Abstract
Objective
To examine the predictors of the occurrence of hypertension in a large multiethnic US cohort.
Patients and methods
There were 614 patients with systemic lupus erythematoses (SLE; ⩾4 American College of Rheumatology revised criteria) with ⩽5 years of disease duration at entry into the cohort (T0) and of Hispanic (Texan or Puerto Rican), African–American or Caucasian ethnicity. T0 variables were compared between patients who did and did not develop hypertension (blood pressure ⩾140/90 mm Hg on at least two occasions and/or the use of antihypertensive drugs) after T0. Significant and clinically relevant variables were then examined by a stepwise logistic regression model.
Results
A total of 379 patients without hypertension at T0 were included (patients who developed hypertension prior to SLE diagnosis (n = 126) or before T0 (n = 109) were excluded). Predictors of hypertension were African–American and Texan–Hispanic ethnicities, renal involvement and a higher body mass index.
Conclusions
Traditional cardiovascular risk factors, disease‐related factors and ethnicity play a role in the occurrence of hypertension in patients with SLE. Controlling renal involvement and optimising body weight may prevent the occurrence of hypertension.
It has been well established that systemic lupus erythematosus (SLE) is associated with an increased risk of cardiovascular disease; compared with the general population, women with SLE have a 5–6‐fold increased risk of coronary heart disease and the risk in women aged 35–44 years is approximately 50 times higher.1 As early as 1976, cardiovascular disease was recognised as a major cause of death, particularly late in the course of SLE.2,3 Both traditional (age, hypertension, hypercholesterolaemia, obesity, tobacco use and diabetes mellitus) and SLE‐related (disease duration, increased C reactive protein levels, the presence of antiphospholipid (aPL) antibodies and glucocorticoid use) factors have been identified as contributors to the occurrence of premature atherosclerosis in SLE.1,4,5,6
According to data from the National Health and Nutrition Examination Survey IV, the prevalence of hypertension in the general US population was 15% in 1999–2000.7 However, hypertension occurs more frequently in patients with SLE than in the general population as evidenced by data from the Hopkins Lupus Cohort (41% and 46% in 1992 and 2000, respectively),4,8 the Toronto Risk Factor Study (33% vs 13% in age‐matched controls)9 and a study from Vanderbilt University (48% vs 25% in age, gender and ethnic‐matched controls).10
Hypertension is a well‐established risk factor for cardiovascular mortality11 and it has been shown to be a continuous, consistent risk for cardiovascular disease, independent of other risk factors.12 The association between hypertension and cardiovascular disease, both clinically and subclinically, in SLE had also been confirmed by several studies.4,5,8,9,13,14,15,16
Given the importance of hypertension as a risk factor for cardiovascular morbidity, and possibly mortality, in patients with SLE17 and its frequent occurrence in these patients, we used the large and comprehensive LUMINA (Lupus in Minorities: Nature vs Nurture) database to study the factors predictive of the occurrence of hypertension in SLE. We hypothesised that, in addition to known risk factors for hypertension, disease‐related risk factors and possibly ethnicity play a role in the occurrence of hypertension in these patients.
Patients and methods
LUMINA is a multiethnic (Hispanic, Caucasian and African–American) longitudinal study of lupus outcome being conducted in three medical centres in the US (the University of Alabama, the University of Texas; Houston and the University of Puerto Rico) and their affiliated practices. Patients with SLE as per the revised and updated American College of Rheumatology (ACR) criteria18 with ⩽5 years of disease duration at entry into the cohort (T0) and of well‐defined ethnicity are eligible to participate in LUMINA. Visits are conducted every 6 months during the first year and yearly thereafter. The other time points that are referred to in this article are the time patients meet four ACR criteria for SLE (TD) and the time of last visit (TL). For those patients who did not develop hypertension, total disease duration was defined as the interval between TD and TL whereas follow‐up time was defined as the interval between T0 and TL. For patients who developed hypertension, the end point was not TL but the visit in which hypertension first occurred.
The LUMINA study conforms to the guidelines of the Declaration of Helsinki for the use of human subjects in research and was approved at the three participating institutions; all patients gave written informed consent. Details relative to the constitution of this cohort and its main features have been described previously.19,20,21,22
Variables
The LUMINA database includes variables from the following domains: socioeconomic–demographic, clinical, immunological, genetic, behavioural and psychological. These variables were measured at T0 and at the follow‐up visits. Only the variables included in these analyses will be described:
Socioeconomic–demographic variables: age, gender, ethnicity, smoking, years of education, health insurance status and poverty (as defined by the US Federal Government, adjusted for the number of subjects in the household).23
Clinical variables: disease activity ascertained using the Systemic Lupus Activity Measure Revised (SLAM‐R),24 damage accrual ascertained using the Systemic Lupus International Collaborating Clinics Damage Index (SDI),25 disease onset type (defined as acute if time to the accrual of four ACR criteria is <4 weeks and insidious if otherwise), body mass index (BMI) calculated from the patients' height and weight, and diabetes mellitus (self‐reported and/or physician‐diagnosed and/or the use of oral hypoglycemic agents or insulin). Renal involvement was defined as nephritis by histopathology (World Health Organization class II or higher and/or >2+ proteinuria).
Laboratory variables: levels of serum creatinine recorded at T0, non‐fasting low‐density lipoprotein (LDL) cholesterol (high if >130 mg/dl) and triglycerides (high if >205 mg/dl). Autoantibodies included are antinuclear antibodies (by immunofluorescence using HEp‐2 cell line as a substrate), anti double‐stranded‐DNA (ds DNA; by immunofluorescence against Crithidia luciliae)26 and aPL antibodies (IgG and/or IgM aPL antibodies by ELISA technique27 and the lupus anticoagulant by Staclot test (Diagnostica Stago, Asnieres‐sur‐Seine, France)).28
Medication use: from T0 to TL in patients who did not have hypertension and from T0 to the visit in which hypertension occurred in those who developed hypertension: non‐steroidal anti‐inflammatory drugs, hydroxychloroquine, low‐dose aspirin and glucocorticoids. The use of glucocorticoids was recorded as current or past and also as the weighted average dose of prednisone equivalent used up to T0.
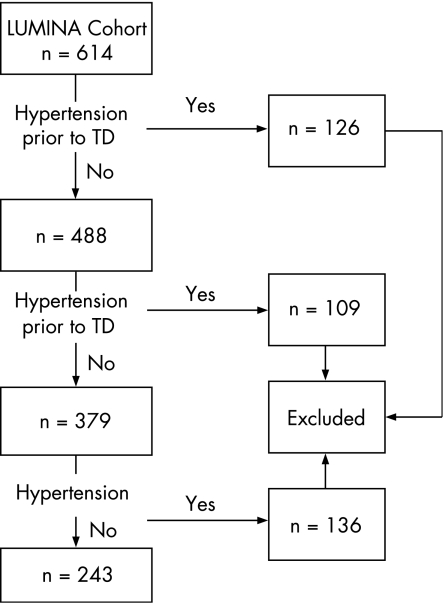
Dependent variable: Hypertension (regardless of the cause) was defined as a systolic blood pressure of 140 mm Hg and/or a diastolic blood pressure of 90 mm Hg on at least two occasions and/or patient's self‐reported use antihypertensive drugs. Patients who were taking antihypertensive drugs for other indications such as proteinuria or Raynaud's symptoms were not included. As noted in fig 1, only patients who developed hypertension after T0 (n = 136) were included in these analyses; those who developed hypertension prior to TD (n = 126) or T0 (n = 109) were not included (n = 235).
Figure 1 Flow diagram of Lupus in Minorities. Nature vs Nurture (LUMINA) cohort of patients studied. TD, time of systemic lupus erythematosus diagnosis.
Statistical analysis
T0 variables (except for disease activity in which the average of all SLAM‐R scores were used and damage accrual in which the first available SDI score for patients with disease duration ⩽6 months at T0 was used) were compared between patients with and without hypertension by using descriptive statistical tests, χ2 test for categorical (and Fisher's exact test, if appropriate) and Student's t test for continuous variables. For both SLAM‐R and SDI, the scores from the renal domain were excluded; for SLAM‐R, hypertension was also excluded. The association between hypertension and those variables significant at a p value ⩽0.10 in the univariable analyses and those felt to be clinically relevant, regardless of their level of significance, were further examined by multivariable logistic regression; first, domain‐specific regression: demographic (age, gender and ethnicity), socioeconomic (poverty, years of education, insurance status, smoking, drinking and using recreational drugs) and clinical (SLAM‐R, renal involvement, BMI, diabetes mellitus and LDL cholesterol, anti‐dsDNA and aPL antibodies and current or past use of glucocorticoids) was performed. Then, a combined regression in which variables significant in the domain‐specific regressions was examined. All analyses were performed using SPSS V.14.0 or SAS V.8.1.
Results
Out of a total of 614 patients, 488 patients were studied: of them, 90% were women; 99 (20%) were Texan Hispanics, 85 (17%) were Puerto Rican Hispanics, 169 (35%) were African–Americans and 135 (28%) were Caucasians. The patients' mean (SD) age at T0 was 34.9 (11.7) years, the mean (SD) total disease duration (TD–TL) was 5.4 (3.6) years and the mean (SD) total follow‐up time (T0–TL) was 3.9 (3.2) years and the number of follow‐up visits was 5.1 (3.0) years; the estimated rate of loss to follow‐up in the LUMINA cohort is 29%.29 Of the 243 (50%) patients who developed hypertension after TD, 136 (56%) patients developed it after T0 and are included in these analyses.
Univariable analyses
Table 1 depicts the main socioeconomic–demographic features for those patients who developed hypertension after T0 and those who did not. Patients who developed hypertension were more likely to be African–American and Hispanic Texan, below the poverty line and less educated; however, they were less likely to have health insurance or to drink alcoholic beverages as compared with those who did not develop hypertension; the proportion of smokers was comparable in both groups.
Table 1 Socioeconomic–demographic features of patients in the LUMINA cohort as a function of the occurrence of hypertension any time after enrollment into the LUMINA cohort.
| Variables | Hypertension | p Value | |
|---|---|---|---|
| Present, n = 136 | Absent, n = 243 | ||
| Mean (SD) age, years | 34.3 (11.5) | 35.3 (12.0) | 0.463 |
| Gender, % women | 90 | 92 | 0.412 |
| Ethnicity (%) | |||
| Hispanic Texan | 24 | 15 | |
| Puerto Rican Hispanic | 9 | 26 | <0.001 |
| African–American | 45 | 25 | |
| Caucasian | 22 | 35 | |
| Mean (SD) education, years | 12.7 (3.0) | 13.5 (3.0) | 0.006 |
| Below poverty level (%) | 40 | 26 | 0.006 |
| Health insurance (%) | 82 | 74 | 0.058 |
| Smoking (%) | 11 | 14 | 0.463 |
| Drinking (%) | 6 | 12 | 0.055 |
Table 2 depicts the clinical and laboratory features. Patients who developed hypertension were more likely to develop renal involvement more frequently, to experience higher disease activity over time and to have accrued more damage, a higher BMI and diabetes mellitus.
Table 2 Clinical and laboratory features of patients in the LUMINA cohort as a function of the occurrence of hypertension any time after enrollment into the LUMINA cohort.
| Variables | Hypertension | p Value | |
|---|---|---|---|
| Present, (n = 136) | Absent, (n = 243) | ||
| Acute onset type (%) | 84 | 87 | 0.285 |
| Mean (SD) follow‐up time, (years) | 2.6 (2.4) | 3.0 (2.5) | 0.112 |
| Mean (SD), total disease duration, (years) | 4.1 (2.9) | 4.2 (3.0) | 0.716 |
| Mean (SD) disease activity* | 8.0 (3.8) | 7.1 (4.2) | 0.046 |
| Mean (SD) damage accrual† | 0.6 (0.9) | 0.5 (1.0) | 0.337 |
| Renal involvement (%) | 62 | 30 | <0.001 |
| Diabetes mellitus (%) | 6 | 2 | 0.032 |
| Body mass index (kg/m2) | 26.4 (4.8) | 25.3 (4.5) | 0.026 |
| Mean (SD) baseline serum creatinine, (mg/dl) | 0.7 (0.4) | 0.7 (0.3) | 0.307 |
| Mean (SD) LDL cholesterol, (mg/dl) | 99 (38) | 94 (39) | 0.193 |
| LDL cholesterol >130 mg/dl (%) | 20 | 12 | 0.061 |
| Mean (SD) triglyceride, (mg/dl) | 133 (69) | 122 (66) | 0.152 |
| Mean (SD) high sensitivity‐ C reactive protein | 13.3 (21.9) | 12.2 (30.3) | 0.712 |
| Anti‐dsDNA antibodies (%) | 57 | 47 | 0.070 |
| Antiphospholipid antibodies (%) | 34 | 22 | 0.014 |
| Low‐dose aspirin use (%) | 11 | 17 | 0.102 |
| Use of NSAID (%) | 56 | 57 | 0.864 |
| Hydroxychloroquine use (%) | 85 | 86 | 0.762 |
| Glucocorticoid use (%) | 96 | 89 | 0.035 |
| Weighted average glucocorticoid dose (in mg of prednisone/day) | 7.1 (10.3) | 6.7 (11.7) | 0.738 |
LDL, low‐density lipoprotein; NSAID, non‐steroidal anti‐inflammatory drugs.
*As per the Systemic Lupus Activity Measure‐Revised over time, scores from the renal domain and hypertension were excluded.
†As per the Systemic Lupus International Collaborating Clinics Damage Index, scores from the renal domain were excluded.
Anti‐dsDNA antibodies and aPL antibodies were more frequently found in patients with hypertension whereas the baseline creatinine, LDL cholesterol and triglyceride levels were comparable in both patient groups. However, the proportion of patients who have high LDL was higher in those patients who developed hypertension than in those who did not, although the difference did not reach statistical significance.
The use of hydroxychloroquine, non‐steroidal anti‐inflammatory drugs and low‐dose aspirin were comparable in both patient groups whereas the use of glucocorticoids was more frequent in those who developed hypertension; however, there was no difference in the average dose of prednisone equivalent used by patients in the two groups.
Multivariable analyses
Table 3 shows the multivariable logistic regression analyses data. African–American and Hispanic Texan ethnicities, renal involvement and a higher BMI were found to be independently associated with the occurrence of hypertension.
Table 3 Predictors of hypertension in patients from the LUMINA cohort by multivariable logistic regression analyses (n = 382).
| Features | OR (95% CI) | p Value |
|---|---|---|
| Hispanic Texan | 3.143 (1.398 to 7.063) | 0.006 |
| African–American ethnicity | 3.780 (1.797 to 7.952) | <0.001 |
| Caucasian | 1.845 (0.858 to 3.967) | 0.117 |
| Renal involvement | 2.953 (1.835 to 4.752) | <0.001 |
| Obesity, body mass index (kg/m2) | 1.060 (1.009 to 1.114) | 0.020 |
In addition to the variables listed in the table, the following were adjusted for in these analyses: age, gender, disease activity over time (Systemic Lupus Activity Measure‐Revised scores from the renal domain and hypertension were excluded), diabetes mellitus, glucocorticoid use, low‐density lipoprotein cholesterol and the presence of antiphospholipid and anti‐dsDNA antibodies.
Discussion
We carried out this study in the LUMINA cohort to assess the risk factors associated with the occurrence of hypertension after patients entered the cohort, hence “incident hypertension”. The strength of our study rests in that LUMINA is a longitudinal cohort with a large number of patients. Our study confirms previous reports from North American SLE cohorts that hypertension occurs frequently in SLE; the overall frequency of hypertension in our cohort was 60%. The prevalence of hypertension in other lupus cohorts with different ethnic composition from New York City (African–Americans), Toronto (Caucasians), Baltimore (African–Americans and Caucasians) and Pittsburgh (Caucasians) were 75%, 50%, 46% and 37%, respectively.8,13,15,17 The longer follow‐up time in the Toronto lupus cohort might explain the higher prevalence rate in this cohort than in the Pittsburgh cohort, despite the fact that both are constituted primarily of Caucasians. Given the ethnic composition of the LUMINA cohort and the total disease duration, our rate is consistent with the data from these cohorts. However, the prevalence of hypertension in SLE in other parts of the world including Europe (Eurolupus, Spain and Greece) and Latin America (GLADEL, for Grupo Latinoamericano de Estudio del Lupus or Latin American Group for the Study of Lupus) are much lower (17%, 21%, 14% and 27%, respectively).2,30,31,32 This is paradoxical given that the reported prevalence of hypertension in the general population of selected European and Latin American countries is higher than in North America.33,34
The risk factors for hypertension have been examined previously in two smaller studies: one in 112 Spanish patients and the other in 150 patients from the Toronto lupus cohort. In the first study, use of glucocorticoids, disease duration and age were found to be predictive of hypertension30 whereas in the second study hypercholesterolaemia was found to be the best predictor of hypertension.13 In our study, we failed to identify any of these previously reported factors as independently contributing to hypertension. However, we have identified factors that were not previously by other investigators such as renal involvement, higher BMI and African–American and Hispanic Texan ethnicities.
Hypertension has been described to be associated with worse renal outcomes in patients with lupus nephritis.17,35 We have now found renal involvement to be a strong predictor of hypertension even after adjusting for possible confounding variables; although this finding seems quite logical, such association has not been reported previously.
Although aPL antibodies were found to be associated with hypertension in the univariable analyses, this variable was not retained in the multivariable analyses. It should be noted, however, that in patients with SLE without renal involvement, these antibodies may still be important predictors of hypertension. It is known, for example, that these antibodies may upregulate endothelin‐1 mRNA in endothelial cells.36 These antibodies have also been shown to contribute to atherogenesis; patients with SLE with IgG anticardiolipin antibodies have significantly lower high‐density lipoprotein cholesterol and Apo A‐1 concentrations compared with those without them;37 anti‐β2‐glycoprotein 1 antibodies form a complex that promotes the uptake of oxidised LDL by macrophages, thus facilitating the formation of foam cells.38 Furthermore, aPL antibodies have been found to be associated with poor renal outcomes in patients with lupus nephritis, a strong predictor of the occurrence of hypertension in our study.39
The association of higher BMI with hypertension has been recognised previously in the general population;40,41,42,43 nevertheless, our data reinforce the notion that body weight should be optimised in patients with SLE to prevent the occurrence of hypertension; in fact, the data from the Nurses' Health Study have shown that women who lost at least 5 kg of body weight had a significantly lower risk of developing hypertension than women who did not.44
Although LUMINA patients of African–American and Hispanic ethnicity residing in Texas (of Mexican ancestry, predominantly) are known to have renal involvement more frequently than Caucasians (and Hispanics from the Island of Puerto Rico),45,46 it is interesting to note that the association with hypertension was more significant than renal involvement, suggesting that other ethnic‐associated factors may be operative.
There have been many studies investigating the role of glucocorticoids in clinical and subclinical cardiovascular disease in SLE; however, the role of glucocorticoids in hypertension have not been examined as extensively. We examined the effects of glucocorticoids using two approaches: first by categorising patients into past or current users vs non‐users, and second by examining the weighted average dose of glucocorticoids as prednisone dose equivalent used up to T0. In contrast with the results from the Hopkins Lupus cohort and the cohort from Spain in which after adjustment for age, weight and antihypertensive drug use, incremental doses of prednisone led to increased blood pressure,30,47 we could not document any such association. However, disease activity was not adjusted for in the two aforementioned studies, which may explain the discrepant results observed between them and ours.
This study is not without limitations. First, visits were annual, thus records of the exact time at which hypertension first occurred are not totally accurate; therefore, we chose not to perform time‐dependent analyses to confirm the findings from the logistic regression model. Second, lipid profiles were measured in stored non‐fasting sera; this is less than ideal as per the recommendations from the National Cholesterol Education Program, which may cause an overestimation of LDL and triglyceride levels and a false‐positive result. Nevertheless, unlike the Toronto lupus cohort in which hypercholesterolaemia was found to be a predictor of hypertension,13 we could not show such an association. Third, medication in the LUMINA cohort is recorded as current or past use; thus use of drugs recorded at T0 might not necessarily explain hypertension that occurred a few years later. Finally, although these risk factors are associated with the occurrence of hypertension, a causal relationship cannot be concluded from this study.
In summary, our data have direct applicability to the management of patients with SLE. Traditional cardiovascular risk factors SLE‐related factors and African–American and Hispanic ethnicities seem to play a predictive role in the occurrence of hypertension in patients with SLE. This is important because hypertension is a strong risk factor for cardiovascular disease, which is one of the major causes of death in SLE. Recognising this might prompt clinicians to closely monitor blood pressure in all patients with lupus, aiming for its tight control. Finally, body weight is also modifiable; optimising it may further prevent the occurrence of hypertension and its deleterious consequences in patients with SLE.
Acknowledgements
We thank all LUMINA patients without whom this study would have not been possible, our supporting staff (Martha L Sanchez and Ellen Sowell at UAB, Carmine Pinilla‐Diaz, MT at UPR, and Robert Sandoval at UTH) for their efforts in securing our patients' follow up and performing other LUMINA‐related tasks and Ms Maria Tyson, for her expert assistance in the preparation of this manuscript.
Abbreviations
ACR - American College of Rheumatology
aPL - antiphospholipid
BMI - body mass index
ds DNA - double stranded DNA
LDL - low‐density lipoprotein
LUMINA - Lupus in Minorities: Nature vs Nurture
SDI - Systemic Lupus International Collaborating Clinics Damage Index
SLAM‐R - Systemic Lupus Activity Measure‐Revised
SLE - systemic lupus erythematosus
Footnotes
Funding: Supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases #R01‐AR42503, General Clinical Research Centers #M01‐rr02558 (UTH‐HSC) and M01‐RR00032 (UAB), the National Center for Research Resources (NCRR/NIH) RCMI clinical research infrastructure initiative (RCRII) award 1p20RR11126 (UPR‐MSC), the Mary Kirkland Scholars Award Program (UABP) and the Stellar Program (Supporting Training Efforts in Lupus for Latino American Rheumatologists) funded by Rheuminations (UAB).
Competing interests: None declared.
References
- 1.Manzi S, Meilahn E N, Rairie J E, Conte C G, Medsger T A, Jr, Jansen‐McWilliams L.et al Age‐specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am J Epidemiol 1997145408–415. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Khamashta M A, Font J, Sebastiani G D, Gil A, Lavilla P.et al Morbidity and mortality in systemic lupus erythematosus during a 10‐year period: a comparison of early and late manifestations in a cohort of 1,000 patients. European Working Party on Systemic Lupus Erythematosus. Medicine 200382299–308. [DOI] [PubMed] [Google Scholar]
- 3.Urowitz M B, Bookman A A, Koehler B E, Gordon D A, Smythe H A, Ogryzlo M A. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med 197660221–225. [DOI] [PubMed] [Google Scholar]
- 4.Petri M, Perez‐Gutthann S, Spence D, Hochberg M C. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med 199293513–519. [DOI] [PubMed] [Google Scholar]
- 5.Gladman D D, Urowitz M B. Morbidity in systemic lupus erythematosus. J Rheumatol 198714223–226. [PubMed] [Google Scholar]
- 6.Toloza S M, Uribe A, McGwin G, Alarcón G S, Fessler B, Bastian H M.et al Systemic lupus erythematosus in a multiethnic US cohort (LUMINA) XXIII. Baseline predictors of vascular events. 7th International Congress on SLE and related conditions, New York 2004M32B. [DOI] [PubMed]
- 7.Gregg E W, Cheng Y J, Cadwell B L, Imperatore G, Williams D E, Flegal K M.et al Secular trends in cardiovascular disease risk factors according to body mass index in US adults. J Am Med Assoc 20052931868–1874. [DOI] [PubMed] [Google Scholar]
- 8.Petri M. Detection of coronary artery disease and the role of traditional risk factors in the Hopkins lupus cohort. Lupus 20009170–175. [DOI] [PubMed] [Google Scholar]
- 9.Bruce I N, Urowitz M B, Gladman D D, Ibañez D, Steiner G. Risk factors for coronary heart disease in women with systemic lupus erythematosus. The Toronto Risk Factor Study. Arthritis Rheum 2003483159–3167. [DOI] [PubMed] [Google Scholar]
- 10.Asanuma Y, Oeser A, Shintani A K, Turner E, Olsen N, Fazio S.et al Premature coronary‐artery atherosclerosis in systemic lupus erythematosus. N Engl J Med 20033492407–2415. [DOI] [PubMed] [Google Scholar]
- 11.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 20023601903–1913. [DOI] [PubMed] [Google Scholar]
- 12.Chobanian A V, Bakris G L, Black H R, Cushman W C, Green L A, Izzo J L., Jret al The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. J Am Med Assoc 20032892560–2572. [DOI] [PubMed] [Google Scholar]
- 13.Rahman P, Aguero S, Gladman D D, Hallett D, Urowitz M B. Vascular events in hypertensive patients with systemic lupus erythematosus. Lupus 20009672–675. [DOI] [PubMed] [Google Scholar]
- 14.Manzi S, Selzer F, Sutton‐Tyrrell K, Fitzgerald S G, Rairie J E, Tracy R P.et al Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum 19994251–60. [DOI] [PubMed] [Google Scholar]
- 15.Selzer F, Sutton‐Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension 2001371075–1082. [DOI] [PubMed] [Google Scholar]
- 16.Doria A, Shoenfeld Y, Wu R, Gambari P F, Puato M, Ghirardello A.et al Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis 2003621071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginzler E M, Felson D T, Anthony J M, Anderson J J. Hypertension increases the risk of renal deterioration in systemic lupus erythematosus. J Rheumatol 1993201694–1700. [PubMed] [Google Scholar]
- 18.Hochberg M C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997401725. [DOI] [PubMed] [Google Scholar]
- 19.Reveille J D, Moulds J M, Ahn C, Friedman A W, Baethge B, Roseman J.et al Systemic lupus erythematosus in three ethnic groups: I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity at disease onset. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 1998411161–1172. [DOI] [PubMed] [Google Scholar]
- 20.Alarcón G S, Friedman A W, Straaton K V, Moulds J M, Lisse J, Bastian H M.et al Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs, Nurture. Lupus 19998197–209. [DOI] [PubMed] [Google Scholar]
- 21.Alarcón G S, McGwin G, Jr, Bastian H M, Roseman J M, Lisse J, Fessler B J.et al Systemic lupus erythematosus in three ethnic group VII: Predictors of early mortality in the LUMINA cohort. Arthritis Rheum (Arthritis Care Res) 200145191–202. [DOI] [PubMed] [Google Scholar]
- 22.Alarcón G S, McGwin G, Jr, Bartolucci A A, Roseman J, Lisse J, Fessler B J.et al Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum 2001442797–2806. [DOI] [PubMed] [Google Scholar]
- 23.US Department of Commerce, Bureau of the Census Current population reports. Series P‐23, No. 28 and Series P‐60, No. 68 and subsequent years. Washington, DC: Housing and Household Economic Statistics Division, 1995
- 24.Gladman D D, Goldsmith C H, Urowitz M B, Bacon P, Bombardier C, Isenberg D.et al Sensitivity to change of 3 systemic lupus erythematosus disease activity indices: International validation. J Rheumatol 1994211468–1471. [PubMed] [Google Scholar]
- 25.Gladman D D, Urowitz M B, Goldsmith C H, Fortin P, Ginzler E, Gordon C.et al The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 199740809–813. [DOI] [PubMed] [Google Scholar]
- 26.Aarden L A, De Groot E R, Feltkamp T E. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti‐dsDNA with the immunofluorescence technique. Ann NY Acad Sci 1975254505–515. [DOI] [PubMed] [Google Scholar]
- 27.Harris E N. Special report. The second international anti‐cardiolipin standardization workshop/the Kingston anti‐phospholipid antibody study (KAPS) group. Am J Clin Pathol 199094476–484. [DOI] [PubMed] [Google Scholar]
- 28.Roisin J ‐ P, Contant G, Martinoli J ‐ L. Detection of lupus‐like anticoagulants (LA). Use of hexagonal phosphatidylethanolamine and of an APTT reagent sensitive to LA. Thromb Haemost 1991652023 [Google Scholar]
- 29.Bertoli A M, Fernandez M, Calvo‐Alen J, Vila L M, Sanchez M L, Reveille J D.et al Systemic lupus erythematosus in a multiethnic U.S. cohort (LUMINA) XXXI: factors associated with patients being lost to follow‐up, Lupus 20061519–25. [DOI] [PubMed] [Google Scholar]
- 30.Sabio J M, Mediavilla J D, Fernández‐Torres C, Aliaga L, Jiménez‐Alonso J J. Risk factors related to hypertension in a Spanish systemic lupus erythematosus cohort. Lupus 200110451–452. [DOI] [PubMed] [Google Scholar]
- 31.Vlachoyiannopoulos P G, Karassa F B, Karakostas K X, Drosos A A, Moutsopoulos H M. Systemic lupus erythematosus in Greece. Clinical features, evolution and outcome: a descriptive analysis of 292 patients, Lupus 19932303–312. [DOI] [PubMed] [Google Scholar]
- 32.Garcia M A, Marcos J C, Marcos A I, Pons‐Estel B A, Wojdyla D, Arturi A.et al Male systemic lupus erythematosus in a Latin‐American inception cohort of 1214 patients. Lupus 200514938–946. [DOI] [PubMed] [Google Scholar]
- 33.Kearney P M, Whelton M, Reynolds K, Muntner P, Whelton P K, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005365217–223. [DOI] [PubMed] [Google Scholar]
- 34.Wolf‐Maier K, Cooper R S, Banegas J R, Giampaoli S, Hense H W, Joffres M.et al Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. J Am Med Assoc 20032892363–2369. [DOI] [PubMed] [Google Scholar]
- 35.Contreras G, Pardo V, Cely C, Borja E, Hurtado A, De La C C.et al Factors associated with poor outcomes in patients with lupus nephritis. Lupus 200514890–895. [DOI] [PubMed] [Google Scholar]
- 36.Atsumi T, Khamashta M A, Haworth R S, Brooks G, Amengual O, Ichikawa K.et al Arterial disease and thrombosis in the antiphospholipid syndrome: a pathogenic role for endothelin 1. Arthritis Rheum 199841800–807. [DOI] [PubMed] [Google Scholar]
- 37.Lahita R G, Rivkin E, Cavanagh I, Romano P. Low levels of total cholesterol, high‐density lipoprotein, and apolipoprotein A1 in association with anticardiolipin antibodies in patients with systemic lupus erythematosus. Arthritis Rheum 1993361566–1574. [DOI] [PubMed] [Google Scholar]
- 38.Hasunuma Y, Matsuura E, Makita Z, Katahira T, Nishi S, Koike T. Involvement of beta 2‐glycoprotein I and anticardiolipin antibodies in oxidatively modified low‐density lipoprotein uptake by macrophages. Clin Exp Immunol 1997107569–573. [DOI] [PubMed] [Google Scholar]
- 39.Moroni G, Ventura D, Riva P, Panzeri P, Quaglini S, Banfi G.et al Antiphospholipid antibodies are associated with an increased risk for chronic renal insufficiency in patients with lupus nephritis. Am J Kidney Dis 20044328–36. [DOI] [PubMed] [Google Scholar]
- 40.Klag M J, Whelton P K, Randall B L, Neaton J D, Brancati F L, Stamler J. End‐stage renal disease in African‐American and white men. 16‐year MRFIT findings. J Am Med Assoc 19972771293–1298. [PubMed] [Google Scholar]
- 41.Qureshi A I, Suri M F, Kirmani J F, Divani A A. Prevalence and trends of prehypertension and hypertension in United States: National Health and Nutrition Examination Surveys 1976 to 2000. Med Sci Monit 200511CR403–CR409. [PubMed] [Google Scholar]
- 42.Must A, Spadano J, Coakley E H, Field A E, Colditz G, Dietz W H. The disease burden associated with overweight and obesity. J Am Med Assoc 19992821523–1529. [DOI] [PubMed] [Google Scholar]
- 43.Brown C D, Higgins M, Donato K A, Rohde F C, Garrison R, Obarzanek E.et al Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res 20008605–619. [DOI] [PubMed] [Google Scholar]
- 44.Huang Z, Willett W C, Manson J E, Rosner B, Stampfer M J, Speizer F E.et al Body weight, weight change, and risk for hypertension in women. Ann Intern Med 199812881–88. [DOI] [PubMed] [Google Scholar]
- 45.Bastian H M, Roseman J M, McGwin G, Jr, Alarcón G S, Friedman A W, Fessler B J.et al Systemic lupus erythematosus in three ethnic groups: XII. Risk factors for lupus nephritis after diagnosis. Lupus 200211152–160. [DOI] [PubMed] [Google Scholar]
- 46.Bastian H M, Alarcon G S, Roseman J M, McGwin G, Jr, Vila L M, Fessler B J.et al Systemic lupus erythematosus in a multiethnic US cohort (LUMINA) XLII: factors predictive of new and worsening proteinuria. Rheumatilogy (Oxford). Published Online First: 28 November 2006, doi: 10. 1093/rheumatology/Kel347 [DOI] [PubMed]
- 47.Petri M, Lakatta C, Magder L, Goldman D. Effect of prednisone and hydroxychloroquine on coronary artery disease risk factors in systemic lupus erythematosus: a longitudinal data analysis. Am J Med 199496254–259. [DOI] [PubMed] [Google Scholar]