Abstract
Background
Rheumatoid arthritis is a chronic autoimmune disease of unknown aetiology characterised by chronic inflammation in the joints and subsequent destruction of the cartilage and bone.
Aim
To propose a new strategy for the treatment of arthritis based on the administration of cortistatin, a newly discovered neuropeptide with anti‐inflammatory actions.
Methods
DBA/1J mice with collagen‐induced arthritis were treated with cortistatin after the onset of disease, and the clinical score and joint histopathology were evaluated. Inflammatory response was determined by measuring the levels of various inflammatory mediators (cytokines and chemokines) in joints and serum. T helper cell type 1 (Th1)‐mediated autoreactive response was evaluated by determining the proliferative response and cytokine profile of draining lymph node cells stimulated with collagen and by assaying the content of serum autoantibodies.
Results
Cortistatin treatment significantly reduced the severity of established collagen‐induced arthritis, completely abrogating joint swelling and destruction of cartilage and bone. The therapeutic effect of cortistatin was associated with a striking reduction in the two deleterious components of the disease—that is, the Th1‐driven autoimmune and inflammatory responses. Cortistatin downregulated the production of various inflammatory cytokines and chemokines, decreased the antigen‐specific Th1‐cell expansion, and induced the production of regulatory cytokines, such as interleukin 10 and transforming growth factor β1. Cortistatin exerted its effects on synovial cells through both somatostatin and ghrelin receptors, showing a higher effect than both peptides protecting against experimental arthritis.
Conclusion
This work provides a powerful rationale for the assessment of the efficacy of cortistatin as a novel therapeutic approach to the treatment of rheumatoid arthritis.
Rheumatoid arthritis (RA) is an autoimmune disease that leads to chronic inflammation in the joints and subsequent destruction of the cartilage and erosion of the bone. Although the contribution of T helper cell type 1 (Th1) responses in RA is not completely understood, several studies in animal models point to a pathogenic role for Th1‐derived cytokines.1,2 Th1 cells reactive to components of the joint infiltrate the synovium, release proinflammatory cytokines and chemokines, and promote macrophage and neutrophil infiltration and activation. Inflammatory mediators, such as cytokines and free radicals, produced by infiltrating inflammatory cells, play a critical role in joint damage.2 The fact that the inflammatory process in RA is chronic suggests that immune regulation in the joints is disturbed. Available therapies based on immunosuppressive agents inhibit the inflammatory component of RA and have the potential to slow progressive clinical disability by delaying erosions and deformity.3 However, they neither reduce the relapse rate nor delay disease onset, and because continued treatment is required to maintain a beneficial effect, they have multiple side effects.4 This illustrates the need for novel therapeutic approaches to prevent the inflammatory and autoimmune components of the disease.
Cortistatin (CST) is a recently discovered cyclic neuropeptide related to somatostatin, which shares many of somatostatin's pharmacological and functional properties, including the depression of neuronal activity and inhibition of cell proliferation.5 However, CST also has many properties distinct from somatostatin, such as slow‐wave sleep induction and locomotor activity reduction.5 Various human immune cells, including lymphocytes, monocytes, macrophages and dendritic cells, produce CST but not somatostatin, and its levels correlate with cell differentiation and activation state,6,7 suggesting that CST might be a major endogenous regulatory factor in the immune system. Indeed, we have recently reported a new role of CST as a potent anti‐inflammatory factor. CST prevents sepsis‐induced mortality by inhibiting the production of inflammatory mediators by activated macrophages and decreasing the recruitment of neutrophils and monocytes to inflamed organs.8 Therefore, the aim of this study is to investigate the potential therapeutic action of CST in an experimental model of RA. Here, we show that treatment with CST has great benefit at the clinical and pathological levels, as the therapeutic effect of CST was exerted at multiple levels, being associated with the downregulation of inflammatory and Th1‐mediated autoimmune components of the disease.
Methods
Arthritis induction and treatment
Animal experimental protocols were reviewed and approved by the ethics committee of the Spanish Council of Scientific Research. For the induction of collagen‐induced arthritis (CIA), DBA/1J mice (7–10 weeks old, Jackson Laboratory, Bar Harbor, Maine, USA) were injected subcutaneously with 200 μg of collagen type II (CII) (Sigma, St Louis, Missouri, USA) emulsified in complete Freund's adjuvant (CFA) containing 200 μg of Mycobacterium tuberculosis H37 RA (Difco, Detroit, Michigan, USA). At day 21 after primary immunisation, mice were given subcutaneous booster doses of 100 μg of CII in CFA. CST (American Peptides Company, Sunnyvale, California, USA) treatment consisted of intraperitoneal administration of 0.1, 1, 2, 5 or 10 nmol/mouse/day of rat CST1–29 on five consecutive days starting at 25 days after immunisation, when all mice showed established arthritis (clinical score >2). These doses of CST were chosen on the basis of previous experiments with CST in a model of inflammatory bowel disease or with other anti‐inflammatory neuropeptides in CIA.9,10 To see whether a unique pulse of the neuropeptide was therapeutic, CST (2 nmol) was administered once at day 25. In each experiment, a control group of mice was injected intraperitoneally with phosphate‐buffered saline (untreated). Because CST is structurally related to somatostatin and exerts some of its immunomodulatory effect through the receptor of the anti‐inflammatory hormone ghrelin, in some experiments we injected ghrelin or somatostatin intraperitoneally (Sigma; 15 μg/mouse/day for five consecutive days after day 25). Clinical arthritis was assessed by two independent, blinded examiners as described,9 by using the following system: grade 0, no swelling; grade 1, slight swelling and erythema; grade 2, moderate swelling and oedema; grade 3, extreme swelling and pronounced oedema; and grade 4, joint rigidity. Each limb was graded, giving a maximum possible score of 16 per animal. For histological analysis, the paws were randomly collected by two independent experimenters at day 45 after primary immunisation, fixed in 4% buffered formaldehyde, decalcified, paraffin‐embedded, sectioned and stained with H&E or Masson–Goldner trichromic stain. Histopathological changes were scored in a blinded manner based on cell infiltration, cartilage destruction and bone erosion parameters as described.11 For determination of cytokine in joints, protein extracts were isolated by homogenisation of joints (50 mg tissue/ml) in 50 mM Tris–HCl, pH 7.4, with 0.5 mM dithiothreitol, and proteinase inhibitor cocktail (10 μg/ml, Sigma). Serum samples were collected at the peak of disease (day 35) and the levels of anti‐CII IgG, IgG1 and IgG2a antibodies were measured by ELISA as described.9 Cytokine and chemokine levels in the serum and joint protein extracts prepared at the disease peak (day 35) were determined by specific sandwich ELISAs using capture/biotinylated detection antibodies from BD Pharmingen (San Diego, California, USA) according to the manufacturer's recommendations. Neutrophil infiltration in the joint was monitored by measuring myeloperoxidase activity in joint extracts isolated at day 35 after immunisation as described.11
Assessment of T cell autoreactive response
Because the T cell autoreactive response precedes the maximal clinical manifestations of the disease, single‐cell suspensions (106 cells/ml) from draining lymph nodes (DLN) and the synovial membrane of knee joints were obtained at 30 days after immunisation. Cells were stimulated in complete medium (RPMI 1640 containing 10% fetal calf serum, 2 mM L‐glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin) with different concentrations of heat‐inactivated CII for 48 h (for the determination of cytokine) or for 72 h (for proliferative response).9 Cell proliferation was evaluated by using a cell proliferation assay with 5‐bromo‐2'‐deoxy‐uridine (Roche Diagnostics GmbH, Mannheim, Germany). Cytokine content in culture supernatants was determined by specific sandwich ELISAs as above. For intracellular analysis of cytokines, DLN and synovial cells were stimulated with inactivated CII (10 μg/ml) for 8 h, in the presence of monensin, and then stained with PerCP‐anti‐CD4 monoclonal antibodies at 4°C, washed, fixed/saponin permeabilised, stained with fluorescein isothiocyanate‐ and phycoerythrin‐conjugated anti‐cytokine‐specific monoclonal antibodies (BD Pharmingen), and analysed on a FACScalibur flow cytometer (Becton Dickinson, Mountain View, California, USA). To distinguish between monocyte/macrophage and T cell sources, intracellular cytokine analysis was done exclusively in the PerCP‐labelled CD4 T cell population. As a recall antigen control, 30 μg of purified protein derivative (PPD) was injected subcutaneously in the CII–CFA emulsion, and in vitro T cell function after culture stimulation with 10 μg/ml PPD was assessed as above.
Alternatively, synovial cells (106 cells/ml) isolated from CIA mice at day 30 after immunisation were stimulated with inactivated CII (10 μg/ml) in the absence or presence of different concentrations of CST, ghrelin or somatostatin, with or without 10−6 M cyclosomatostatin or [D‐Lys3]‐growth hormone releasing peptide 6 (Lys‐GHRP‐6; Sigma). Cytokine levels were determined in supernatants after 48 h culture.
Data analysis
All values are expressed as mean (SD). The differences between groups were analysed using the Mann–Whitney U test and, if appropriate, the Kruskal–Wallis analysis of variance test.
Results
CST decreases the severity of collagen‐induced arthritis
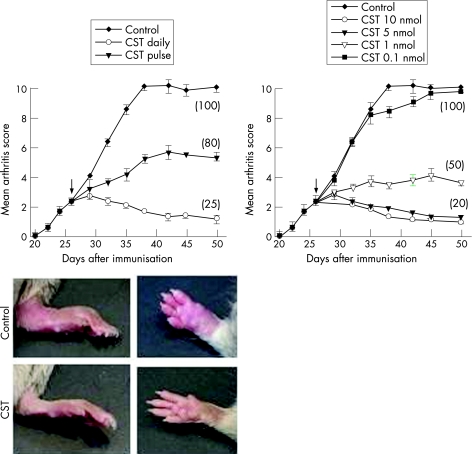
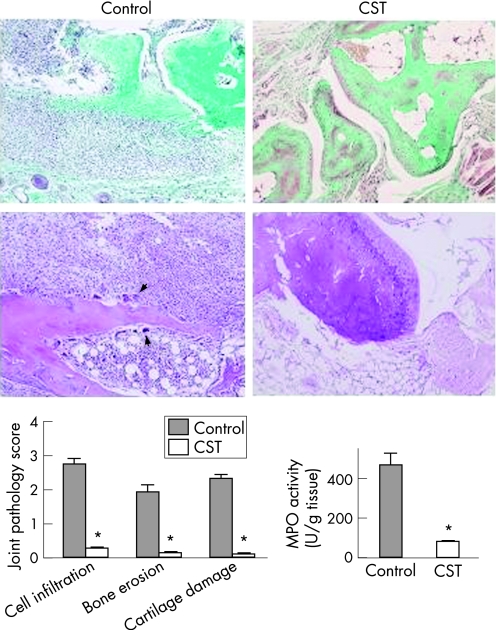
CIA is a murine experimental disease model that shares a number of clinical, histological and immunological features with RA, and it is used as a model system to test potential therapeutic agents. CST treatment of mice with established clinical signs of arthritis progressively attenuated the severity of CIA and decreased the percentage of mice with arthritis, as compared with untreated mice (fig 1). Daily administration of 2 nmol of CST for five days offered the best therapeutic effect, although a single injection was enough to significantly ameliorate the pathological signs of arthritis (fig 1). The beneficial effect was dose‐dependent (fig 1). Because we observed few differences between the 2 nmol and 10 nmol doses, all further experiments used five administrations of the 2 nmol dose on consecutive days. We saw no loss of the therapeutic effects 3 weeks after cessation of CST treatment (fig 1). In addition, throughout our study, we did not observe any overt toxicity or lethality caused by daily peptide injection. Histopathological analyses of joints showed that CST treatment completely abrogated CIA—characteristic chronic inflammation of synovial tissue (infiltration of inflammatory cells—lymphocytes, plasma cells, macrophages and neutrophils—into the joint cavity and periarticular soft tissue, pannus formation, cartilage destruction and bone erosion (fig 2). The CST‐mediated inhibition of neutrophil infiltration was confirmed with decreased joint myeloperoxidase activity. In addition, CST treatment inhibited the osteoclast‐inducing activity observed in the CIA mice with recruitment of osteoclast in basic multicellular units that produce focal subchondral bone erosion (fig 2, arrows).
Figure 1 Cortistatin (CST) decreases the severity of collagen‐induced arthritis. DBA1 mice with established CIA were injected intraperitoneally (arrow) either with phosphate‐buffered saline (control) or with different doses of CST (2 nmol/mouse for left panel) daily for five days or with a single administration (pulse) on day 25. The severity of arthritis was assessed by clinical scoring. Numbers in parenthesis are the frequency of arthritis (percentage of mice with an arthritis score >2 at day 50). Images show representative examples of the paw swelling in mice of the different experimental groups. n = 8–16 mice per group. p<0.001 versus control for 1, 2, 5 and 10 nmol CST treatments after day 32.
Figure 2 Cortistatin (CST) ameliorates the histopathology of collagen‐induced arthritis (CIA). DBA1 mice with established CIA were injected intraperitoneally either with phosphate‐buffered saline (control) or with CST (2 nmol/mouse) daily for five days after day 25 post immunisation. Histological analysis of trichrome‐stained (upper) or H&E‐stained (lower) sections of joints obtained at day 45 was performed. Arrows point to osteoclasts destroying bone. Scoring of inflammation, cartilage damage and bone erosion of paws from untreated (control) and CST‐treated CIA mice is shown. Neutrophil infiltration in the joints was determined by measuring myeloperoxidase (MPO) activity in protein extracts isolated at day 35. *p<0.001 versus control.
CST inhibits inflammatory response in CIA
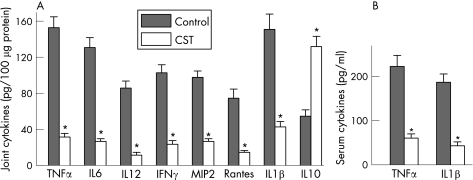
Next, we investigated the mechanisms underlying the decrease in the severity of CIA following CST treatment. Several pieces of evidence have shown the involvement of a wide array of cytokines and chemokines in joint inflammation and arthritis progression.1,2 Because CST has been recently suggested to be a potent anti‐inflammatory factor,8 we evaluated its effect on the production of inflammatory mediators that are mechanistically linked to the severity of CIA. CST treatment significantly reduced protein expression of inflammatory cytokines (tumour necrosis factor (TNF)α, interferon (IFN)γ, interleukin (IL)6, IL1β and IL12) and chemokines (Rantes and MIP‐2) in the joint of mice with arthritis (fig 3A). In addition, joints of CST‐treated mice showed increased levels of the regulatory cytokine IL10 (fig 3A). The broad anti‐inflammatory activity of CST in the inflamed joint was accompanied by downregulation of the systemic inflammatory response. CST decreased CIA‐induced serum levels of the proinflammatory cytokines TNFα and IL1β (fig 3B).
Figure 3 Cortistatin (CST) inhibits the inflammatory response in collagen‐induced arthritis (CIA). DBA1 mice with established CIA were injected intraperitoneally either with phosphate‐buffered saline (control) or with CST (2 nmol/mouse) daily for five days after day 25 post immunisation. Systemic and local expression of inflammatory mediators was assayed by ELISA in joint protein extracts (A) and sera (B) isolated at day 35 after immunisation. A paw from an unimmunised mouse was analysed simultaneously for the assessment of the basal response. n = 6–8 mice/group. *p<0.001 versus controls. IFN, interferon; IL, interleukin; MIP, macrophage inflammatory protein; TNF, tumour necrosis factor.
CST downregulates Th1‐mediated CII‐specific response in CIA
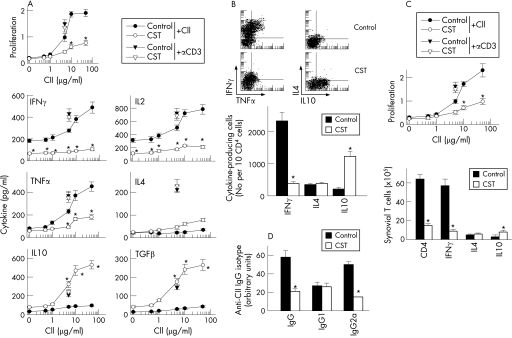
Although macrophages and neutrophils are the major sources of inflammatory mediators, CD4 T cells play a key role in the initiation and perpetuation of CIA by producing IFNγ, a potent inducer of the inflammatory response. In fact, CIA is considered an archetypal example of Th1‐type cell‐mediated autoimmune disease.2 Therefore, CST could ameliorate CIA by reducing autoreactive T cell responses and/or migration to the joints. We determined the proliferation and the cytokine profile of DLN cells isolated from CST‐treated mice with arthritis in response to antigen (CII) in vitro. DLN cells obtained from CIA mice showed marked CII‐specific proliferation and effector T cells producing high levels of Th1‐type cytokines (IFNγ, IL2 and TNFα) and low levels of Th2‐type cytokines (IL4 and IL10) (fig 4A). In contrast, DLN cells from CST‐treated mice proliferated much less, produced low levels of Th1 cytokines and high amounts of suppressive cytokines (IL10 and transforming growth factor (TGF)β1); the Th2‐type cytokine IL4 was not significantly affected (fig 4A). This effect was antigen‐specific, because CST treatment did not affect proliferation and cytokine production by PPD‐stimulated spleen cells from PPD/CFA‐immunised CIA mice (not shown). This suggests that CST administration during CIA progression partially inhibits CII‐specific Th1‐cell clonal expansion. In order to distinguish whether the decrease in Th1 cytokine production induced by CST treatment is a consequence of either downregulation of cytokine release or inhibition of Th1 cell expansion, and to identify the source of IL10 (macrophages or CD4 T cells), we determined the intracellular expression of these cytokines by flow cytometry in sorted CD4 T cells. CST significantly decreased the number of TNFα/IFNγ‐producing Th1 cells, and increased the number of IL10‐producing CD4 T cells in DLN (fig 4B). Thus, CST administration to CIA mice regulates the expansion of autoreactive/inflammatory Th1 cells and presumably IL10‐secreting T cells. We observed similar effects on synovial cells (fig 4C).
Figure 4 Cortistatin (CST) downregulates T helper cell type 1 (Th1)‐mediated response in collagen‐induced arthritis (CIA). DBA1 mice with established CIA were injected intraperitoneally either with with phosphate‐buffered saline (control) or with CST (2 nmol/mouse) daily for five days after day 25 post immunisation. (A) Proliferative response and cytokine production of draining lymph nodes (DLN) cells isolated at day 30 from untreated (control) or CST‐treated CIA mice were determined after in vitro stimulation with various concentrations of collagen type II (CII). Stimulation of DLN cells with anti‐CD3 antibodies (?, for untreated CIA mice; ?, for CST‐treated CIA mice) was used for assessment of nonspecific stimulation. A pool of three non‐immunised DBA/1 DLN cell samples was used for assessment of the basal response. No proliferation or cytokine production by T cells was detectable in the presence of an unrelated antigen (OVA). n = 5 mice/group. (B) Number of CII‐specific cytokine‐producing T cells. DLN cell samples from untreated (control) or CST‐treated CIA mice were restimulated in vitro with CII (10 μg/ml) and analysed for CD4 and intracellular cytokine expression by flow cytometry. Dot plots show representative double staining for interferon (IFN)γ/tumour necrosis factor (TNF)α or interleukin (IL)4/IL10 expression in gated CD4 T cells. The number of IFNγ‐expressing, IL4‐expressing and IL10‐expressing T cells relative to 104 CD4 T cells is shown in the lower panel. Data shown represent pooled values from two independent experiments. (C) CII‐specific proliferative response and the number of cytokine‐producing CD4 T cells were determined in synovial membrane cells isolated from untreated (control) or CST‐treated CIA mice and stimulated in vitro with CII (10 μg/ml) for 48 h. Data show the results of pooled synovial cells from three animals per group. (D) The levels of CII‐specific IgG, IgG1 and IgG2a antibodies in sera collected at day 35 from untreated (control) or CST‐treated CIA mice were determined by ELISA (8–12 mice/group). *p<0.001 versus controls. TGF, transforming growth factor.
High levels of circulating antibodies directed against collagen‐rich joint tissue invariably accompany the development of RA and CIA, and their production is a major factor in determining susceptibility to the disease.12 CST administration resulted in reduced serum levels of CII‐specific IgG, particularly autoreactive IgG2a antibodies (fig 4D), generally reflective of Th1 activity.13 These data provide further evidence that CST administration during CIA reduces the Th1 autoreactive responses both in the joint and peripherally.
CST deactivates synovial cells through both somatostatin and ghrelin receptors
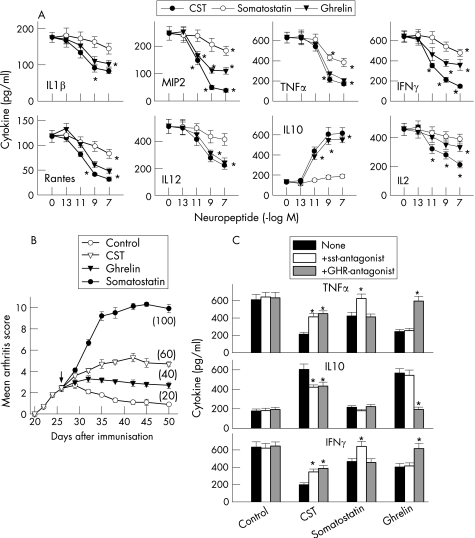
The decrease in inflammatory mediators observed in the CST‐treated CIA mice (fig 3) could be a consequence of the diminished infiltration of inflammatory cells in the inflamed joints. However, CST inhibited the production of pro‐inflammatory mediators by synovial cells isolated from CIA mice on in vitro CII restimulation (fig 5A). This suggests that, in addition to the reduction in inflammatory infiltration, CST administration could deactivate the inflammatory response of infiltrating/proliferating synovial cells.
Figure 5 Cortistatin (CST) inhibits synovial cell activation through both somatostatin and ghrelin receptors. (A) Synovial membrane cells isolated from collagen‐induced arthritis (CIA) mice at peak of disease were activated with collagen type II (CII) (10 μg/ml) in the absence or presence of different concentrations of CST, somatostatin or ghrelin, and the levels of chemokines and cytokines in the culture supernatants were determined by ELISA. n = 3 experiments performed in duplicate. *p<0.001 versus control. (B) The severity and frequency (parenthesis) of arthritis in mice with CIA treated intraperitoneally (arrow) for five consecutive days with phosphate‐buffered saline (control), CST (5 nmol), somatostatin (5 nmol) or ghrelin (5 nmol). n = 8 mice/group. p<0.001 versus control after day 32. (C) Synovial membrane cells isolated from CIA mice were activated with CII (10 μg/ml) and treated with medium (control), CST (10−8 M), somatostatin (10−8 M) or ghrelin (10−8 M) in the absence (none, black bars) or presence of the somatostatin‐receptor antagonist cyclosomatostatin (sst‐antagonist, white bars; 10−6 M) or the ghrelin‐receptor antagonist Lys‐GHRP‐6 (GHR‐antagonist, grey bars; 10−6 M). Cytokine contents in the culture supernatants were determined by ELISA. n = 3 experiments performed in duplicate. *p<0.001 versus neuropeptide‐treated cells in the absence of antagonist. IFN, interferon; IL, interleukin; MIP, macrophage inflammatory protein; TNF, tumour necrosis factor.
CST shows a high homology with somatostatin, binds to somatostatin receptors (sst) and shares some functions with somatostatin.5 In addition to binding to sst in immune cells, CST, but not somatostatin, can also bind to other receptors, including the receptor for the growth‐hormone secretagogue ghrelin,14 a hormone recently described as a potent anti‐inflammatory factor.15,16 Therefore, next we compared the effect of the three peptides in synovial cell activation and CIA progression, and investigated the receptor involved in the effect of CST. CST showed higher inhibitory effect on the inflammatory response of synovial cells and on the severity of CIA than somatostatin (fig 5A,B). In addition, the sst‐antagonist cyclosomatostatin partially reversed the effect of CST, although it fully blocked the effect of somatostatin (fig 5C). This suggests that CST could exert its effects through both sst‐dependent and sst‐independent mechanisms. Indeed, a ghrelin‐receptor antagonist partially blocked the inhibitory effect of CST, but not of somatostatin, on synovial cell activation (fig 5C), pointing to ghrelin receptor as a putative candidate for the sst‐independent mechanism. In this sense, ghrelin showed a similar potency to that of CST on CIA protection and synovial inflammation (fig 5B,C).
Discussion
The initial stages of RA and CIA involve multiple steps, which can be divided into two main phases: initiation and establishment of autoimmunity, and later events associated with the evolving immune and inflammatory responses. The crucial process underlying the initiation of disease is the induction of autoimmunity to collagen‐rich joint components; later events involve a destructive inflammatory process.1,2 Progression of the autoimmune response involves the development of autoreactive Th1 cells, their entry into the joint tissues, and future recruitment of inflammatory cells through multiple mediators. Certain therapeutic approaches address the autoimmune component of CIA and RA, complementing existing anti‐inflammatory therapies. In this study, we show that the neuropeptide CST provides a highly effective therapy for CIA. Its therapeutic effect is associated with a striking reduction in the two deleterious components of the disease—that is, the autoimmune and inflammatory responses. CST treatment decreased the presence of autoreactive Th1 cells in the periphery and the joint. In addition, CST strongly reduced the inflammatory response during CIA progression by downregulating the production of several inflammatory mediators, such as various cytokines and chemokines in the joints. As a consequence, CST reduced the frequency of arthritis, ameliorated symptoms and avoided joint damage. From a therapeutic point of view, it is important to take into account the ability of delayed administration of CST to ameliorate ongoing disease, which is an essential prerequisite for an anti‐arthritic agent, as treatment is started after the onset of arthritis in patients. The fact that we did not observe a loss in the beneficial effect of CST with time suggests that an initial treatment with CST could induce remission of the disease. Therefore, in contrast to other treatments for RA with potential side effects, long‐term treatment may not be required with CST.
The capacity of CST to regulate a wide spectrum of inflammatory mediators might offer a therapeutic advantage over other treatments directed against a single mediator, such as the new biological agents. Chemokines are responsible for the infiltration into the joint and activation of various leucocyte populations, which contribute to the pathology of CIA.1,2,17 The fact that CST treatment reduced the expression of a plethora of chemokines could partially explain the absence of inflammatory infiltrates in the joint tissues of CST‐treated mice, being especially relevant for chemokines such as MIP‐2 (chemotactic for neutrophils) and Rantes (for macrophages and T cells), all involved in CIA pathogenesis.17,18 In addition to regulating cell recruitment to the joints, CST also regulates the activation of inflammatory cells in the joints. Thus, CST downregulated the production of the proinflammatory/cytotoxic cytokines TNFα, IFNγ, IL6, IL1β and IL12 in the inflamed joint and increased the levels of the anti‐inflammatory cytokines IL10 and TGFβ, which ameliorate the disease.19,20 The decrease in inflammatory mediators could be the consequence of a diminished infiltration of inflammatory cells in the synovium. However, the fact that CST inhibited the production of proinflammatory mediators by synovial cells isolated from CIA mice on in vitro CII‐specific response argues against this hypothesis. This suggests that, in addition to the reduction in inflammatory infiltration, CST deactivates the inflammatory response. A recent study demonstrated that CST acts as a macrophage‐deactivating factor by downregulating the production of a wide range of inflammatory mediators,8 suggesting that the deactivation of resident and infiltrating macrophages is a major mechanism involved in the anti‐inflammatory action of CST in CIA.
CIA is also a Th1‐mediated disease, and the bias towards Th1 cytokines (mainly IFNγ and TNFα) is crucial in the establishment of chronic inflammation in the joint.1,2 Our findings shows that the administration of CST to mice with arthritis results in a decreased CII‐specific Th1‐mediated response. It appears that the inhibition of the Th1 response is caused by a direct action on synovial and DLN cells, as synovial and DLN cells obtained from CST‐treated animals are refractory to Th1 cell stimulation. In contrast to IFNγ and TNFα, CST increased the production of IL10 and TGFβ1. However, the fact that CST increased IL10, but not IL4, production in synovial and DLN CD4 T cells argues against a shift towards Th2 responses. IL10 has been recently recognised as a signature cytokine for a subset of CD4 T cells that exert regulatory functions and are involved in the restoration of the immune tolerance.21
CST shares receptors, and many structural and functional properties, with somatostatin. However, the lack of increased CST expression in somatostatin‐deficient mice and the exclusive roles described for CST in nervous system5,22,23 argue against a compensatory role of CST. Our work supports this hypothesis, as CST was significantly more efficient in protecting from CIA development than somatostatin. The superior potency of CST in reducing inflammation may reside in its capacity to activate different receptors and transduction pathways. Whereas somatostatin binds only to sst, CST can also activate other receptors, including the ghrelin receptor, associated with anti‐inflammatory actions.14,15 In this study, ghrelin was also therapeutic against CIA. Therefore, the possibility exists that CST is exerting its therapeutic effect on CIA at least partially through ghrelin receptor. Indeed, effects of CST on synovial inflammatory response were partially reversed by both somatostatin‐ and ghrelin‐receptor antagonists. However, the participation of CST‐specific receptors not yet identified cannot be ruled out. It is important to note that somatostatin has been extensively tested in human subjects, including patients with RA.24,25 Therefore, based on its somatostatin‐like structure, CST should be well tolerated in doses similar to those that are able to prevent CIA. Indeed, it has been reported that the clinical use of CST in humans has been without any toxic effects.26
In summary, this work identifies CST as a new immunomodulatory factor with the capacity to deactivate the inflammatory response in vivo at multiple levels and to maintain immune tolerance, and provides a powerful rationale for the assessment of the efficacy of CST as a novel therapeutic approach to the treatment of RA and other chronic autoimmune disorders.
Acknowledgements
This work was supported by grants from the Spanish Ministry of Health (PI04/0674, PI06/1291) and from Ramon Areces Foundation.
Abbreviations
CFA - complete Freund's adjuvant
CIA - collagen‐induced arthritis
CII - collagen type II
CST - cortistatin
DLN - draining lymph nodes
IFN - interferon
IL - interleukin
PPD - purified protein derivative
RA - rheumatoid arthritis
sst - somatostatin receptors
Th1 - T helper cell type 1
TGF - transforming growth factor
TNF - tumour necrosis factor
Footnotes
Competing interests: None declared.
References
- 1.Tremoulet A H, Albani S. Novel therapies for rheumatoid arthritis. Expert Opin Investig Drugs 2006151427–1441. [DOI] [PubMed] [Google Scholar]
- 2.Brand D D, Kang A H, Rosloniec E F. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol 2003253–18. [DOI] [PubMed] [Google Scholar]
- 3.Finckh A, Simard J F, Duryea J, Liang M H, Huang J, Daneel S.et al The effectiveness of anti‐tumor necrosis factor therapy in preventing progressive radiographic joint damage in rheumatoid arthritis: a population‐based study. Arthritis Rheum 20065454–59. [DOI] [PubMed] [Google Scholar]
- 4.Scott D L, Kingsley G H. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med 2006355704–712. [DOI] [PubMed] [Google Scholar]
- 5.Spier A D, de Lecea L. Cortistatin: a member of the somatostatin neuropeptide family with distinct physiological functions. Brain Res Rev 200033228–241. [DOI] [PubMed] [Google Scholar]
- 6.Dalm V A. Expression of somatostatin, cortistatin, and somatostatin receptors in human monocytes, macrophages, and dendritic cells. Am J Physiol Endocrinol Metab 2003285E344–E353. [DOI] [PubMed] [Google Scholar]
- 7.Dalm V A. Cortistatin rather than somatostatin as a potential endogenous ligand for somatostatin receptors in the human immune system. J Clin Endocrinol Metab 200388270–276. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez‐Rey E, Chorny A, Robledo G, Delgado M. Cortistatin, a new anti‐inflammatory peptide with therapeutic effect on lethal endotoxemia. J Exp Med 2006103563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgado M, Abad C, Martinez C, Receta J, Gomariz R P. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med 20017563–568. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez‐Rey E, Varela N, Sheibanie A F, Chorny A, Ganea D, Delgado M. Cortistatin, an anti‐inflammatory peptide with therapeutic action in inflammatory bowel disease. Proc Natl Acad Sci USA 20061034228–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasama T, Strieter R M, Lukacs N W, Lincoln P M, Burdick M D, Kunkel S L. Interleukin‐10 and chemokine regulation during the evolution of murine type‐II collagen‐induced arthritis. J Clin Invest 1995952868–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seki N, Sudo Y, Yoshioka T, Sugihara S, Fujitsu T, Sakuma S.et al Type II collagen‐induced murine arthritis induction and perpetuation of arthritis require synergy between humoral and cell‐mediated immunity. J Immunol 19881401477–1487. [PubMed] [Google Scholar]
- 13.O'Garra A, Robinson D. Development and function of T helper 1 cells. Adv Immunol 200483133–162. [DOI] [PubMed] [Google Scholar]
- 14.Deghenghi R, Papotti M, Ghigo E, Muccioli G. Cortistatin, but not somatostatin, binds to growth hormone secretagogue (GHS) receptors of human pituitary gland. J Endocrinol Invest 200124RC1–RC3. [DOI] [PubMed] [Google Scholar]
- 15.Dixit V D, Schaffer E M, Pyle R S, Collins G D, Sakthivel S K, Palaniappan R.et al Ghrelin inhibits leptin‐ and activation‐induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest 200411457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez‐Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in an experimental model of colitis. Gastroenterology 20061301707–1720. [DOI] [PubMed] [Google Scholar]
- 17.Koch A E. Chemokines and their receptors in rheumatoid arthritis: future targets? Arthritis Rheum 200552710–721. [DOI] [PubMed] [Google Scholar]
- 18.Garcia‐Vicuna R, Gomez‐Gaviro M V, Dominguez‐Luis M J, Pec M K, Gonzalez‐Alvaro I, Alvaro‐Gracia J M.et al CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast‐like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum 2004503866–3877. [DOI] [PubMed] [Google Scholar]
- 19.Kuruvilla A P, Shah R, Hochwald G M, Liggitt H D, Palladino M A, Thorbecke G J.et al Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci USA 1991882918–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walmsley M, Shah R, Hochwald G M, Liggitt H D, Palladito M A, Thorbecke G J. Interleukin‐10 inhibition of the progression of established collagen‐induced arthritis. Arthritis Rheum 199639495–503. [DOI] [PubMed] [Google Scholar]
- 21.Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol 20044408–414. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez J L, Mouchantaf R, Kumar U, Otero Corchon V, Rubinstein M, Low M.et al Brain somatostatin receptors are up‐regulated in somatostatin‐deficient mice. Mol Endocrinol 2002161951–1963. [DOI] [PubMed] [Google Scholar]
- 23.de Lecea L, Criado J R, Prospero‐Garcia O, Gautvik K M, Schweitzer P, Danielson P E.et al A cortical neuropeptide with neuronal depressant and sleep‐modulating properties. Nature 1996381242–245. [DOI] [PubMed] [Google Scholar]
- 24.Paran D, Elkayam O, Mayo A, Paran H, Amit M, Yaron M.et al A pilot study of a long acting somatostatin analogue for the treatment of refractory rheumatoid arthritis. Ann Rheum Dis 200160888–891. [PMC free article] [PubMed] [Google Scholar]
- 25.Takeba Y, Suzuki N, Takeno M, Asai T, Tsuboi S, Hoshino T.et al Modulation of synovial cell function by somatostatin in patients with rheumatoid arthritis. Arthritis Rheum 1997402128–2138. [DOI] [PubMed] [Google Scholar]
- 26.Broglio F, Koetsveld P P, Benso A, Gottero C, Prodam F, Papotti M.et al Ghrelin secretion is inhibited by either somatostatin or cortistatin in humans. J Clin Endocrinol Metab 2002874829–4832. [DOI] [PubMed] [Google Scholar]