Abstract
Objectives
To identify the mechanism of interleukin (IL)7‐stimulated tumour necrosis factor α (TNFα) production and to determine the relationship between intra‐articular IL7 and TNFα expression levels in patients with rheumatoid arthritis (RA). In addition, the effect of TNFα blockade on IL7 activity and on IL7 levels was studied.
Methods
The effect of IL7 on isolated CD4 T cells and CD14 monocytes/macrophages was studied. IL7 and TNFα levels were measured in the synovial fluid of patients with RA. In RA synovial tissue, IL7 and TNFα expression was assessed in addition to IL1β, numbers of inflammatory cells and adhesion molecule expression. The extent to which TNFα blockade could prevent IL7‐induced lymphocyte responses was studied in vitro. In addition, regulation of serum IL7 levels on anti‐TNFα therapy (adalimumab) was studied.
Results
IL7 induced cell contact‐dependent TNFα production by cocultures of T cells and monocytes, but not by T cells and monocytes cultured separately. IL7 and TNFα levels in RA synovial fluid and synovial tissue significantly correlated. IL7‐stimulated lymphocyte responses were not inhibited by TNFα blockade. Circulating IL7 levels were significantly reduced in patients who successfully responded to anti‐TNFα treatment. However, IL7 levels persisted in non‐responders.
Conclusion
The present data suggest that IL7 is an important inducer of T cell‐dependent TNFα production in RA joints. This may contribute to the correlation of intra‐articular IL7 and TNFα in these joints. Furthermore, the persistence of IL7‐induced inflammatory activity on TNFα blockade in vitro and persistence of IL7 levels and disease activity in anti‐TNFα non‐responders suggest that IL7 might additionally promote TNFα‐independent inflammation.
Rheumatoid arthritis (RA) is a chronic disabling type of arthritis that affects >1% of the adult population. RA is characterised by persistent inflammation of the joints, often resulting in continuously progressing tissue destruction.1 Numerous studies revealed a pivotal role for CD4 T cells and macrophages in RA synovitis2,3,4,5,6 associated with the abundant production of catabolic enzymes and proinflammatory cytokines,2,7 including tumour necrosis factor α (TNFα).8,9,10,11,12,13,14,15 Clinical studies have supported the importance of TNFα in the inflammatory and tissue‐destructive processes in patients with RA.16 Despite the success of anti‐TNFα treatment, a considerable number of patients do not respond or only improve partially.16,17,18 The lack of efficacy of anti‐TNFα treatment in certain patients might be due to persisting TNFα‐independent proinflammatory activity induced by mediators other than TNFα. Additionally, such mediators may contribute to continuous induction of TNFα, preventing an adequate response to anti‐TNFα treatment. Recently, several studies indicated that interleukin (IL)7 might be such a mediator, contributing to chronic inflammation in RA.
IL7 belongs to the IL2 family of cytokines that includes IL2, IL4, IL9, IL15, IL21 and thymic stromal lymphopoietin. IL7 mediates its effects through the IL7R, which consists of the common cytokine γ chain (γc) and the IL7Rα chain.19 IL7 is produced by stromal cells at lymphopoietic sites and plays a role in the regulation of peripheral homeostasis of the CD4 T cell pool. IL7 is a growth factor for T cells in early T cell development, and promotes proliferation, survival and differentiation of mature naive and memory T cells.20 In addition, high concentrations of IL7 were shown to induce cytokine production by monocytes from healthy individuals.21
In patients with arthritis (RA and juvenile idiopathic arthritis (JIA)), increased levels of IL7 have been shown compared with healthy controls22,23,24 and correlated with increased disease activity.22,24 In addition, recently, strongly increased IL7 levels were found in the synovial fluid (SF) of patients with RA and patients with JIA compared with patients with osteoarthritis and oligoarticular patients, respectively.25,26 Furthermore, abundant expression of IL7 by macrophages, endothelial cells and fibroblasts was detected in the synovial tissue of patients with RA.25,27
The purpose of this study was to define the mechanism by which IL7 induces TNFα production by monocytes and CD4 T cells, and to investigate the relationship between intra‐articular IL7 and TNFα levels. The TNFα dependency of IL7‐induced lymphocyte activation was tested in vitro by TNFα blockade. Finally, the persistence of IL7 levels on TNFα blockade was studied in patients treated with the anti‐TNFα monoclonal antibody adalimumab.
Methods
Patients
Table 1 shows the demography of patients with RA. Patients with RA were classified according to the 1987 revised American College of Rheumatology criteria.28 Patients who donated peripheral blood (PB) or synovial fluid for cell cultures or analysis of IL7 and TNFα by ELISA were randomly selected from our outpatient clinic. Synovial tissue biopsy specimens were taken from a cohort of patients with persistent synovitis of the knee. Anti‐TNFα‐treated patients had previously failed to at least three conventional anti‐rheumatic drugs. Written consent was obtained from the patients according to the Helsinki declaration, and the University Medical Center Utrecht medical ethics committee approved the design of the studies.
Table 1 Demography of patients with rheumatoid arthritis (RA).
| Synovial fluid | Synovial tissue | Serum anti‐TNFα study baseline values | |
|---|---|---|---|
| Number | 30 | 23 | 22 |
| Age, mean (SD) | 64 (10) | 60 (11) | 52 (13) |
| Disease duration | 12±15 | 9±12 | 14±9 |
| Sex ratio (female/male) | 23/7 | 16/7 | 17/5 |
| RF (+/−) | 19/11 | 13/10 | 16/6 |
| ESR (mm/1st h) | 32.1±20.8* | 42.3±33.4† | 44.1±29.1 |
| CRP (mg/l) | 27.2±34.5* | 36.8±41.4† | 24.9±28.6 |
CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; RF, rheumatoid factor.
For age, disease duration, ESR, and CRP levels, mean (SD) values are given. Numbers of female/male and RF positive/negative patients are also given.
*ESR and CRP levels were available for 25 patients.
†ESR and CRP levels were available for 19 patients.
Cytokine assessment by enzyme‐linked immunosorbent assay
Prior to cytokine analysis, SF of patients with RA (n = 30) was treated with hyaluronidase (20 U/ml; type IV, Sigma, Munich, Germany) for 20 min at 37°C to reduce viscosity. IL7 and TNFα in SF were measured with a commercially available ELISA according to the manufacturer's instructions (Diaclone, Besancon, France, and Biosource Europe, Nivelles, Belgium, respectively). Specificity was tested as described previously.25
IL7 levels in serum samples from anti‐TNFα‐treated patients with RA were measured using a different ELISA kit (R&D, Minneapolis, Minnesota, USA) as this measures IL7 in the serum more sensitively than the above‐described ELISA kit (Diaclone). One possible explanation for the observed difference in sensitivity is the use of different IL7‐specific monoclonal antibodies recognising dissimilar epitopes that could be influenced in their own way by IL7‐binding molecules such as those previously described (eg, glycoseaminoglycans such as heparin and chondroitin sulphate).29
To demonstrate the specificity of anti‐TNFα treatment on IL7 levels, circulating levels of IL15 (R&D) and IL18 (MBL, Woburn, Massachusetts, USA) were also measured with commercially available ELISA kits.
Immunohistology of synovial tissue
RA knee synovial tissue biopsy specimens were obtained, stored and prepared for immunohistochemical analysis as described previously.25,30 Biopsy sections (n = 23) were incubated with polyclonal rabbit anti‐human IL7 antibody (H‐151, Santa Cruz Biotechnology, Santa Cruz, California, USA), followed by a two‐step immunophosphatase staining method as described previously.
Numbers of IL7 cells were counted independently by two observers (JAGvR, MWvW) who were blinded to the patient's identity. Cells were counted in 3−5 tissue sections per patient that included in each section intimal lining layer and synovial sublining. Numbers of cells were calculated per mm2 as an average of the analysed tissue sections.
In an additional set of slides, serial sections were stained with the following mouse monoclonal antibodies (mAbs): anti‐CD3 (SK7; Becton‐Dickinson, San Jose, California, USA) to detect T lymphocytes, anti‐CD68 (EBM11; Dako, Glostrup, Denmark) for macrophages in the intimal lining layer and synovial sublining, and anti‐CD55 (clone 67; Serotec, Oxford, UK), which recognises fibroblast‐like synoviocytes. Staining was also done with mAbs against the proinflammatory cytokines TNFα (52B83; Monosan, Uden, The Netherlands) and IL1β (2D8; Immunokontact, Frankfurt, Germany). Staining was performed according to a three‐step immunoperoxidase method, as described previously.31 For control sections, the primary antibodies were omitted or irrelevant isotype‐matched antibodies were used. Sections stained were coded and randomly analysed by one blinded observer (MWvW) using digital image analysis, as described previously.32 Measurements for CD markers were expressed in cell counts/mm2 and for cytokines in integrated optical density/mm2.
Fluorescence‐activated cell sorting analysis
The expression of IL7Rα (CD127) on CD4 T cells and CD14 monocytes/macrophages from PB was analysed directly after isolation by fluorescence‐activated cell sorting (FACS) analysis. Cells were triple stained with CD4‐PE‐Cy5, CD14‐FITC (Dako) and CD127‐PE (Immunotech, Marseille, France). FITC/PE‐labelled isotype controls (Immunotech) were used for control staining.
For intracellular TNFα detection of co‐cultured CD4 and CD14 cells, FACS analysis was used. During the last 4 h of a 3‐day culture period in the presence or absence of IL7 (Peprotech, Rocky Hill, New Jersey, USA), cells were exposed to 10 μg/ml Brefeldin A (ICN Pharmaceuticals, Costa Mesa, California, USA) to block protein secretion and enhance intracellular cytokine staining. The cells were fixed and permeabilised with a fixation and permeabilisation kit, according to the manufacturer's instructions (Caltag Laboratories, Burlingame, California, USA). During fixation, cells were stained with fluorochrome‐labelled CD4 and CD14 surface antibodies. Fluorochrome‐labelled anti‐TNFα‐PE and isotype control antibody (R&D) were added during the permeabilisation step to stain TNFα intracellular. Fluorescence was analysed by FACS analysis. The mean fluorescence intensity of TNFα produced by CD4 and CD14 cells in the presence of IL7 was expressed as the percentage compared with cells in the absence of IL7.
Cell isolation
Heparinised PB or SF was diluted 1:1 with RPMI 1640 medium (Gibco BRL, Life Technologies, Mezelbeke, Belgium) containing penicillin (100 U/ml), streptomycin (100 μg/ml) and glutamine (2 mM). Mononuclear cells (MC) were isolated by density centrifugation using Ficoll‐Paque (Pharmacia, Uppsala, Sweden).
CD4 and CD14 cells were isolated from peripheral blood mononuclear cells through negative selection by means of microbead‐activated cell sorting as described previously.25
Cell cultures
To determine proliferation of synovial fluid mononuclear cells (SFMC), these cells were cultured in 96‐well plates (1×106 cells/ml; 20 μl/well) for 3 days. The cells were cultured in RPMI supplemented with penicillin, streptomycin, glutamine and 10% pooled fetal calf serum (Gibco BRL) in the presence or absence of IL7 (Preprotech), in the presence or absence of anti‐TNFα (cA2, 10 μg/ml, Centocor, Malvern, Pennsylvania, USA). Proliferation was measured as described previously.25
To analyse cytokine production, isolated cells (0.5×106 cells/ml; 1 ml/well) were also cultured in 24‐well plates. To study the influence of direct cell–cell contact, CD4 and CD14 cells were co‐cultured for 3 days in these 24‐well plates in the presence or absence of a transwell (6.5 mm, 0.4 μm pore size, Corning, New York, USA) and with or without IL7. CD14 cells were placed in the lower compartment and CD4 cells in the upper compartment, preventing direct cell–cell contact between the two cell fractions, but allowing effects mediated via soluble factors.
Statistical analysis
Statistical analysis of paired evaluations was performed using the non‐parametric Wilcoxon signed ranks test. Correlation analysis between the numbers of IL7 cells and inflammatory markers was done by Spearman's correlation analysis for non‐parametric data and Pearson's correlation analysis for parametric data. For all analyses data were considered statistically significant at p<0.05.
Results
IL7 stimulates T cell‐dependent TNFα production by monocytes/macrophages
Since monocytes/macrophages are major producers of TNFα, we investigated how IL7 influences TNFα production by monocytes and compared this with production by CD4 T cells.
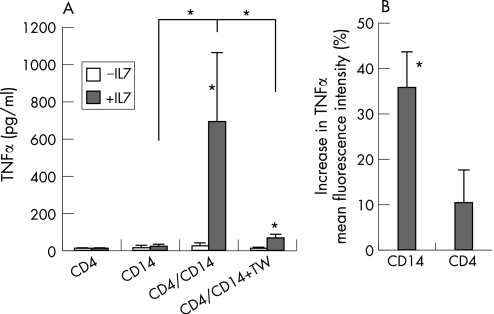
IL7 did not stimulate TNFα production of CD14 monocytes/macrophages or CD4 T cells cultured alone (fig 1A). However, IL7 did stimulate TNFα production when monocytes/macrophages were co‐cultured with CD4 T cells. To measure the specificity of this TNFα induction, IL1β was also measured. In all cases, IL1β levels stayed below the detection limit.
Figure1 Interleukin (IL)7 induces contact‐dependent tumour necrosis factor α (TNFα) production primarily by CD14 monocytes/macrophages co‐cultured with CD4 T cells. (A) CD4 (5×105/ml) and CD14 (5×105/ml) cells from patients with rheumatoid arthritis (RA) (n = 6) were exposed to IL7 (10 ng/ml) when cultured alone for 3 days or when co‐cultured in the absence or the presence of a transwell (TW) to prevent cell contact. IL7 did not significantly stimulate TNFα production by separated CD4 or CD14 cultures, but did significantly increase TNFα production in the IL7‐stimulated co‐culture. (B) Mean fluorescence intensity of TNFα produced by co‐cultured CD4 and CD14 cells in the presence of IL7 was measured by fluorescence‐activated cell sorting analysis. IL7‐induced TNFα was expressed as a percentage of co‐cultured cells in the absence of IL7. Data are means (SEM) of three patients with RA. *p<0.05.
Interruption of direct cell–cell contact (by use of a semipermeable membrane in a transwell culture system) almost completely prevented IL7‐stimulated TNFα production (fig 1A). TNFα production was associated with T cell activation (measured by proliferation and major histocompatibility complex class II expression) and monocyte activation (measured by CD40 and CD80 induction) (data not shown).
To detect whether CD4 T cells or CD14 monocytes/macrophages produced TNFα, intracellular TNFα was measured (by FACS analysis, fig 1B). In patients with RA who secreted high TNFα levels in the IL7‐stimulated co‐cultures, we detected a consistent and significant increase in TNFα production by CD14 monocytes in all individuals (35.9 (8.2%), expressed as percentage vs unstimulated co‐cultures, p<0.05), whereas TNFα production by CD4 T cells was not significantly altered (10.1 (8.9%) vs unstimulated co‐cultures).
IL7 levels correlate with TNFα levels in RA synovial fluid and tissue
Because of the stimulation of TNFα production by IL7, we investigated the association of IL7 and TNFα in the joints of patients with RA.
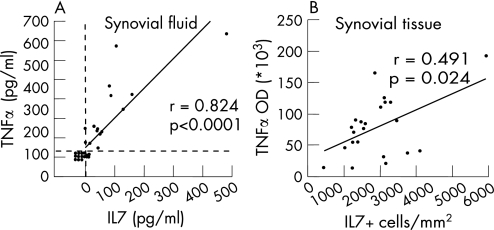
IL7 and TNFα levels in the SF of patients with RA significantly correlated with each other (n = 30, fig 2A). In addition, the relationship between expression of IL7 on the one hand and TNFα, IL1β, numbers of inflammatory cells and adhesion molecule expression on the other hand was investigated in RA synovial tissue. Numbers of IL7+ cells correlated significantly with TNFα (fig 2B) but not with IL1β (table 2). Furthermore, the number of IL7+ cells correlated significantly with numbers of CD68 cells and the expression of E‐selectin (table 2). The expression of IL7 did not correlate significantly with expression of either intercellular adhesion molecule 1 or vascular cell adhesion molecule 1, or with numbers of CD3, CD4, and CD8 T cells, or with CD22 B cells.
Figure 2 Significant correlation between interleukin (IL)7 and tumour necrosis factor α (TNFα) levels in the synovial fluid (SF) and synovial tissue of patients with rheumatoid arthritis (RA). Table 1 shows the characteristics of the patients with RA. (A) In RA, SF (n = 30) levels of IL7 and TNFα levels were measured using ELISA. (B) Numbers of IL7 cells (average of the number cells/mm2 tissue area) and TNFα expression levels (optical density (OD)) in synovial tissue of patients with RA (n = 22, one missing value) were assessed immunohistologically. Spearman's correlation coefficient r and p value are given.
Table 2 Correlation between synovial tissue interleukin (IL)7 expression and cytokines, inflammatory cells and adhesion molecules.
| r | p | |
|---|---|---|
| TNFα | 0.493 | 0.024* |
| IL1β | −0.244 | 0.262 |
| CD68 | 0.621 | <0.001* |
| CD3 | 0.117 | 0.596 |
| CD4 | 0.266 | 0.219 |
| CD8 | 0.227 | 0.298 |
| CD22 | −0.017 | 0.939 |
| ICAM‐1 | 0.108 | 0.623 |
| VCAM‐1 | 0.207 | 0.344 |
| E‐selectin | 0.430 | 0.040* |
ICAM, intercellular adhesion molecule; TNF, tumour necrosis factor; VCAM, vascular cell adhesion molecule.
*Statistically significant correlations.
Persistent IL7‐induced lymphocyte responses on TNFα blockade
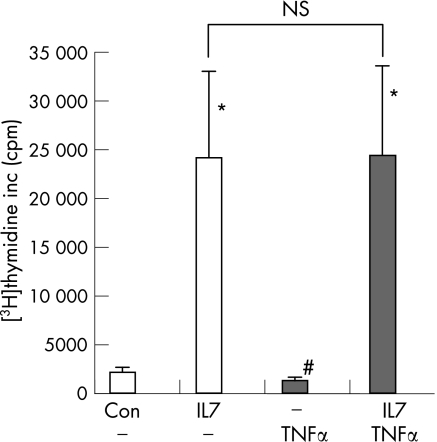
To investigate to what extent IL7‐stimulated responses are TNFα‐dependent, we tested whether IL7‐induced activity of MC was prevented by TNFα blockade. Although spontaneous proliferation was reduced significantly by anti‐TNFα treatment (from 2059 (498) to 1276 (341), p<0.05, fig 3), IL7‐induced proliferation of SFMC was not blocked by anti‐TNFα mAb treatment (fig 3). Similar to cells from the SF, IL7 stimulated proliferation of MC from the peripheral blood (n = 3, from 372 (115) to 2881 (1262)). Also, this proliferation was not significantly inhibited by TNFα blockade (on average with 31%, to 1974 (677)).
Figure 3 Tumour necrosis factor α (TNFα) blockade in vitro does not prevent interleukin (IL)7‐induced proliferation of synovial fluid mononuclear cells from patients with rheumatoid arthritis (n = 5). Mononuclear cells (5×105/ml) were cultured in the presence of IL7 (10 ng/ml), anti‐TNFα momoclonal antibodies (cA2, 10 μg/ml, black bars) or the combination of both. Lymphocyte proliferation was measured by [3H]‐thymidine incorporation. * and # indicate a statistically significant increase or decrease, respectively, of p<0.05 compared to control cultures. NS = not significant.
Persistent IL7 levels on anti‐TNFα mAb treatment in non‐responding patients
Apart from the TNFα‐independent induction of proinflammatory activity by IL7 in vitro, it was investigated whether IL7 persisted in patients with RA who were treated with anti‐TNFα mAb. This is of particular interest as previously TNFα was shown to stimulate IL7 production (of RA fibroblasts) in vitro.27
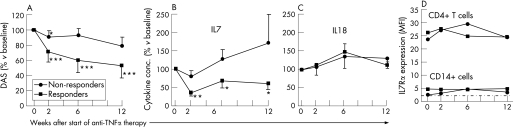
According to the European League Against Rheumatism response criteria,33 7 non‐responders and 15 moderate/good responders were identified (disease activity scores (DAS) are shown in fig 4A). In responders, IL7 levels significantly decreased upon anti‐TNFα treatment (at all time points after the start of treatment). However, in non‐responders, IL7 levels did not change significantly on treatment (fig 4B). After 2 weeks of treatment, the change in IL7 levels significantly correlated with the change in erythrocyte sedimentation rate (ESR) and DAS (r = 0.633, p<0.01; r = 0.438, p<0.05, respectively). These correlations were not observed at weeks 6 and 12.
Figure 4 Anti‐tumour necrosis factor α (TNFα) monoclonal antibody treatment (adalimumab, 40 mg subcutaneous, every other week) reduces serum interleukin (IL)7 levels in clinical responders, whereas in clinical non‐responders IL7 levels persist. IL7Rα expression levels on T cells and monocytes/macrophages in responders were not significantly changed. (A) Based on the European League Against Rheumatism (EULAR) response criteria, patients were judged as non‐responders (n = 7) and responders (n = 15). As expected, in responders, a strong suppression of disease activity score (DAS) was observed, which was significantly different from that in non‐responders, who showed only modest changes in disease activity. The average absolute DAS score at baseline did not differ significantly between responders and non‐responders (mean (SD), 6.7 (1) vs 5.8 (1.3), respectively). (B) Serum IL7 levels in responders at 2, 6 and 12 weeks significantly decreased on anti‐TNFα treatment compared with those at baseline (100%). Non‐responders did not show significant changes in IL7 levels compared with those at baseline. The difference in percentage change of IL7 levels between responders and non‐responders was significantly different at 2 and 6 weeks after the start of treatment. Baseline IL7 values between anti‐TNFα responders and non‐responders were not significantly different (14.9 (13.6) and 13.2 (10.7) pg/ml, respectively). (C) Serum IL18 levels in responders at 2, 6 and 12 weeks did not significantly alter on anti‐TNFα treatment compared with those at baseline (100%). Baseline IL18 values between anti‐TNFα responders and non‐responders were not significantly different (358 (300) and 557 (487) pg/ml, respectively). (D) IL7Rα expression levels (mean fluorescence intensity (MFI)) on CD4 T cells and CD14 monocytes/macrophages on treatment. No significant change in IL7Rα expression on T cells was observed. The modest IL7Rα expression on monocytes (compared to isotype control, indicated by the dashed line) was only slightly increased in non‐responders after 2 and 6 weeks of anti‐TNFα treatment (both p<0.05). *, ** and *** indicate statistically significant differences of DAS and IL7 compared with those at baseline of p<0.05, p<0.01 and p<0.001, respectively.
As IL7 correlated with disease activity, we tested whether mere reduction of disease activity caused a generalised decrease of inflammatory cytokines, in particular those with capacity to induce TNFα, such as IL1534 and IL18.35 In contrast with IL7, there were insignificant changes in IL18 levels on anti‐TNFα treatment in both non‐responders and responders (fig 4C). Serum levels of IL15 were undetectable in all patients and were not affected by anti‐TNFα treatment.
As TNFα has been reported to decrease IL7Rα expression,36 it was tested whether anti‐TNFα also affected IL7Rα expression. The expression of this receptor was measured on circulating CD4 T cells and CD14 monocytes/macrophages before and after treatment. The IL7Rα expression was much higher on CD4 T cells than on monocytes/macrophages (MFI 25 (2) vs 3 (0.5), respectively, fig 4D). Although levels increased on CD4 T cells in non‐responding patients, anti‐TNFα treatment both in responders and non‐responders did not significantly affect IL7Rα expression on CD4 T cells (fig 4D, upper lines). IL7Rα expression on monocytes in responders and non‐responders was not significantly changed after 2 weeks of treatment. In non‐responders, the receptor level was statistically significantly increased after 6 and 12 weeks of treatment, although the effect was marginal (fig 4D, lower lines).
Discussion
T cell activation has been observed to induce IL7 secretion by dendritic cells. Blockade of IL7 in these cultures prevented T cell activation.37,38 Recently, we have found that maturation of RA dendritic cells in vitro by activation through Toll‐like receptors is associated with significantly increased IL7 protein levels. Although the exact triggers for the IL7 production that induce inflammation in RA are unknown, recently the cell types producing IL7 have been identified. Apart from fibroblasts25,27 and endothelial cells, professional antigen‐presenting cells such as macrophages and dendritic‐like cells also produce IL7 in RA synovial tissue.25,38 The present study demonstrates that IL7 stimulates the production of TNFα by monocytes requiring cell contact with CD4 T cells, a mechanism that has been recognised to be crucial in RA.13,14,15 Furthermore, in RA, SF and tissue IL7 expression correlates with expression of TNFα. Apart from inducing TNFα, IL7 can induce proinflammatory activity that persists on blockade of TNFα. The most important finding is that non‐responsiveness to anti‐TNFα treatment is related to persistent IL7 levels.
Previously, a high concentration of IL7 (100 ng/ml) was shown to induce cytokine secretion (including TNFα) by isolated monocytes from healthy controls.21 Lower concentrations (⩽10 ng/ml) did not induce TNFα secretion. The present data are in line with this study, indicating that RA monocytes/macrophages when cultured separately cannot be stimulated by IL7 to induce TNFα secretion in a concentration up to 10 ng/ml. However, in the presence of CD4 T cells, this lower IL7 concentration induces high amounts of TNFα production. The above‐described T cell contact‐dependent effects may be related to the expression of IL7Rα primarily on CD4 T cells, in contrast with monocytes that lack IL7Rα expression. This indicates that IL7 (produced by cells such as antigen‐presenting cells) may primarily act on T cells to induce T cell contact‐dependent activation of other cell types such as monocytes. This mechanism of action could also occur in RA joints, since synovial CD4 T cells and macrophages from the SF show similar IL7Rα expression patterns as their circulating counterparts (data not shown).
The correlation of IL7 and TNFα expression in RA joints may be due to the capacity of IL7 to induce TNFα (this study,24) or, vice versa, due to the capacity of TNFα to induce IL7.27 Alternatively, common or distinct triggers may induce both IL7 and/or TNFα, independent of the mutual action of both cytokines. Our data show that in a substantial proportion of patients TNFα blockade results in a decrease of serum IL7. This reduction may be due to the prevention of a direct effect of TNFα on several cell types to produce IL7. Reduction of IL7 in case of anti‐TNFα treatment may subsequently contribute to reduction of inflammation and disease activity. In our study, a reduction in IL7 levels correlated with a reduction in disease parameters (ESR, DAS) after 2 weeks of treatment. Based on these data, it is suggested that downregulation of IL7 by anti‐TNFα may contribute to disease inhibition. In addition, considering the potent proinflammatory effects of IL7, it is indicated that insufficient IL7 reduction could contribute to persistent disease activity in a substantial number of patients.
Although anti‐TNFα treatment downregulates circulating IL7 levels, it can be questioned whether anti‐TNFα treatment leads to sufficient suppression of IL7 expression at the site of inflammation. Previously we have reported increased IL7 levels in the SF of patients with RA who were using anti‐TNFα drugs when compared with patients who were not treated with TNFα blocking agents.25 Since circulating levels of IL7 may have different sources other than the joint (eg, lymphopoietic sites), there may be dissociation between intra‐articular and circulating IL7 levels. Persistent local IL7 could thus mediate persisting and residual inflammation. Measurement of IL7 in the synovial tissue of anti‐TNFα‐treated patients in a controlled study will be needed to demonstrate whether indeed IL7 production persists in RA joints on anti‐TNFα treatment.
The persistence of serum IL7 levels in patients who do not respond clinically to anti‐TNFα treatment is an interesting observation. In these patients, IL7 production seems to be induced by a TNFα‐independent pathway. As shown in the present study, IL7 may subsequently induce proinflammatory responses that are also TNFα independent. IL7‐driven pathways may be present both in responding and non‐responding patients, explaining either the partial responses or the lack of response to anti‐TNFα treatment. Apart from patients with RA, increased IL7 levels are found in the circulation or at the inflammatory site of several other (auto) immune‐mediated diseases, such as psoriasis and JIA.22,39 Since in these diseases anti‐TNFα treatment is used as an anti‐inflammatory drug and IL7 may be an important proinflammatory mediator, detailed analysis of the role of IL7 in the immunopathogenesis of RA and these diseases may lead to novel treatment strategies.
Acknowledgements
We thank Dr Nazira Jahangier and Dr Andre van Rijthoven for providing patient material, and Dr D Fitzpatrick and Dr C Willis (Amgen) for critical reading of the manuscript. The Dutch Arthritis Association and Amgen contributed financially to this work.
Abbreviations
FACS - fluorescence‐activated cell sorting
IL - interleukin
JIA - juvenile idiopathic arthritis
mAbs - monoclonal antibodies
MC - mononuclear cells
PB - peripheral blood
RA - rheumatoid arthritis
SF - synovial fluid
SFMC - synovial fluid mononuclear cells
TNFα - tumour necrosis factor α
Footnotes
Competing interests: None declared.
References
- 1.Feldmann M, Brennan F M, Maini R N. Rheumatoid arthritis. Cell 199685307–310. [DOI] [PubMed] [Google Scholar]
- 2.Burmester G R, Stuhlmuller B, Keyszer G, Kinne R W. Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? Arthritis Rheum 1997405–18. [DOI] [PubMed] [Google Scholar]
- 3.Kremer J M, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S.et al Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. N Engl J Med 20033491907–1915. [DOI] [PubMed] [Google Scholar]
- 4.Morita Y, Yamamura M, Kawashima M, Harada S, Tsuji K, Shibuya K.et al Flow cytometric single‐cell analysis of cytokine production by CD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum 1998411669–1676. [DOI] [PubMed] [Google Scholar]
- 5.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum 199639115–124. [DOI] [PubMed] [Google Scholar]
- 6.Tak P P, Smeets T J, Daha M R, Kluin P M, Meijers K A, Brand R.et al Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum 199740217–225. [DOI] [PubMed] [Google Scholar]
- 7.Firestein G S, Alvaro‐Gracia J M, Maki R, Alvaro‐Garcia J M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol 19901443347–3353. [PubMed] [Google Scholar]
- 8.Feldmann M, Maini R N. Anti‐TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 200119163–196. [DOI] [PubMed] [Google Scholar]
- 9.Paleolog E M, Hunt M, Elliott M J, Feldmann M, Maini R N, Woody J N. Deactivation of vascular endothelium by monoclonal anti‐tumor necrosis factor alpha antibody in rheumatoid arthritis. Arthritis Rheum 1996391082–1091. [DOI] [PubMed] [Google Scholar]
- 10.To S S, Newman P M, Hyland V J, Robinson B G, Schrieber L. Regulation of adhesion molecule expression by human synovial microvascular endothelial cells in vitro. Arthritis Rheum 199639467–477. [DOI] [PubMed] [Google Scholar]
- 11.Firestein G S. Invasive fibroblast‐like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum 1996391781–1790. [DOI] [PubMed] [Google Scholar]
- 12.Romas E, Gillespie M T, Martin T J. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor‐alpha in bone destruction in rheumatoid arthritis. Bone 200230340–346. [DOI] [PubMed] [Google Scholar]
- 13.Li J M, Isler P, Dayer J M, Burger D. Contact‐dependent stimulation of monocytic cells and neutrophils by stimulated human T‐cell clones. Immunology 199584571–576. [PMC free article] [PubMed] [Google Scholar]
- 14.Lacraz S, Isler P, Vey E, Welgus H G, Dayer J M. Direct contact between T lymphocytes and monocytes is a major pathway for induction of metalloproteinase expression. J Biol Chem 199426922027–22033. [PubMed] [Google Scholar]
- 15.Vey E, Burger D, Dayer J M. Expression and cleavage of tumor necrosis factor‐alpha and tumor necrosis factor receptors by human monocytic cell lines upon direct contact with stimulated T cells. Eur J Immunol 1996262404–2409. [DOI] [PubMed] [Google Scholar]
- 16.Olsen N J, Stein C M. New drugs for rheumatoid arthritis. N Engl J Med 20043502167–2179. [DOI] [PubMed] [Google Scholar]
- 17.Moreland L W, Schiff M H, Baumgartner S W, Tindall E A, Fleischmann R M, Bulpitt K J.et al Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med 1999130478–486. [DOI] [PubMed] [Google Scholar]
- 18.van de Putte L B, Atkins C, Malaise M, Sany J, Russell A S, van Riel P L.et al Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis 200463508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soumelis V, Reche P A, Kanzler H, Yuan W, Edward G, Homey B.et al Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 20023673–680. [DOI] [PubMed] [Google Scholar]
- 20.Fry T J, Mackall C L. Interleukin‐7: from bench to clinic. Blood 2002993892–3904. [DOI] [PubMed] [Google Scholar]
- 21.Alderson M R, Tough T W, Ziegler S F, Grabstein K H. Interleukin 7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J Exp Med 1991173923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Benedetti F, Massa M, Pignatti P, Kelley M, Faltynek C R, Martini A. Elevated circulating interleukin‐7 levels in patients with systemic juvenile rheumatoid arthritis. J Rheumatol 1995221581–1585. [PubMed] [Google Scholar]
- 23.Stabler T, Piette J C, Chevalier X, Marini‐Portugal A, Kraus V B. Serum cytokine profiles in relapsing polychondritis suggest monocyte/macrophage activation. Arthritis Rheum 2004503663–3667. [DOI] [PubMed] [Google Scholar]
- 24.van Roon J A, Glaudemans K A, Bijlsma J W, Lafeber F P. Interleukin 7 stimulates tumour necrosis factor alpha and Th1 cytokine production in joints of patients with rheumatoid arthritis. Ann Rheum Dis 200362113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Roon J A, Verweij M C, Wijk M W, Jacobs K M, Bijlsma J W, Lafeber F P. Increased intraarticular interleukin‐7 in rheumatoid arthritis patients stimulates cell contact‐dependent activation of CD4(+) T cells and macrophages. Arthritis Rheum 2005521700–1710. [DOI] [PubMed] [Google Scholar]
- 26.Ruprecht C R, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A.et al Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med 20052011793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harada S, Yamamura M, Okamoto H, Morita Y, Kawashima M, Aita T.et al Production of interleukin‐7 and interleukin‐15 by fibroblast‐like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum 1999421508–1516. [DOI] [PubMed] [Google Scholar]
- 28.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 29.Clarke D, Katoh O, Gibbs R V, Griffiths S D, Gordon M Y. Interaction of interleukin 7 (IL‐7) with glycosaminoglycans and its biological relevance. Cytokine 19957325–330. [DOI] [PubMed] [Google Scholar]
- 30.Jahangier Z N, Jacobs J W, Kraan M C, Wenting M J, Smeets T J, Bijlsma J W.et al Pre‐treatment macrophage infiltration of the synovium predicts the clinical effect of both radiation synovectomy and intra‐articular glucocorticoids. Ann Rheum Dis 2006651286–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tak P P, van der Lubbe P A, Cauli A, Daha M R, Smeets T J, Kluin P M.et al Reduction of synovial inflammation after anti‐CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum 1995381457–1465. [DOI] [PubMed] [Google Scholar]
- 32.Haringman J J, Vinkenoog M, Gerlag D M, Smeets T J, Zwinderman A H, Tak P P. Reliability of computerized image analysis for the evaluation of serial synovial biopsies in randomized controlled trials in rheumatoid arthritis. Arthritis Res Ther 20057R862–R867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Gestel A M, Prevoo M L, 't Hof M A, van Rijswijk M H, van de Putte L B, van Riel P L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 19963934–40. [DOI] [PubMed] [Google Scholar]
- 34.McInnes I B, Leung B P, Sturrock R D, Field M, Liew F Y. Interleukin‐15 mediates T cell‐dependent regulation of tumor necrosis factor‐alpha production in rheumatoid arthritis. Nat Med 19973189–195. [DOI] [PubMed] [Google Scholar]
- 35.Dai S M, Matsuno H, Nakamura H, Nishioka K, Yudoh K. Interleukin‐18 enhances monocyte tumor necrosis factor alpha and interleukin‐1beta production induced by direct contact with T lymphocytes: implications in rheumatoid arthritis. Arthritis Rheum 200450432–443. [DOI] [PubMed] [Google Scholar]
- 36.Park J H, Yu Q, Erman B, Appelbaum J S, Montoya‐Durango D, Grimes H L.et al Suppression of IL7Ralpha transcription by IL‐7 and other prosurvival cytokines: a novel mechanism for maximizing IL‐7‐dependent T cell survival. Immunity 200421289–302. [DOI] [PubMed] [Google Scholar]
- 37.Vasir B, Avigan D, Wu Z, Crawford K, Turnquist S, Ren J.et al Dendritic cells induce MUC1 expression and polarization on human T cells by an IL‐7‐dependent mechanism. J Immunol 20051742376–2386. [DOI] [PubMed] [Google Scholar]
- 38.van Roon J A, van Rossum S, Wenting‐van Wijk M, Bijlsma J W, Lafeber F P. IL‐7 blockade inhibits maturation of functional antigen‐presenting cells from healthy controls and ra patients. Ann Rheum Dis 200665(Suppl II)467 [Google Scholar]
- 39.Bonifati C, Trento E, Cordiali‐Fei P, Carducci M, Mussi A, D'Auria L.et al Increased interleukin‐7 concentrations in lesional skin and in the sera of patients with plaque‐type psoriasis. Clin Immunol Immunopathol 19978341–44. [DOI] [PubMed] [Google Scholar]