Abstract
Objective
To investigate the predictive value of the distribution of inflamed joints at first presentation for the severity of the disease course in rheumatoid arthritis (RA).
Methods
Of the 1009 consecutive patients included in the Leiden Early Arthritis Clinic (Leiden, The Netherlands), 285 patients fulfilled the American College of Rheumatology criteria for RA within 1 year of follow‐up. Of these, 28 patients achieved remission. Radiographs of hands and feet were scored according to the Sharp–van der Heijde method, and the 28 patients with the most destructive disease were selected. The distribution of inflamed joints of the patients with the extreme disease courses was compared. The association between the distribution of inflamed joints and the level of destruction of the joints of hands and feet in the whole group of patients with RA was assessed using regression analysis.
Results
Comparison of patients with extreme disease courses using univariate and logistic regression analyses showed that arthritis of the large joints—in particular, the knee—was associated with severe RA. In the whole group of patients with RA, the total number of swollen joints and the presence of knee arthritis were associated independently with the level of destruction of the small joints. Patients with RA with knee arthritis had higher C reactive protein (CRP) levels than patients without knee arthritis, and investigating the distribution of inflamed joints together with other variables yielded the number of swollen joints, CRP, presence of anti‐cyclic citrullinated peptide antibodies and symptom duration as predictors for severity of RA.
Conclusion
Arthritis of large joints—in particular, the knee—at first presentation is associated with a destructive course of RA.
The initial clinical presentation of rheumatoid arthritis (RA) is variable, and the number as well as the distribution of inflamed joints may vary between a monoarthritis and an extensive polyarthritis. In general, RA is considered to be a chronic potentially destructive disease, but the severity of the disease course for an individual patient is difficult to predict at baseline. Patients with RA who present with an extensive polyarthritis may have a mild disease course or remit spontaneously, whereas patients who initially present with a monoarthritis may experience a severe destructive course of the disease. The implication of being able to predict the disease course in RA is obvious, given the value of early treatment and the common use of aggressive treatment strategies.1,2,3 Several studies have assessed associations between clinical variables and RA severity.4,5,6,7,8,9,10,11,12,13,14,15,16,17 In these studies, the presence of morning stiffness, symptom duration >6 months, rheumatoid factor (RF), antibodies against cyclic‐citrullinated peptides (CCPs), early radiological erosions and an elevated C reactive protein (CRP) level were correlated with a more severe outcome of the disease.4,5,6,7,8,9,10,11,12,13,14,15,16,17 So far, it is not known whether the distribution of inflamed joints is associated with the disease outcome in RA. Therefore, the present study aimed to investigate the predictive value of the distribution of inflamed joints at first presentation for the severity of the disease course in RA. To identify the joints that are associated with a severe disease outcome, the distributions of swollen joints of patients with RA with extreme disease courses, sustained remission and progressive erosive disease were compared. The comparison of the extremes of phenotypes may reduce the risk of missing risk factors caused by regression to the mean and this approach, in addition to studying the whole group of patients, may lead to the detection of additional prognostic factors. Subsequently, in the whole group of patients with RA, the association between the distribution of inflamed joints at baseline and the level of radiological destruction of the small joints of the hands and feet during follow‐up was determined and the ability of the identified joints to predict RA severity in relation with other clinical parameters was assessed.
Patients and methods
Patients
For this study, patients from the Leiden Early Arthritis Clinic (Leiden, The Netherlands)—a population‐based inception cohort of patients with newly diagnosed early arthritis—were selected. This cohort presented in 1993 at the Department of Rheumatology of the Leiden University Medical Center, the only referral centre for rheumatology in a healthcare region of approximately 400 000 inhabitants in The Netherlands. General practitioners were encouraged to refer patients directly when arthritis was suspected; patients were included if physical examination revealed arthritis.18 In the study period (1993–9), 1009 patients with early arthritis were included. After 2 weeks of follow‐up, 182 patients had fulfilled the 1987 American College of Rheumatology (ACR) criteria for RA19 and 326 patients had arthritis that could not be readily classified (undifferentiated arthritis (UA)). After 1 year of follow‐up, a total of 285 patients fulfilled the 1987 ACR criteria for RA. In all, 103 of these patients had been classified as UA at 2 weeks of follow‐up. From these patients with RA, two categories of patients with extreme disease courses were selected: those who entered sustained remission (remitting RA) and those who had progressed to the most destructive disease (severe RA). Remission was defined as satisfying the proposed American Rheumatism Association criteria for clinical remission20 after having discontinued the use of disease‐modifying antirheumatic drugs (DMARDs) for at least 1 year. After one additional year of follow‐up, the presence of sustained remission was confirmed. 28 patients had achieved sustained remission after a mean disease duration of 3.7 years (range 210–3159 days, SD 852 days).21 The remitting patients are further referred to as “remitting RA”. Radiographs of the hands and feet were taken at baseline and yearly thereafter, and the level of radiological joint destruction was scored according to the Sharp–van der Heijde method.22,23 The rheumatologist who scored the radiographs was unaware of the study question. The patients with the most destructive disease course were identified by selecting the 28 patients who had the highest Sharp–van der Heijde scores after 1 year of follow‐up. This corresponded with a Sharp–van der Heijde score >34 (mean 59). These patients are further referred to as “most severe RA”.
Methods
At inclusion, the rheumatologist questioned the patient about the initial symptoms as well as the presence of morning stiffness and the symptom duration. Physical examination at baseline included a swollen joint count, scaling each joint on a 0–1 scale (0 denotes no swelling and 1 indicates the presence of swelling). Subsequently, the joints or joint groups were categorised (eg, all metacarpophalangeal joints on one side were counted as one joint). The following joints (groups) were studied: shoulders, elbows, wrists, metacarpophalangeal joints, proximal and distal interphalangeal joints, knees, ankles, and metatarsophalangeal joints. For the analysis, a dichotomous approach that indicated the presence or absence of swelling per joint group was used.
Baseline laboratory parameters included eosinophil sedimentation rate, CRP, IgM rheumatoid factor ELISA as described previously24 and anti‐CCP2 antibodies (ELISA, Immunoscan RA Mark 2, Euro‐Diagnostica, Arnhem, The Netherlands). According to the manufacturer's instruction, a level >25 arbitrary units was considered positive.
Statistical analysis
The baseline parameters of the patients with remitting and severe RA were compared using the Mann–Whitney U test for continuous variables and the χ2 test for nominal variables. Subsequently, a logistic regression analysis with backward selection procedure (p>0.10 as removal criterion, using a likelihood ratio test) was performed, with the presence of remitting RA or most severe RA as the dependent variable and the individual joint groups that were significantly associated with the disease outcome in the univariate analysis and the total number of swollen joints as possible explanatory variables.
To validate the findings, the association between the joint groups and radiological joint destruction was assessed in the total group of patients with RA. The Sharp–van der Heijde scores at 1‐year follow‐up were entered as a dependent variable in a linear regression analysis (backward selection procedure, p>0.10 as removal criterion), with the total number of swollen joints and the joint count for the individual joint groups being possible explanatory variables. To assess the association between the distribution of swollen joints and the level of radiological joint destruction in relation to other clinical variables that might be associated with RA severity, another linear regression analysis (backward selection procedure, p>0.1 as removal criteria) was performed. In this analysis, the Sharp–van der Heijde score at 1‐year follow‐up was entered as a dependent variable, and the total number of swollen joints, the swollen joint count for the individual joint groups, gender, age, the presence of anti‐CCP antibodies, RF, morning stiffness, the CRP level and symptom duration entered as possible explanatory variables.
To assess whether the findings from these analyses were specific for RA or were also applicable to arthritis of various causes (UA), a logistic regression analysis with backward selection procedure (p>0.10 as removal criterion, using likelihood ratio test) was performed, with the outcome RA after 1 year (RA yes or no) being the dependent variable and the baseline parameters and individual joint groups being possible explanatory variables in patients who were classified as UA at 2 weeks follow‐up.
SPSS V.11.0 was used to analyse the data. A p value ⩽0.05 was considered to be significant.
Results
Univariate comparison of patient characteristics at first presentation of patients with RA with extreme disease courses
The patients in the remitting and most severe RA group had no significant differences in the distribution of gender, age and morning stiffness. The patients with the most severe disease course harboured RF and anti‐CCP antibodies more frequently, had higher levels of CRP and had higher Sharp–van der Heijde scores at inclusion compared with patients with remitting RA (table 1). The patients with the most severe disease course also had a significantly higher number of swollen joints at first presentation than the patients with remitting RA. The distribution of swollen joints was different between the two groups: the patients with the most severe RA had, significantly more often, arthritis of the shoulders, elbows, proximal interphalangeal joints, knees and ankles (table 2). There was no difference in the prevalence of swollen metacarpophalangeal and metatarsophalangeal joints between the two groups of patients with RA.
Table 1 Baseline characteristics of patients with rheumatoid arthritis with the extreme disease courses.
| Remitting RA n = 28 | Most severe RA n = 28 | p Value | |
|---|---|---|---|
| Female, n (%) | 18 (64) | 17 (61) | 0.7 |
| Age (years), median (IQR) | 59 (48–71) | 59 (50–72) | 1.0 |
| Morning stiffness (min), median (IQR) | 127 (30–180) | 127 (30–180) | 1.0 |
| Symptom duration, (days), median (IQR) | 127 (57–207) | 152 (87–281) | 0.1 |
| C reactive protein (mg/l), median (IQR) | 29 (7–46) | 56 (26–75) | 0.01 |
| IgM RF positive, n (%) | 6 (21) | 23 (82) | <0.05 |
| Anti‐CCP‐2 positive, n (%) | 3 (11) | 21 (75) | <0.05 |
| Total number of swollen joints, median (IQR) | 5.3 (3–7) | 7.3 (5–9) | <0.05 |
| Sharp–van der Heijde score, median (IQR) | 0 (0–2) | 10 (1–17) | <0.05 |
CCP, cyclic‐citrullinated peptide; RA, rheumatoid arthritis; RF, rheumatoid factor.
Table 2 Distribution of swollen joints of the patients with rheumatoid arthritis with extreme disease courses.
| Joint group n (%)* | Remitting RA n = 28 | Most severe RA n = 28 | OR | 95% CI | p Value |
|---|---|---|---|---|---|
| Shoulder | 1 (3%) | 6 (21%) | 6.4 | 2.0 to 26.4 | <0.05 |
| Elbow | 4 (14%) | 8 (29%) | 2.5 | 1.2 to 5.5 | <0.05 |
| Wrist | 19 (68%) | 21 (75%) | 1.4 | 0.7 to 2.8 | 0.3 |
| Metacarpophalangeal | 23 (82%) | 23 (82%) | 1.0 | 0.5 to 2.2 | 1.0 |
| Proximal interphalangeal | 17 (61%) | 23 (82%) | 2.9 | 1.5 to 5.9 | <0.05 |
| Distal interphalangeal | 1 (4%) | 1 (4%) | 1.0 | 0.2 to 4.1 | 1.0 |
| Knee | 7 (25%) | 17 (61%) | 4.7 | 2.5 to 9.0 | <0.05 |
| Ankle | 4 (14%) | 9 (32%) | 2.9 | 1.4 to 6.3 | <0.05 |
| Metatarsophalangeal | 8 (29%) | 9 (32%) | 1.2 | 0.6 to 2.7 | 0.6 |
RA, rheumatoid arthritis.
*The numbers (%) indicate the number (percentage) of patients with swelling of (at least one of) the joints of the specific joint groups.
Regression analysis in patients with RA with extreme disease courses
To investigate which joints (groups) were independently associated with the disease outcome, a logistic regression analysis with backward selection procedure was performed with the presence of remitting RA or most severe RA as the dependent variable and the individual joint groups that were significantly associated with the disease outcome in the univariate analysis (shoulder, elbow, proximal interphalangeal, knee and ankle) and the total number of swollen joints as possible explanatory variables. In this analysis, only the presence of a swollen knee (OR 7, p = 0.004) was found to be significantly associated with the disease outcome (table 3).
Table 3 Results of logistic regression analysis using a backward selection procedure and remitting or severe rheumatoid arthritis as the dependent variable and the total number of swollen joints and the joints with a significant result in univariate analysis as possible explanatory variables.
| Joint group | p Value | OR | 95% CI |
|---|---|---|---|
| Shoulder | 0.07 | 8.7 | 0.8 to 90.1 |
| Elbow | 0.08 | 4.1 | 0.9 to 19.4 |
| Knee | 0.004 | 7.0 | 1.9 to 25.9 |
Regression analysis in all 285 patients with RA
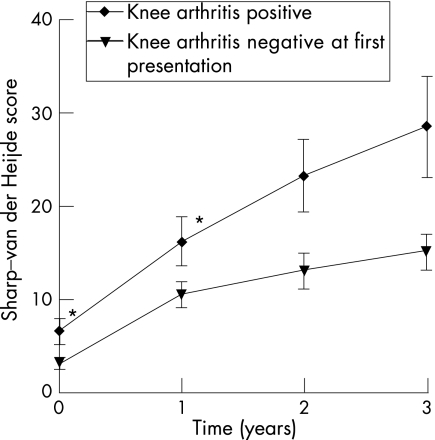
To validate the findings, the association between the distribution of swollen joints and radiological joint destruction was assessed in the total group of patients with RA . The Sharp–van der Heijde score at 1‐year follow‐up was entered as a dependent variable in a linear regression analysis with a backward selection procedure and the total number of swollen joints and all evaluated joint groups as possible explanatory variables. This analysis showed that, in the total group of patients with RA, the total number of swollen joints (p = 0.004) and swelling of the knee (p = 0.03) were independently associated with the level of radiological joint destruction of hands and feet (table 4). A similar analysis was performed with the Sharp–van der Heijde scores at 2‐ and 3‐year follow‐up as dependent variable (x rays were available of, respectively, 202 and 162 patients). In these analyses, only swelling of the knee was found to be associated independently with the level of radiological joint destruction (B = 7.2, SE = 3.9, p = 0.05 for the 2‐year time point and B = 3.5, SE = 4.7, p = 0.005 for the 3‐year time point). Figure 1 displays the level of radiological destruction of the small joints of the hands and feet during 3 years of follow‐up of the patients with RA who at first presentation did have or did not have arthritis of the knee (fig 1).
Table 4 Results of linear regression analysis using a backward selection procedure in the 285 patients with rheumatoid arthritis with a Sharp–van der Heijde score at 1‐year follow‐up as the dependent variable and distribution and total number of swollen joints as possible explanatory variables.
| Joint group | B | SE | p Value |
|---|---|---|---|
| Knee | 1.4 | 0.5 | 0.004 |
| Total number of swollen joints | 6.1 | 2.9 | 0.03 |
B indicates the regression coefficient.
Figure 1 The level of radiological destruction of the small joints of hands and feet during 3 years of follow‐up of patients with rheumatoid arthritis with or without arthritis of the knee at first presentation. *p<0.05 (Mann–Whitney U test). At the 2‐ and 3‐year time points, radiographs of hands and feet were available of 202 and 162 patients, respectively.
In conclusion, regression analyses in both the whole group of patients with RA and using the approach of the extremes of the disease courses yielded the total number of swollen joints and, particularly, the presence of arthritis of the knee as independently associated with the level of joint destruction of the small joints of hands and feet during follow‐up.
Predictive ability of distribution of swollen joints in relation to other clinical characteristics for RA severity
Next, we investigated the predictive ability of the presence of arthritis of the knee and the total number of swollen joints in relation to other clinical variables that are observed or described to be associated with a severe disease outcome: CRP, RF, anti‐CCP antibodies, morning stiffness and symptom duration (table 1).4,17 A linear regression analysis with a backward selection procedure revealed that the total number of swollen joints (B = 0.9, SE = 0.4, p = 0.03), the presence of anti‐CCP antibodies (B = 8.4, SE = 2.4, p<0.001), the CRP level (B = 0.02, SE = 0.007, p<0.01) and the symptom duration (B = 0.2, SE = 0.04, p<0.001) were independently associated with the level of joint destruction after 1‐year of follow‐up. In this analysis, the presence of arthritis of the knee was not an independent predictor for disease severity. The fraction of explained variation of this model was 0.2. As, after correction for clinical variables in a regression analysis, the presence of arthritis of the knee was not found to be independently associated with RA severity (in contrast with the CRP level, the presence of anti‐CCP antibodies and symptom duration), we hypothesised that these variables might be distributed differently among the patients with RA with and without arthritis of the knee. Arthritis of the knee was present in 106 (39%) patients at first presentation. The presence of anti‐CCP antibodies and symptom duration, and also the variables age, gender, RF and morning stiffness, were not significantly different between the patients with RA with or without arthritis of the knee. However, there was a significant difference in the level of CRP: patients with RA with arthritis of the knee at first presentation had higher CRP levels (mean (SD) 48 (35) mg/l) compared with patients with RA without involvement of the knee (mean (SD) 22 (24) mg/l, p<0.001).
Discussion
The present study aimed to investigate the predictive value of the distribution of inflamed joints at first presentation for the severity of the disease course in RA and demonstrated, using two different approaches, that the presence of arthritis of large joints, and arthritis of the knee in particular, is independently associated with the level of joint destruction. In regression analysis, both arthritis of the knee and the total number of swollen joints at baseline were significantly associated with a severe disease outcome. As all patients with RA fulfilled the ACR criteria and as per definition had involvement of the small joints, and as the presence/absence of arthritis was evaluated as a dichotomous variable per joint group, in the present study, a difference in the total number of swollen joints reflects a difference in the number of swollen large joint groups. Among all joints, the presence of arthritis of the knee at baseline was found to be the strongest predictor for a higher level of radiological destruction of the small joints of the hands and feet.
After the identification of the knee and the total number of swollen joints as predictors for severe radiological destruction of small joints, we were interested in knowing whether these parameters were of additional value to established parameters for disease severity (anti‐CCP antibodies, RF, CRP, morning stiffness and symptom duration). Therefore, a linear regression analysis was performed that included not only the distribution and number of swollen joints but also other clinical variables. This analysis revealed a significant association between the level of radiological joint destruction and the total number of swollen joints, CRP, anti‐CCP antibodies and symptom duration. The presence of knee arthritis was not significantly associated with RA severity in this analysis. A post hoc analysis showed that patients with arthritis of the knee had higher levels of CRP compared with the patients without arthritis of the knee. Thus, apparently, the CRP level is a stronger predictor for the level of joint destruction than the presence of an inflamed knee. Interestingly, the presence of anti‐CCP antibodies was not different between patients with and without knee arthritis. These data suggest that inflammation of large synovial joints, such as the knee, induces a higher amount of proinflammatory cytokines (among others, interleukin‐6) that subsequently triggers an increased production of CRP by the liver. Holt et al previously showed that, in patients with inflammatory arthritis, the concentration of synovial interleukin‐6 in knee joints was associated with the plasma level of interleukin‐6 and also with plasma level of CRP.25 In experimental studies in rabbits, interleukin‐1β infusion by intra‐articular knee injection with a retroviral vector containing a DNA fragment encoding for mature interleukin‐1β26,27 yielded not only a local reaction in the knee resembling human RA with the formation of pannus and bony erosions, but also a dramatic systemic response with severe systemic manifestations. These findings also support the notion that the presence of a large area of inflamed synovium (ie, involvement of a large joint as the knee) is correlated with higher systemic level of proinflammatory cytokines and a more severe disease outcome. The correlation between the extension of synovitis and the level of CRP is not exclusive for RA. In 54 patients with reactive arthritis who were included in the Leiden Early Arthritis Cohort, the 24 patients who at first presentation had arthritis of the knee had higher CRP levels compared with the 30 patients who presented without knee arthritis (mean (SD) 49.3 (56.5) vs 27.4 (41.9) mg/l, respectively).
Although having arthritis of the knee was also correlated with a high level of CRP in the present study and the CRP level seemed to be a better predictor for disease severity compared with knee arthritis, in clinical practice—especially in primary care, where decisions on referral are often made only on the basis of physical examination—a physician establishes the presence of arthritis of large joints such as the knee directly during the first visit, whereas laboratory results are not immediately available. Furthermore, the presence of an elevated CRP level can also be caused by non‐rheumatological factors such as a transient infection. As the present study aimed to investigate the predictive value of the distribution of inflamed joints for the disease outcome in RA, the finding that the presence of arthritis of large joints (and the knee in particular) is associated with a more destructive disease might be of help in clinical practice.
Studies that investigate the natural disease course in RA are currently hampered by the fact that aggressive and effective disease‐modifying antirheumatic drugs, including biologicals, are used. The effective reduction of disease activity diminishes the level of joint destruction. Despite the indisputable benefit for patients with RA, the natural course of the disease is altered and the patients who are currently treated for RA are less suitable for studies that aim to identify variables that predict disease outcome. The patients included in the present study were treated for RA between 1993 and 1999. In this time era, treatment with DMARDs began at a relatively late stage and drugs of choice were, among others, malaria drugs, the ability of which to halt disease progression is limited. None of the included patients were treated with biological drugs. Out of the 28 patients who achieved sustained remission, 16 patients had received no DMARD treatment, 6 were treated with malaria drugs and the remaining 7 were prescribed methotrexate or sulphasalazine. The patients included in the present study are more suitable for a study assessing risk factors for RA severity than the patients who are currently included in our Early Arthritis Clinic.
In conclusion, early recognition of patients with RA with a potentially severe disease course is important as these patients, in particular, may benefit from the therapeutic options that are currently available. It is already known that the presence of anti‐CCP antibodies, symptom duration and CRP are associated with RA severity. This study reveals that the presence of arthritis of large joints—and, particularly, arthritis of the knee—are also predictive for a destructive disease course.
Abbreviations
ACR - American College of Rheumatology
CCP - cyclic‐citrullinated peptide
CRP - C reactive protein
DMARD - disease‐modifying antirheumatic drug
RA - rheumatoid arthritis
RF - rheumatoid factor
UA - undifferentiated arthritis
Footnotes
Competing interests: None declared.
References
- 1.Quinn M A, Emery P. Potential for altering rheumatoid arthritis outcome. Rheum Dis North Am 200531763–772. [DOI] [PubMed] [Google Scholar]
- 2.Quinn M A, Cox S. The evidence for early intervention. Rheum Dis North Am 200531575–589. [DOI] [PubMed] [Google Scholar]
- 3.Emery P. Treatment of rheumatoid arthritis. BMJ 2006332152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser H, le Cessie S, Vos K, Breedveld F C, Hazes J M. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum 200246357–365. [DOI] [PubMed] [Google Scholar]
- 5.Visser H. Early diagnosis of rheumatoid arthritis. Best Pract Res Clin Rheumatol 20051955–72. [DOI] [PubMed] [Google Scholar]
- 6.Brennan P, Harrisson B. A simple algorithm to predict the development of radiological erosions in patients with early rheumatoid arthritis: prospective cohort study. BMJ 1996316471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorman J D, Lum R F, Chen J J, Suarez‐Almazor M E, Thomson G, Criswell L A. Impact of shared epitope genotype and ethnicity on erosive disease: a meta‐analysis of 3,240 rheumatoid arthritis patients. Arthritis Rheum 200450400–412. [DOI] [PubMed] [Google Scholar]
- 8.Van der Helm‐van Mil A H, Verpoort K N, Breedveld F C, Toes R E, Huizinga T W J. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther 20057R949–R958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Gaalen F A, Linn‐Rasker S P, Van Venrooij W J, de Jong B A, Breedveld F C, Verweij C L.et al Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum 200450709–715. [DOI] [PubMed] [Google Scholar]
- 10.Raza K, Breese M, Nightingale P, Kumar K, Potter T, Carruthers D M.et al Predictive value of antibodies to cyclic citrullinated peptide in patients with very early inflammatory arthritis. J Rheumatol 200532231–238. [PMC free article] [PubMed] [Google Scholar]
- 11.Kaarela K, Luukkainen R, Koskimies S. How often is seropositive rheumatoid arthritis an erosive disease? A 17 year followup study. J Rheumatol 1993201670–1673. [PubMed] [Google Scholar]
- 12.Bas S, Genevay S, Meyer O, Gabay C. Anti‐cyclic citrullinated peptide antibodies, IgM and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis (Oxford). Rheumatology 200342677–680. [DOI] [PubMed] [Google Scholar]
- 13.Stockman A, Tait B D, Wolfe R, Brand C A, Rowley M J, Vanney M D.et al Clinical laboratory and genetic markers associated with erosions and remission in patients with early inflammatory arthritis: a prospective cohort study. Rheumatol Int 200571–10. [DOI] [PubMed] [Google Scholar]
- 14.Dixey J, Solymossy C, Young A, Early RA Study Is it possible to predict radiological damage in early rheumatoid arthritis? A report on the occurrence, progression and prognostic factors of radiological erosions over the first 3 years in 866 patients from the early RA study (ERAS). J Rheumatol 200469(Suppl)48–54. [PubMed] [Google Scholar]
- 15.Scott D L. Radiological progression in established rheumatoid arthritis. J Rheumatol 200469(Suppl)55–65. [PubMed] [Google Scholar]
- 16.Goronzy J J, Matteson E L, Fulbright J W, Warrington K J, Chang‐Miller A, Hunder G G.et al Prognostic markers of radiologic progression in early rheumatoid arthritis. Arthritis Rheum 20045043–54. [DOI] [PubMed] [Google Scholar]
- 17.Berglin E, Johansson T, Sundin K, Jidell E, Wadell G, Hallmans G.et al Radiological outcome in rheumatoid arthritis is predicted by the presence of antibodies against cyclic citrullinated peptide before and at disease onset, and by IgA‐rheumatoid factor at disease onset. Ann Rheum Dis 200665453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Aken J, van Bilsen J H, Allaart C F, Huizinga T W, Breedveld F C. The Leiden Early Arthritis Clinic. Clin Exp Rheumatol 200321(Suppl 31)S100–S105. [PubMed] [Google Scholar]
- 19.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 20.Pinals R A, Masai A T, Larsen R A. Preliminary criteria for clinical remission in rheumatoid arthritis. Arthritis Rheum 1981241308–1315. [DOI] [PubMed] [Google Scholar]
- 21.Linn‐Rasker S P, Allaart C F, Kloppenburg M, Breedveld F C, Huizinga T W J. Sustained remission in a cohort of patients with rheumatoid arthritis: association with absence of IgM RF and absence of anti‐CCP antibodies. Int J Adv Rheumatol 200424–6. [Google Scholar]
- 22.Van der Heide D M. Plain X‐rays in rheumatoid arthritis: overview of scoring methods, their reliability and applicability. Bailliere's Clin Rheumatol 199610435–453. [DOI] [PubMed] [Google Scholar]
- 23.Van der Heide D. How to read radiographs according to the Sharp‐van der Heijde method. J Rheumatol 200027261–263. [PubMed] [Google Scholar]
- 24.Visser H, Gelinck L B, Kampfraath A H, Breedveld F C, Hazes J M. Diagnostic and prognostic characteristics of enzyme linked immunosorbent rheumatoid factor assays in rheumatoid arthritis. Ann Rheum Dis 199655157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holt I, Cooper R G, Hopkins S J. Relationship between local inflammation, interleukin‐6 concentration and the acute phase protein response in arthritis patients. Eur J Clin Invest 199121479–484. [DOI] [PubMed] [Google Scholar]
- 26.Ghivizzani S C, Kang R, Georgescu H I, Lechman E R, Jaffurs D, Engle J M.et al Constitutive intra‐articular expression of human IL‐1β following gene‐transfer to rabbit synovium produces all major pathologies of human rheumatoid arthritis. J Immunol 19971593604–3612. [PubMed] [Google Scholar]
- 27.Kay J, Calabrese L. The role of interleukin‐1 in the pathogenesis of rheumatoid arthritis. Rheumatology 200443(Suppl 3)iii2–iii9. [DOI] [PubMed] [Google Scholar]