Abstract
Background
Several clinical and experimental lines of evidence suggest that leucotriene B4 (LTB4), an arachidonic acid derivative with potent proinflammatory properties, plays a key role in the pathophysiology of rheumatoid arthritis (RA).
Objective
To evaluate the efficacy and safety of BIIL 284, an oral long‐acting LTB4 receptor antagonist, as monotherapy for the treatment of patients with active RA.
Methods
This was a multi‐centre, randomised, double‐blind, placebo‐controlled trial of patients with active RA of 3 months' duration. A total of 342 patients were randomised to receive 5 mg, 25 mg or 75 mg of BIIL 284 or placebo. The primary end point was the percentage of patients achieving an American College of Rheumatology (ACR) 20.
Results
Although a higher percentage of ACR 20 responders was observed in the groups treated with 25 mg and 75 mg of BIIL 284 compared with those treated with placebo, no statistically significant differences were found between any of the three active treatment groups compared with the placebo group with regard to the primary or secondary end points. All trial treatments were safe and well tolerated.
Conclusions
This clinical trial demonstrates that treatment of patients with active RA with a potent oral long‐acting LTB4 receptor antagonist produced only modest improvements in disease activity. The results of this trial support the conclusion that LTB4 is not a major contributor to the inflammatory process in RA.
Leucotriene B4 (LTB4) is a product of the metabolism of arachidonic acid via the 5‐lipoxygenase (5‐LO) pathway that plays a key role in the immediate inflammatory response.1 It has been shown to be an effective chemotactic factor for neutrophils, eosinophils and monocytes/macrophages.2 It promotes neutrophil activation, aggregation and adherence to endothelial cells, natural killer cell cytotoxicity, and potentiates cytokine and matrix metalloproteinase release from T cells.3,4 Two distinct G protein‐coupled receptors, BLT1 and BLT2, mediate the effect of LTB4.5,6 BLT1 is a high‐affinity receptor expressed in most leucocytes, and BLT2 is a low‐affinity LTB4 receptor more widely expressed in human tissues.
Several clinical observations have suggested that LTB4 may be an important mediator of joint inflammation in rheumatoid arthritis (RA). High concentrations of LTB4 have been found in both synovial fluid7,8 and serum9 of patients with active RA. Treatment with either intra‐articular injection of corticosteroid7 or methotrexate10 results in rapid reduction in the synthesis of LTB4 and other products of the 5‐LO pathway in neutrophils of patients with RA. The potential importance of the products of the 5‐LO pathway in joint inflammation has been also supported by animal models of RA. The severity of collagen‐induced arthritis (CIA) is decreased in both 5‐LO‐deficient11 and 5‐LO‐activating protein‐deficient mice compared with wild‐type animals.12 A specific role for LTB4 in the development and progression of joint destruction in vivo was suggested by the observation that administration of an LTB4 receptor antagonist, CP‐105696, resulted in dramatic reductions in both the clinical severity and histological evidence of joint damage in the CIA model.12 These data suggest that products of the 5‐LO pathway, including LTB4, might be important mediators of inflammation in RA and, consequently, potential targets for therapeutic intervention. However, the clinical experience with 5‐LO and LTB4 inhibitors in RA has been very limited. The 5‐LO inhibitor zileuton was studied in a small (n = 24) 4‐week randomised double‐blind trial in patients with RA. The drug was found to induce a non‐significant trend towards improvement in the number of tender and swollen joints, number of painful joints, and patient and physician assessments compared with controls.13 No studies on the adequate duration to assess efficacy have yet been performed in RA with an LTB4 receptor antagonist.
BIIL 284 is a prodrug, which is metabolised after oral administration by ubiquitous esterases to BIIL 260 and its glucuronidated metabolite BIIL 315.14 BIIL 260 and BIIL 315 interact with the LTB4 receptors in a saturable, reversible and competitive manner. In vivo, BIIL 284 administered orally inhibited LTB4‐induced mouse ear inflammation, LTB4‐induced transdermal chemotaxis in guinea pigs and LTB4‐induced neutropenia in monkeys.14 BIIL 284 at a dose of 10 mg/kg once daily orally significantly inhibited disease progression and joint destruction in a therapeutic murine CIA model (unpublished observation). BIIL 315 was found to be the predominant active metabolite in human plasma after oral administration, with an apparent terminal half‐life of 13 h. A phase I pharmacokinetics–pharmacodynamics study in patients with RA found that doses of 25 mg and 150 mg BIIL 284 once daily could achieve 100% inhibition of the ex vivo LTB4‐induced CD11b/CD18 upregulation on peripheral blood neutrophils.15 Pharmacokinetic studies of BIIL 315 showed that the plasma concentration of this metabolite is proportional to the dosage, after multiple dosing of 25–250 mg of BIIL 284 daily in the fed state. Steady state is reached within 3–4 days, with average plasma concentrations being about 30% higher compared with the first dose.
This study was designed to evaluate the efficacy and safety of BIIL 284 at three different dose regimens (5, 25 and 75 mg once daily) versus placebo for 3 months as monotherapy for the treatment of patients with active RA.
Patients and methods
Study design
This was a multi‐centre, randomised, double‐blind, placebo‐controlled and parallel‐group study designed to determine the clinical effect of three oral doses of BIIL 284 (5, 25 and 75 mg once a day) and its safety in patients with RA. The duration of the study was 3 months. Patients were recruited from May 2001 to November 2002 by rheumatologists working in 55 centres from Belgium, Canada, France, Germany, Italy and Spain, and for a planned total sample size of 400 patients, with the objective of obtaining 372 evaluable patients for efficacy analysis. The study protocol was approved by the institutional review board of each of the participating centres, and written informed consent was obtained from all participants.
Eligibility
Patients of either sex, aged 18–70 years, having active RA16 diagnosed for at least 6 months were eligible. At baseline, active RA was defined by at least 6 swollen joints out of 28 joints examined; at least 8 tender joints out of 28 joints examined; and by 2 of the 3 following criteria: pain assessed by the patient (visual analogue scale (VAS)) ⩾40 mm, investigator's assessment of disease activity (VAS) ⩾40 mm, sedimentation rate ⩾28 mm in the first hour or serum C reactive protein level ⩾20 mg/l.
The principal exclusion criteria were RA functional class IV (American College of Rheumatology (ACR) criteria for classification of functional status in RA); lack of efficacy of more than three previous different disease‐modifying anti‐rheumatic drugs (DMARDs); any of the following treatments within 4 weeks before the baseline study or during the study: methotrexate, gold agents, D‐penicillamine, sulphasalazine, antimalarial drugs, azathioprine, ciclosporin A, alkylating agents, minocycline, etanercept, leflunomide; parenteral treatment with corticosteroids, intra‐articular corticosteroid injections, oral corticosteroids (>10 mg/day or 0.2 mg/kg/day prednisone equivalent) and other leucotriene inhibitors; change in treatment with non‐steroidal anti‐inflammatory drugs or initiation of physiotherapy in the previous 2 weeks; synovectomy and/or surgical treatment for RA in the previous 3 months; synoviorthesis in the previous 4 weeks.
Medication
Eligible patients were sequentially assigned to treatment with one of three doses of BIIL 284 (5, 25 or 75 mg) or placebo in a 1:1:1:1 allocation ratio. Patients were instructed to take the study medication orally once daily after breakfast. Patients were allowed to use their usual analgesic medication as rescue medication during the screening and treatment phases. It was necessary to have at least a 12 h wash‐out period of rescue medication before any visit.
Study end points
The primary efficacy end point was the percentage of patients achieving the ACR 20 improvement criteria.17 Secondary efficacy end points included the percentage of patients achieving ACR 50 and ACR 70 criteria,17 morning stiffness, number of drop‐outs for any reason, number of withdrawals due to lack of efficacy, health‐related quality of life assessed by the 36‐item short‐form health survey questionnaire,18 disease activity index and consumption of rescue medication. Safety end points included incidence and intensity of adverse events (AEs), number of withdrawals due to AEs, assessment of tolerability by the investigator, patient's assessment of fatigue, laboratory evaluation, vital functions and a 12‐lead ECG.
Patient evaluation and study procedures
Swollen and tender joint counts were assessed in 28 joints.19 A 100 mm horizontal VAS was used for the assessment of pain, disease activity, general health status and fatigue by the patient, as well as disease activity by the investigator. Functional disability was measured using the disability section of the Health Assessment Questionnaire.20 Morning stiffness was measured in minutes. Disease activity index (disease activity score (DAS) 28) was calculated by the validated modified DAS that included 28‐joint counts as described by Prevoo et al.21 The intensity of AEs was judged by the investigator, who determined the relationship between the study medication and AE.
The assessments were performed at visit 1 (screening), visit 2 (baseline, day 0), visit 3 (day 14), visit 4 (day 28), visit 5 (day 56), visit 6 (end of treatment, day 84) and at follow‐up visit (day 98). At baseline, RA activity (as defined in the section Eligibility) was confirmed. Blood samples for the determination of BIIL 315 were taken on visits 2, 4 and 6 during two prespecified time windows. Descriptive statistics for BIIL 315 plasma concentrations were calculated by treatment group, visit and sampling window. For visits 4 and 6, descriptive statistics were also calculated separately for ACR 20 responders and ACR 20 non‐responders.
Statistical analysis
The primary statistical analysis was performed for the full analysis set population (patients who had received at least one dose of treatment and who had at least a baseline value and a post‐treatment value for the end point). Additionally, a per‐protocol analysis was conducted (as a secondary analysis), excluding 98 patients for important protocol violations, poor compliance (<60%) or insufficient exposure (duration of treatment <50 days). All patients receiving at least one dose of trial medication were considered valid for the analysis of safety.
The Cochran–Mantel Haenszel test stratified by centre was used for treatment comparison of binary data. For continuous data, the comparison between BIIL 284 and placebo was assessed with an analysis of covariance, with main factor treatment and centre and baseline data as covariates. For missing data (particularly for premature withdrawal), the last observation carried forward method was used. Statistical significance was set at p<0.05 (two‐sided).
Sample size
A placebo response rate of 30% was assumed as the highest response rate reported in the literature. A sample size of 372 evaluable patients (93 per arm) was determined to detect a 20% point difference in ACR 20 response rate between the highest dose of BIIL 284 (75 mg) and placebo for a type I error rate of 5% and a statistical power of 80% using a two‐tailed χ2 test. The sample size was increased to 100 patients per arm for an anticipated 7% drop‐out rate.
Results
Patients and study course
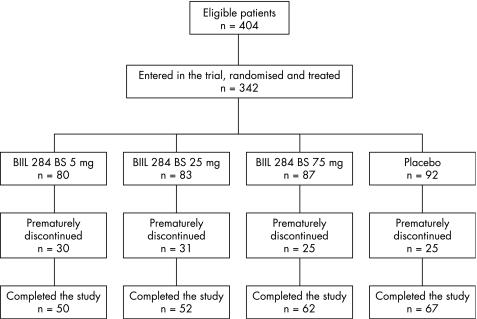
Of a total of 404 eligible patients, 342 were entered into this trial and treated (BIIL 284 5 mg, n = 80; BIIL 284 25 mg, n = 83; BIIL 284 75 mg, n = 87; placebo, n = 92). The treatment trial was prematurely discontinued in 111 patients for the following reasons: AEs in 55, lack of efficacy in 47, consent withdrawn in 6, non‐compliance with the protocol in 2 and other reasons in 1 patient. Figure 1 shows a summary of the patients' disposition by treatment groups. Table 1 shows the demographic data of the full analysis set population (n = 342).
Figure 1 Patients' disposition.
Table 1 Demographic and baseline clinical characteristics of the study population (full dataset).
| Total | BIIL 284 | Placebo | |||
|---|---|---|---|---|---|
| 5 mg | 25 mg | 75 mg | |||
| Study patients | 342 | 80 | 83 | 87 | 92 |
| Sex n (%) | |||||
| Men | 58 (17.0) | 12 (15.0) | 13 (15.7) | 17 (19.5) | 16 (17.4) |
| Women | 284 (83.0) | 68 (85.0) | 70 (84.3) | 70 (80.5) | 76 (82.6) |
| Age (years), mean (SD) | 54.3 (11.4) | 54.8 (11.2) | 53.2 (11.5) | 54.5 (10.6) | 54.9 (12.4) |
| Race n (%) | |||||
| Caucasian | 335 (98.5) | 79 (98.8) | 79 (97.5) | 86 (98.9) | 91 (98.9) |
| Black | 2 (0.6) | 0 | 1 (1.2) | 0 | 1 (1.1) |
| Asian | 3 (0.9) | 1 (1.3) | 1 (1.2) | 1 (1.1) | 0 |
| Weight (kg), mean (SD) | 72.0 (16.3) | 71.4 (16.2) | 74.4 (17.9) | 71.1 (15.2) | 71.2 (16.0) |
| Height (cm), mean (SD) | 162.9 (8.3) | 161.6 (8.6) | 163.9 (8.1) | 163.2 (8.0) | 162.7 (8.4) |
| Duration of RA (years), mean (SD) | 9.6 (9.2) | 10.3 (10.0) | 8.7 (7.1) | 8.4 (8.2) | 10.9 (10.9) |
| Rheumatoid factor + (%) | 58.7 | 53.6 | 63.8 | 58.9 | 58.5 |
| Baseline efficacy assessments, mean (SD) | |||||
| Tender joints | 16.5 (6.1) | 17.0 (6.1) | 16.0 (5.7) | 16.3 (6.7) | 16.7 (6.0) |
| Swollen joints | 11.7 (4.7) | 11.5 (4.0) | 11.7 (4.6) | 11.7 (5.2) | 11.9 (5.0) |
| Morning stiffness | 114.4 (118.1) | 103.8 (90.1) | 114.3 (129.1) | 124.9 (152.3) | 113.7 (89.6) |
| ESR (mm/h) | 34.8 (24.6) | 31.9 (22.2) | 36.2 (27.3) | 36.8 (25.7) | 34.0 (23.3) |
| CRP (mg/l) | 27.0 (31.0) | 24.8 (29.5) | 26.5 (31.7) | 29.2 (33.4) | 27.5 (29.9) |
| Medication history | |||||
| DMARD (%) | 84.2 | 81.3 | 88.0 | 81.6 | 85.9 |
| Different DMARD (median) | 3 | 3 | 3 | 3 | 3 |
| Different DMARD, mean (SD) | 3.0 (1.9) | 3.4 (2.2) | 2.9 (2.0) | 3.0 (1.7) | 2.9 (1.8) |
| MTX (%) | 62.9 | 63.8 | 68.7 | 60.9 | 58.7 |
| Corticosteroids (%) | 61.1 | 57.5 | 67.7 | 62.1 | 57.6 |
| NSAIDs (%) | 80.1 | 75.0 | 86.7 | 85.1 | 73.9 |
| Concurrent medication | |||||
| Corticosteroids (%) | 10.5 | 16.3 | 6.0 | 13.8 | 6.5 |
| NSAIDs (%) | 9.1 | 5.0 | 9.6 | 14.9 | 6.5 |
CRP, C reactive protein; DMARD, disease‐modifying anti‐rheumatic drug; ESR, erythrocyte sedimentation rate; MTX, methotrexate; NSAID, non‐steroidal anti‐inflammatory drug.
Efficacy evaluation
The primary end point of the study (percentage of ACR 20 responders) was analysed for all treatment groups at days 14, 28, 56 and 84 after the start of treatment. As shown in table 2, no statistically significant differences between the four treatment groups at any time point were observed, except for a small but significant increase in the percentage of responders in the 25 mg BIIL 284 group at day 28 compared with the placebo group (27.7% vs 15.2%; p = 0.042). However, after 3 months, the percentages of patients achieving ACR 20 improvement in the 25 mg and 75 mg BIIL 284 groups were almost identical (about 29%) and, although they were somewhat higher than that in the placebo group (18.5%), they did not reach statistical significance. Furthermore, the individual components of ACR 20 did not show any significant differences among treatments.
Table 2 American College of Rheumatology 20 response and p value comparison with placebo by treatment group and time since start of drug administration (last observation carried forward).
| Visit day | BIIL 284 | Placebo | ||
|---|---|---|---|---|
| 5 mg (n = 80) | 25 mg (n = 83) | 75 mg (n = 87) | (n = 92) | |
| Day 14 | ||||
| n (%) | 9 (11.2) | 11 (13.2) | 9 (10.3) | 9 (9.9) |
| p Value* | 0.837 | 0.479 | 0.979 | |
| Day 28 | ||||
| n (%) | 13 (16.2) | 23 (27.7) | 14 (16.1) | 14 (15.2) |
| p Value* | 0.725 | 0.042 | 0.816 | |
| Day 56 | ||||
| n (%) | 16 (20.0) | 24 (28.9) | 23 (26.4) | 16 (17.4) |
| p Value* | 0.624 | 0.070 | 0.165 | |
| Day 84† | ||||
| n (%) | 16 (20.0) | 24 (28.9) | 25 (28.7) | 17 (18.5) |
| p Value* | 0.795 | 0.097 | 0.109 | |
Results of the analysis of efficacy in the full analysis set (n = 342).
*Cochran–Mantel Haenszel test adjusted for centre effects.
†End of treatment.
A variety of subpopulation analyses were performed to identify potential responders to BIIL 284, including patients aged >60 years (n = 131) and the group of patients with >5 years, duration of RA (n = 198). Again, no clinical or statistical differences in the individual components of ACR 20 were observed at the end of the study.
With regard to the secondary end points, no statistically significant difference was found in any of the comparisons performed for each treatment group at any time point.
Safety evaluation
In all, 222 patients experienced at least one AE during the treatment phase of the trial: 58 (72.5%) patients in the 5 mg BIIL 284 group, 51 (61.4%) patients in the 25 mg BIIL 284 group, 55 (63.2%) patients in the 75 mg BIIL 284 and 58 (63.0%) patients in the placebo group. There were also no significant differences in the intensity of the AEs among the treatment groups.
According to the investigator's judgement, the relationship between the AEs and the trial drug medication was positive in 162 patients. The distribution of these patients among the four treatment groups was 39, 37, 36 and 50 patients for the 5, 25, 75 mg and placebo groups, respectively. There were 10 patients with a serious AE. Two patients died. One patient who died owing to a fatal choking episode belonged to the 75 mg BIIL 284 treatment group. The second patient who died had acute leukaemia, but had received placebo. In neither case were these deaths thought to be causally related to the study drug by the investigator. In 21 patients, treatment was discontinued owing to an AE considered to be causally related to study drug treatment by the investigator. Six of these patients had received placebo.
With respect to RA exacerbations, the difference in worsening rates between pooled BIIL 284 arms (16.8%) and placebo (12%) did not reach significance (p = 0.27).
Pharmacokinetics
Plasma concentration data showed the presence of exposure to BIIL 315, and increased with dose after administration of BIIL 284, acheiving steady state at visit 4 (28 days), with no further accumulation. Trough levels for patients with RA were in the same range as found previously for healthy subjects and patients with RA.15 Exposure to BIIL 315 was not significantly different between ACR 20 responders and ACR 20 non‐responders.
Discussion
The results of this multi‐centre, randomised, double‐blind, placebo‐controlled trial failed to show significant clinical efficacy for the long‐acting oral LTB4 receptor antagonist, BIIL 284, as monotherapy in patients with active RA. Although the proportion of patients achieving the ACR 20 criteria was highest in the higher dosing groups, 25 and 75 mg daily, this trend did not reach statistical significance at the end of the study. The difference between the placebo response rate and those of the two highest BIIL 284 dose groups was approximately 10%. In contrast, the difference based on ACR 20 between currently used DMARDs and their placebo control groups in recent clinical trials was at least 20%.22,23,24 The design of the current trial, as well as demographics and baseline clinical characteristics of patients enrolled in this study were comparable to recent trials of synthetic and biological DMARDs.22,23,24
It is unlikely that the failure to show greater efficacy for BIIL 284 was due to inadequate dosing, exposure to active metabolite BIIL 315 or duration of the trial. The exposure to BIIL 315 measured in the study guarantees a nearly complete inhibition of LTB4‐induced upregulated expression of CD11b/CD18 over the dosing interval.15 In addition, BIIL 315 plasma levels were comparable in responders and non‐responders. Furthermore, at the end of the trial (day 84), the proportion of patients responding by ACR 20 criteria in the 25 and 75 mg BIIL 284 dose groups was the same. At earlier time points, 28 and 56 days, the proportion of patients responding in the 25 mg dosing group was slightly higher than that seen in the 75 mg group. The maximal responses to BIIL 284 were observed by day 56 for both the 25 and 75 mg dosing groups, with no further increase by day 84. These findings support the conclusion that dose of drug used and duration of trial were adequate to test the drug's potential efficacy in RA.
Yokomizo et al5,6 described two different functionally active subtypes of the LTB4 receptor. Recently, differential expression of these LTB4 receptor subtypes has been reported in the tissues of patients with RA.25 Although BLT1 was found to be strongly expressed on leucocytes from the synovial fluid of patients with RA, BLT2 was found to be the predominant receptor expressed on synovial macrophages, fibroblast‐like synoviocytes and synovial lymphocytes. Recent studies of the active metabolites of BIIL 284, BIIL 315 and BIIL 260 have revealed that both are potent antagonists of both the BLT1 and BLT2 receptors (Yokomizo, personal observation). Therefore, the differential LTB4 receptor subtype potency of BIIL 315 cannot be an explanation for the lack of relevant clinical efficacy of BIIL 284 found in the current trial.
In conclusion, despite clinical observations in patients with RA and positive studies in animal models such as the murine CIA model suggesting that LTB4 antagonism might be a promising therapeutic strategy in RA,26,27 this clinical trial showed that the effect of a potent long‐lasting oral LTB4 receptor antagonist was modest. The results of this clinical trial are consistent with the conclusion that LTB4 is not a major inflammatory mediator of the rheumatoid inflammatory process in humans.
Acknowledgements
We thank Dr Paul Scholl, Dr Steven Padula and Dr Franz Birke for their review of the manuscript, and Dr Marta Pulido and Judith Christie for their editorial assistance in the preparation of the manuscript.
Abbreviations
ACR - American College of Rheumatology
AE - adverse event
CIA - collagen‐induced arthritis
DMARD - disease‐modifying anti‐rheumatic drug
5‐LO - 5‐lipoxygenase
LTB4 - leucotriene B4
RA - rheumatoid arthritis
VAS - visual analogue scale
Footnotes
Funding: This trial was supported by Boehringer Ingelheim.
Competing interests: None declared.
References
- 1.Henderson W R., Jr The role of leukotrienes in inflammation. Ann Intern Med 1994121684–697. [DOI] [PubMed] [Google Scholar]
- 2.Ford‐Hutchinson A W. Leukotriene B4 in inflammation. Crit Rev Immunol 1990101–12. [PubMed] [Google Scholar]
- 3.Lewis R A, Austen K F, Soberman R J. Leukotrienes and other products of the 5‐lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med 1990323645–655. [DOI] [PubMed] [Google Scholar]
- 4.Leppert D, Hauser S L, Kishiyama J L, An S, Zeng L, Goetzl E J. Stimulation of matrix metalloproteinase‐dependent migration of T cells by eicosanoids. FASEB J 199591473–1481. [DOI] [PubMed] [Google Scholar]
- 5.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G‐protein‐coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 1997387620–624. [DOI] [PubMed] [Google Scholar]
- 6.Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med 2000192421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klickstein L B, Shapleigh C, Goetzl E J. Lipoxygenation of arachidonic acid as a source of polymorphonuclear leukocyte chemotactic factors in synovial fluid and tissue in rheumatoid arthritis and spondyloarthritis. J Clin Invest 1980661166–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson E M, Rae S A, Smith M J. Leukotriene B4, a mediator of inflammation present in synovial fluid in rheumatoid arthritis. Ann Rheum Dis 198342677–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gursel T, Firat S, Ercan Z S. Increased serum leukotriene B4 level in the active stage of rheumatoid arthritis in children. Prostaglandins Leukot Essent Fatty Acids 199756205–207. [DOI] [PubMed] [Google Scholar]
- 10.Sperling R I, Benincaso A I, Anderson R J, Coblyn J S, Austen K F, Weinblatt M E. Acute and chronic suppression of leukotriene B4 synthesis ex vivo in neutrophils from patients with rheumatoid arthritis beginning treatment with methotrexate. Arthritis Rheum 199235376–384. [DOI] [PubMed] [Google Scholar]
- 11.Chen X S, Sheller J R, Johnson E N, Funk C D. Role of leukotrienes revealed by targeted disruption of the 5‐lipoxygenase gene. Nature 1994372179–182. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths R J, Pettipher E R, Koch K, Farrell C A, Breslow R, Conklyn M J.et al Leukotriene B4 plays a critical role in the progression of collagen‐induced arthritis. Proc Natl Acad Sci U S A 199592517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinblatt M E, Kremer J M, Coblyn J S, Helfgott S, Maier A L, Petrillo G.et al Zileuton, a 5‐lipoxygenase inhibitor in rheumatoid arthritis. J Rheumatol 1992191537–1541. [PubMed] [Google Scholar]
- 14.Birke F W, Meade C J, Anderskewitz R, Speck G A, Jennewein H M. In vitro and in vivo pharmacological characterization of BIIL 284, a novel and potent leukotriene B(4) receptor antagonist. J Pharmacol Exp Ther 2001297458–466. [PubMed] [Google Scholar]
- 15.Alten R, Gromnica‐Ihle E, Pohl C, Emmerich J, Steffgen J, Roscher R.et al Inhibition of leukotriene B4‐induced CD11B/CD18 (Mac‐1) expression by BIIL 284, a new long acting LTB4 receptor antagonist, in patients with rheumatoid arthritis. Ann Rheum Dis 200463170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 17.Felson D T, Anderson J J, Boers M, Bombardier C, Furst D, Goldsmith C.et al American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 199538727–735. [DOI] [PubMed] [Google Scholar]
- 18.Ware J E, Jr, Sherbourne C D. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 199230473–483. [PubMed] [Google Scholar]
- 19.Fuchs H A, Brooks R H, Callahan L F, Pincus T. A simplified twenty‐eight‐joint quantitative articular index in rheumatoid arthritis. Arthritis Rheum 198932531–537. [DOI] [PubMed] [Google Scholar]
- 20.Fries J F. The assessment of disability: from first to future principles. Br J Rheumatol 19832248–58. [DOI] [PubMed] [Google Scholar]
- 21.Prevoo M L, van ‘t Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 22.Moreland L W, Baumgartner S W, Schiff M H, Tindall E A, Fleischmann R M, Weaver A L.et al Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)‐Fc fusion protein. N Engl J Med 1997337141–147. [DOI] [PubMed] [Google Scholar]
- 23.Smolen J S, Kalden J R, Scott D L, Rozman B, Kvien T K, Larsen A.et al Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double‐blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet 1999353259–266. [DOI] [PubMed] [Google Scholar]
- 24.Edwards J C, Szczepanski L, Szechinski J, Filipowicz‐Sosnowska A, Emery P, Close D R.et al Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 20043502572–2581. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto A, Endo H, Hayashi I, Murakami Y, Kitasato H, Kono S.et al Differential expression of leukotriene B4 receptor subtypes (BLT1 and BLT2) in human synovial tissues and synovial fluid leukocytes of patients with rheumatoid arthritis. J Rheumatol 2003301712–1718. [PubMed] [Google Scholar]
- 26.Kim N D, Chou R C, Seung E, Tager A M, Luster A D. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med 2006203829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao W H, Del Prete A, Bock C B, Haribabu B. Targeted disruption of leukotriene B4 receptors BLT1 and BLT2: a critical role for BLT1 in collagen‐induced arthritis in mice. J Immunol 20061766254–6261. [DOI] [PubMed] [Google Scholar]