimeric antibodies were not able to do so (fig 2D).
Signal transduction and activation of Ras
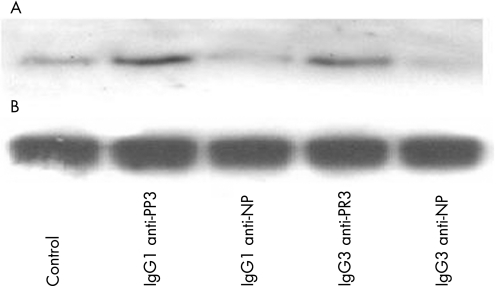
Previous work from our laboratory has shown that patient‐derived PR3‐ANCA can activate the intracellular signalling molecule p21ras.28 We tested for the ability of the chimeric IgG1 and IgG3 PR3‐ANCA to trigger this pathway. Unstimulated neutrophils showed no p21ras activity; the control chimeric antibodies produced minimal levels of activity whereas both the chimeric IgG PR3‐ANCAs gave rise to strong activation (fig 3).
Figure 3 Activation of p21ras in neutrophils stimulated with chimeric IgG1 and IgG3 proteinase 3 (PR3)‐antineutrophil cytoplasmic antibodies: (A) western blotting to detect active p21ras, (B) western blotting to detect total Ras to demonstrate even loading of neutrophil lysate. NP, 4‐hydroxyl‐3‐nitrophenylacetyl.
Discussion
ANCA are strongly associated with development of small‐vessel systemic vasculitis, and autoantibody titres are valuable markers that help in diagnosis and patient management. Additionally, there is increasing evidence for direct involvement of ANCA in disease pathogenesis. Previous work from this laboratory and others has shown in vitro activation of primed neutrophils by ANCA IgG (reviewed in Morgan et al29), but there is little consensus on the distribution or pathogenic implication of IgG subclasses within ANCA. Most studies have reported positive PR3‐ANCA titres for IgG subclasses IgG1, IgG3 and IgG4, while agreeing that IgG2 ANCA constitute a minor component. The relative titres of IgG1, IgG3 and IgG4 ANCA were shown to vary between patients and for sera taken during active disease and remission.9,14,18 Despite this variability between studies, clinical associations have suggested that the IgG3 subclass is particularly associated with active disease10,11 and possibly with renal involvement.9 Theoretical reasons used to support enhanced pathogenicity of IgG3 PR3‐ANCA relate to the longer hinge region of this subclass antibody compared with IgG1 and thus greater potential to co‐ligate PR3 with FcγR on the neutrophil surface to promote neutrophil activation. This study has allowed an in vitro comparison of the functional activities between IgG1 and IgG3 PR3‐ANCA that are both directed towards the same PR3 epitope. Both antibodies displayed a similar profile of functional activities for superoxide production, degranulation, IL8 release and adhesion. However, IgG1 PR3‐ANCA induced greater degranulation, wheras IgG3 PR3‐ANCA induced a greater release of IL8. Both antibodies induced activation of p21ras, a pivotal signal transduction mediator, after activation of neutrophils by ANCA.28 Thus, the two subclasses of chimeric PR3‐ANCA display a similar functional response profile as do human polyclonal PR3‐ANCA, but the two subclasses show subtle differences in quantitative responses. If the clinical observations suggesting greater pathogenicity of IgG3 PR3‐ANCA are correct, then the enhanced release of IL8 induced by the IgG3 PR3‐ANCA chimeric antibody may be relevant, since IL8 may promote further neutrophil accumulation and amplify disease. Although IgG1 PR3‐ANCA enhanced degranulation, the outcome for tissue injury may be more dependent on the protease/anti‐protease balance in vivo than on the level of degranulation itself, although this is speculation. It is important to note, however, that the chimeric antibodies are slightly different from ANCA found in patient sera. The most important difference is that ANCA are polyclonal. The nature of binding of the human purified IgG may be to target multiple sites on a given antigen, leading to clustering of antigen and Fc receptors to form large mats. This may give rise to different functional consequences, which would presumably be largely quantitative given the similar qualitative profiles between the chimeric PR3‐ANCA and their human polyclonal counterparts.
It has been shown that antibodies that have identical variable regions but differ in their subclass, can differ in their fine specificity and affinity.30 Potentially, this is owing to influences of the constant region on antibody structure affecting the variable region. Our chimeric IgG1 and IgG3 PR3‐ANCA have been derived from a murine monoclonal antibody and retain the complementary determining regions and framework regions from this murine IgG1 molecule; the antibodies also share the same light chains. However, the two antibodies differ with respect to their heavy chains. That this affects binding is suggested by the difference between the dissociation of the parent and offspring antibodies from PR3 using surface plasmon resonance. This implies that the chimeric IgG1 and IgG3 PR3‐ANCA could induce different neutrophil functions by differential interactions with antigen, secondary to their differing constant regions. Alternatively, the differences may be a direct effect of the different constant regions since, despite the >95% sequence identity between the constant regions of IgG1 and IgG3,31 each still exhibits a unique profile of effector functions and/or efficacy.32 Of particular interest for effects of IgG ANCA on neutrophil activation is the interaction of the IgG with FcγRs. The interaction site for each of the FcγRs has been “mapped” to the lower hinge region and the hinge proximal region of the CH2 domains. Primary sequence and conformational flexibility differences in this area of the IgG molecule result in each IgG subclass exhibiting unique FcγR‐activating potential (table 2). This may be relevant for fine specificity of functional responses during ANCA activation of neutrophils owing to the important facilitatory role of FcγR during these responses.19,33,34
In conclusion, human/mouse chimeric IgG1 and IgG3 PR3‐ANCA have been produced that recognise human PR3. Both antibodies can induce the in vitro neutrophil functional responses seen with polyclonal patient‐derived PR3‐ANCA even though each is monoclonal in nature. Further, subtle differences in neutrophil‐activating capacity were elucidated, with IgG3 PR3‐ANCA demonstrating greater release of IL8 from neutrophils. It is possible that this enhanced availability of IL8 may promote further neutrophil accumulation at vasculitic sites and might underlie the previous clinical observations suggesting that ANCA of the IgG3 subclass are more pathogenic than those of the IgG1 subclass.
Supplementary Material
Acknowledgements
This study was funded by Arthritis and Rheumatism Council and United Hospitals Birmingham Charities.
We thank Peter Ashton for performing the MS analysis and John Lund and Gerard Nash for advice.
Abbreviations
ANCA - antineutrophil cytoplasmic antibodies
FcγR - Fc γ receptors
fMLP - N‐formyl‐methionyl‐leucyl‐phenylalanine
IL - interleukin
MPO - myeloperoxidase
NP - 4‐hydroxyl‐3‐nitrophenylacetyl
PR3 - proteinase 3
RU - resonance units
TNF - tumour necrosis factor
Footnotes
Competing interests: None declared.
References
- 1.Reumaux D, Duthilleul P, Roos D. Pathogenesis of diseases associated with antineutrophil cytoplasmic autoantibodies. Hum Immunol 2004651–12. [DOI] [PubMed] [Google Scholar]
- 2.Seo P, Stone J H. The antineutophil cytoplasmic antibody‐associated vasculitides. Am J Med 200411739–50. [DOI] [PubMed] [Google Scholar]
- 3.Falk R J, Terrell R S, Charles L A, Jennette J C. Antineutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA 1990874115–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cockwell P, Brooks C J, Adu D, Savage C O. Interleukin‐8: a pathogenic role in antineutrophil cytoplasmic autoantibody (ANCA)‐associated glomerulonephritis. Kidney Int 199955852–863. [DOI] [PubMed] [Google Scholar]
- 5.Tse W Y, Williams J, Pall A, Wilkes M, Savage C O, Adu D.et al Antineutrophil cytoplasmic antibody‐induced neutrophil nitric oxide production is nitric oxide synthase independent. Kidney Int 200159593–600. [DOI] [PubMed] [Google Scholar]
- 6.Calderwood J W, Williams J M, Morgan M D, Nash G B, Savage C O. ANCA induces beta2 integrin and CXC chemokine dependent neutrophil‐endothelial cell interactions that mimic those of highly cytokine activated endothelium. J Leukoc Biol 20057733–43. [DOI] [PubMed] [Google Scholar]
- 7.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y.et al Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase (MPO‐ANCA) cause glomerulonephritis and vasculitis in mice. J Clin Invest 2002110955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little M A, Smyth C L, Yadav R, Ambrose L, Cook H T, Nourshargh S.et al Antineutrophil cytoplasmic antibodies directed against myeloperoxidase augment leukocyte‐microvascular interactions in vivo. Blood 20051062050–2058. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer E, Tervaert J W, Horst G, Huitema M G, van der Giessen M, Limburg P C.et al Predominance of IgG1 and IgG4 subclasses of antineutrophil cytoplasmic autoantibodies (ANCA) in patients with Wegener's granulomatosis and clinically related disorders. Clin Exp Immunol 199183379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esnault V L M, Ronda N, Jayne D R, Lockwood C M. Association of ANCA isotype and affinity with disease expression. J Autoimmun 19936197–205. [DOI] [PubMed] [Google Scholar]
- 11.Jayne D R W, Weetman A P, Lockwood C M. IgG subclass distribution of autoantibodies to neutrophil cytoplasmic antigens in systemic vasculitis. Clin Exp Immunol 199184476–481. [PMC free article] [PubMed] [Google Scholar]
- 12.Segelmark M, Wieslander J. IgG subclasses of antineutrophil cytoplasmic antibodies (ANCA). Nephrol Dial Transplant 19938696–702. [DOI] [PubMed] [Google Scholar]
- 13.Mellbye O J, Mollnes T E, Steen L S. IgG subclass distribution and complement activation ability of autoantibodies to neutrophil cytoplasmic antigens (ANCA). Clin Immunol Immunopathol 19947032–39. [DOI] [PubMed] [Google Scholar]
- 14.Mulder A H L, Stegeman C A, Kallenberg C G M. Activation of granulocytes by antineutrophil cytoplasmic antibodies (ANCA) in Wegener's granulomatosis: a predominant role for the IgG3 subclass of ANCA. Clin Exp Immunol 1995101227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locke I C, Leaker B, Cambridge G. A comparison of the characteristics of circulating anti‐myeloperoxidase autoantibodies in vasculitis with those in non‐vasculitic conditions. Clin Exp Immunol 1999115369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper L, Radford D, Plant T, Drayson M, Adu D, Savage C O. IgG from MPO‐ANCA positive patients stimulates greater activation of primed neutrophils than IgG from PR3‐ANCA positive patients. Arthritis Rheum 200144921–930. [DOI] [PubMed] [Google Scholar]
- 17.Nowack R, Grab I, Flores‐Suarez L F, Schnulle P, Yard B, van der Woude F J. ANCA titres, even of IgG subclasses, and soluble CD14 fail to predict relapses in patients with ANCA‐associated vasculitis. Nephrol Dial Transplant 2001161631–1637. [DOI] [PubMed] [Google Scholar]
- 18.Holland M, Hewins P, Goodall M, Adu D, Jefferis R, Savage C O. Antineutrophil cytoplasmic antibody IgG subclasses in Wegener's granulomatosis: a possible pathogenic role for the IgG4 subclass. Clin Exp Immunol 2004138183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben‐Smith A, Dove S K, Martin A, Wakelam M J, Savage C O. Autoantibodies from patients with systemic vasculitis activate primed neutrophils via Fcγ receptor dependent pathways. Blood 2001981448–1455. [DOI] [PubMed] [Google Scholar]
- 20.Kettritz R, Choi M, Butt W, Rane M, Rolle S, Luft F C, Klein J B. Phosphatidylinositol 3‐kinase controls antineutrophil cytoplasmic antibodies‐induced respiratory burst in human neutrophils. J Am Soc Nephrol 2002131740–1749. [DOI] [PubMed] [Google Scholar]
- 21.Williams J M, Ben‐Smith A, Hewins P, Dove S K, Hughes P, McEwan R.et al Activation of the Gi heterotrimeric G protein by ANCA IgG F(ab')2 fragments is necessary but not sufficient to stimulate the recruitment of those downstream mediators used by intact ANCA IgG. J Am Soc Nephrol 200314661–669. [DOI] [PubMed] [Google Scholar]
- 22.Ling N R, Bishop S, Jefferis R. Use of antibody‐coated red cells for the sensitive detection of antigen and in rosette tests for cells bearing surface immunoglobulins. J Immunol Methods 197715279–289. [DOI] [PubMed] [Google Scholar]
- 23.Goodall M. A simple hollow‐fiber bioreactor for the “in‐house” production of monoclonal antibodies. Methods Mol Biol 19988039–56. [DOI] [PubMed] [Google Scholar]
- 24.Bruggemann M, Williams G T, Bindon C I, Clark M R, Walker M R, Jefferis R.et al Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med 19871661351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jepsen J L, Skottum T. A rapid one‐step method for the isolation of human granulocytes from whole blood. Scand J Clin Invest 198242235–238. [PubMed] [Google Scholar]
- 26.Radford D J, Lord J M, Savage C O S. The activation of the neutrophil respiratory burst by antineutrophil cytoplasmic antibody (ANCA) from patients with systemic vasculitis requires tyrosine kinases and protein kinase C activation. Clin Exp Immunol 1999118171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radford D J, Savage C O S, Nash G B. Treatment of rolling neutrophils with antineutrophil cytoplasmic autoantibodies causes conversion to firm integrin‐mediated adhesion. Arthritis Rheum 2000431337–1344. [DOI] [PubMed] [Google Scholar]
- 28.Williams J M, Savage C O. Characterization of the regulation and functional consequences of p21ras activation in neutrophils by antineutrophil cytoplasmic antibodies. J Am Soc Nephrol 20051690–96. [DOI] [PubMed] [Google Scholar]
- 29.Morgan M D, Harper L, Williams J, Savage C. Antineutrophil cytoplasm‐associated glomerulonephritis. J Am Soc Nephrol 2006171224–1234. [DOI] [PubMed] [Google Scholar]
- 30.Torres M, May R, Scharff M D, Casadevall A. Variable‐region‐identical antibodies differing in isotype demonstrate differences in fine specificity and idiotype. J Immunol 20051742132–2142. [DOI] [PubMed] [Google Scholar]
- 31.Jefferis R, Lund J, Pound J. Molecular definition of interaction sites on human IgG for Fc receptors (huFc gamma R). Mol Immunol 1990271237–1240. [DOI] [PubMed] [Google Scholar]
- 32.Roux K H, Strelets L, Michaelsen T E. Flexibility of human IgG subclasses. J Immunol 19971593372–3382. [PubMed] [Google Scholar]
- 33.Kocher M, Edberg J C, Fleit H B.et al Antineutrophil cytoplasmic antibodies preferentially engage FcγRIIIb on hman neutrophils. J Immunol 19981616909–6914. [PubMed] [Google Scholar]
- 34.Mulder A H L, Heeringa C, Brouwer E, Limburg P C, Kallenberg C G. Activation of granulocytes by antineutrophil cytoplasmic antibodies (ANCA): a FcγRII‐dependent process. Clin Exp Immunol 199498270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.