Abstract
Background
Evidence suggests that both selective cyclooxygenase (COX)‐2 inhibitors and non‐selective non‐steroidal anti‐inflammatory drugs (NSAIDs) increase the risk of cardiovascular events. However, evidence from prospective studies of currently available COX‐2 inhibitors and non‐selective NSAIDs is lacking in patients at high cardiovascular risk who are taking aspirin.
Objective
To determine the cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib.
Methods
The Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET) of 18 325 patients with osteoarthritis comprised two parallel substudies, comparing lumiracoxib (COX‐2 inhibitor) with either ibuprofen or naproxen. A post hoc analysis by baseline cardiovascular risk, treatment assignment, and low‐dose aspirin use was performed. The primary composite end point was cardiovascular mortality, non‐fatal myocardial infarction, and stroke at 1 year; a secondary end point was the development of congestive heart failure (CHF).
Results
In high risk patients among aspirin users, patients in the ibuprofen substudy had more primary events with ibuprofen than lumiracoxib (2.14% vs 0.25%, p = 0.038), whereas in the naproxen substudy rates were similar for naproxen and lumiracoxib (1.58% vs 1.48%, p = 0.899). High risk patients not taking aspirin had fewer primary events with naproxen than with lumiracoxib (0% vs 1.57%, p = 0.027), but not for ibuprofen versus lumiracoxib (0.92% vs 0.80%, p = 0.920). Overall, CHF developed more often with ibuprofen than lumiracoxib (1.28% vs 0.14%; p = 0.031), whereas no difference existed between naproxen and lumiracoxib.
Conclusions
These data suggest that ibuprofen may confer an increased risk of thrombotic and CHF events relative to lumiracoxib among aspirin users at high cardiovascular risk. The study indicates that naproxen may be associated with lower risk relative to lumiracoxib among non‐aspirin users. This study is subject to inherent limitations, and therefore should be interpreted as a hypothesis‐generating study.
Keywords: coronary disease; osteoarthritis; anti‐inflammatory agents, non‐steroidal anti‐inflammatory drugs; COX‐2 inhibitors; aspirin
The association of non‐steroidal anti‐inflammatory drugs (NSAIDs), including cyclooxygenase (COX)‐2 inhibitors, with cardiovascular events is a major public health concern. Most patients treated with these drugs are elderly, and hence those at increased risk of both coronary artery disease and gastrointestinal bleeding due to NSAIDs.1 Since the publication of the Vioxx Gastrointestinal Outcomes Research (VIGOR) trial,2 cardiovascular side effects have been a main concern of this drug class. The excess risk of cardiovascular events in the Adenomatous Polyp Prevention on Vioxx (APPROVe) trial,3 the study of valdecoxib in patients after coronary artery bypass graft surgery,4 and the Adenoma Prevention with Celecoxib (APC) study5 have brought the safety of the entire COX‐2 inhibitor class into question.
The safety of non‐selective NSAIDs has also been questioned. To date, information about cardiovascular toxicity of non‐selective NSAIDs has mainly accrued from observational studies and has frequently yielded conflicting conclusions, particularly for naproxen.6,7,8 Similarly, clinical studies of the cardiovascular safety of non‐selective NSAIDs, particularly ibuprofen, in patients prescribed aspirin have generated conflicting results.6,9,10,11 To date, randomised clinical trials have not examined the long term effects of NSAID agents in high cardiovascular risk populations. Clinical trials of NSAID agents in patients at high cardiovascular risk have been limited to pilot studies of flurbiprofen and meloxicam, and perioperative parecoxib/valdecoxib.12,13,14,15
Mechanistic explanations for the differential cardiovascular effects of aspirin, naproxen, other non‐selective NSAIDs and COX‐2 inhibitors remain unresolved. Although non‐selective NSAIDs inhibit both the COX‐1 and COX‐2 enzymes, selective COX‐2 inhibitors including lumiracoxib have little or no effect on COX‐1 at therapeutic concentrations. The primary hypothesis explaining the increased cardiovascular risk associated with COX‐2 inhibitors is that COX‐2 inhibition creates an imbalance, favouring COX‐1 mediated platelet aggregation while preventing COX‐2‐dependent prostacyclin production by endothelial cells.16 However, there is also mechanistic evidence that non‐selective NSAIDs such as ibuprofen may contribute to this imbalance as well, either by interfering with aspirin acetylation of the COX‐1 binding site on platelets, or by providing insufficient COX‐1 inhibition during the dosing cycle.17 Although the potential for a cardioprotective effect for high‐dose naproxen remains unproved, supporting evidence includes recent observations that regular administration of high‐dose naproxen inhibits platelet COX‐1 activity throughout the dosing cycle.18
The Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET)19,20 was designed to evaluate the gastrointestinal and cardiovascular safety of lumiracoxib, a selective COX‐2 inhibitor, compared with two non‐selective NSAIDs, naproxen and ibuprofen. Given the paucity of cardiovascular safety data among high risk cardiovascular patients receiving non‐selective NSAIDs and COX‐2 inhibitors, we sought to evaluate the safety of these three drugs in high versus low risk patients in TARGET, stratified according to low‐dose aspirin use.
Methods
Patients and design
In brief, TARGET was an international, double blind study of 18 325 patients aged ⩾50 years with primary osteoarthritis comparing lumiracoxib 400 mg once daily (four times the recommended dose) with naproxen 500 mg twice daily (high‐dose naproxen), or with ibuprofen 800 mg three times daily (high‐dose ibuprofen) for 52 weeks.21 All patients were required to provide informed consent. The study was approved by the ethics committees in all participating centres. TARGET was divided into two substudies: one using naproxen, and the other using ibuprofen as the comparator; within each substudy, randomisation was stratified by age (50–64, 65–74, ⩾75 years) and low‐dose aspirin use. By design, the study was intended to include 24% of patients at high cardiovascular risk who were taking low‐dose aspirin for primary or secondary prevention. All patients were followed up for 52 weeks (or until withdrawal) with clinic visits at 4, 13, 20, 26, 39 and 52 weeks and a follow‐up visit (phone contact) 4 weeks after leaving the study.
TARGET predefined high cardiovascular risk as those patients with prior events, including silent myocardial infarction (MI) and a high cardiovascular risk based on the Framingham risk equations.22 Based on accumulating evidence from studies such as the Heart Outcomes Prevention Evaluation (HOPE) trial that subjects with diabetes and a cardiovascular risk factor ⩾1 also constitute a high cardiovascular risk cohort, we broadened the original TARGET definition of high cardiovascular risk to include patients with diabetes and a cardiovascular risk factor ⩾1.23 We conducted a post hoc subanalysis using this broader high cardiovascular risk definition stratified by treatment assignment and low‐dose aspirin use. Patients were excluded from the study if they had an MI, stroke, coronary artery bypass graft surgery, percutaneous coronary intervention, or new‐onset angina within 6 months before screening, ECG evidence of silent myocardial ischaemia, New York Heart Association congestive heart failure class III–IV, or if they were receiving anticoagulation treatment.
Low‐dose aspirin (75–100 mg/day) use was strongly recommended in all high risk patients based upon existing guidelines24,25; however, the decision for administration of aspirin was ultimately left to the discretion of the individual investigator. At baseline the investigator reported whether a patient was taking low‐dose aspirin, and the patient was randomised accordingly on the basis of aspirin use and age using an Interactive Voice Response System.
A composite cardiovascular end point modified from the Antiplatelet Trialists' Collaboration (APTC)26 was the primary end point for this analysis of cardiovascular adverse events. This end point included confirmed silent (ECG‐detected) MI, confirmed or probable clinical MI, stroke (ischaemic and haemorrhagic) and cardiovascular death. All events were adjudicated by an independent Cardiovascular and Cerebrovascular Safety Committee (CCSC). Myocardial infarction was divided into established or acute; established MI was defined as the development of new pathological Q waves on serial ECGs, and acute or recent MI was defined according to current guidelines.27 Serial ECGs were obtained and analysed locally for any cardiac ischaemic events occurring during the study. In addition to clinical events, the CCSC reviewed the cases of ECG‐detected MI (reported as a new finding on the end of study/post‐baseline ECG by the central ECG reading laboratory). These events were categorised as confirmed silent (ECG‐detected) MI or no event of silent (ECG‐detected) MI. Other adverse events of interest included hypertension, measured as described previously,20 and congestive heart failure data collected using case report forms as part of the monitoring and recording of all adverse events.
Role of the funding source
This analysis was designed as collaboration between the CCSC and the sponsor. The sponsor managed the data; the authors had unrestricted access to the data for manuscript preparation.
Statistical analysis
Analysis was by intention to treat. Time‐to‐event data were fitted to subgroups of interest by means of Cox proportional hazards models including age and treatment group as factors. Hazard ratios (HRs) (and 95% confidence intervals (CIs)) were used to assess between‐treatment group differences; in the case of zero events in one of the treatment groups, Fisher's exact tests were used instead. Homogeneity of HRs across relevant strata (substudy, high cardiovascular risk and aspirin use) was tested by including appropriate interaction terms in the above Cox proportional hazards models. All p values were two sided and considered significant if <0.05.
Results
Patients
The TARGET study included 18 325 patients with osteoarthritis: 3042 (16.6%) met the definition of high cardiovascular risk and 15 283 (83.4%) were considered patients at low cardiovascular risk. Among the 3042 patients at high cardiovascular risk, 1699 (56%) were in the naproxen substudy and 1343 (44%) in the ibuprofen substudy (p<0.0001). Overall, 60% of high risk patients were taking low‐dose aspirin for cardiovascular protection (57% in the ibuprofen and 62% in the naproxen substudy). Consistent with the overall TARGET trial, the 1 year drop out rate in this high risk cohort was 43%; most withdrawals occurred with ibuprofen (48%).
Baseline characteristics
Baseline characteristics differed across substudies in both high and low cardiovascular risk cohorts. The ibuprofen substudy included, on average, a significantly higher number of patients with diabetes and dyslipidaemia but a lower number of patients with a history of cerebrovascular or cardiovascular disease than the naproxen substudy (p<0.0001 for all comparisons). In both substudies, the patients receiving low‐dose aspirin were older, had a higher incidence of cerebrovascular or cardiovascular disease than patients without aspirin treatment (table 1).
Table 1 Characteristics of patients in the ibuprofen and naproxen substudies stratified by cardiovascular risk.
| Low CV risk without aspirin | Low CV risk with aspirin | High CV risk without aspirin | High CV risk with aspirin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ibuprofen (n = 3181) | Lumiracoxib (n = 3075) | Ibuprofen (n = 593) | Lumiracoxib (n = 581) | Ibuprofen (n = 250) | Lumiracoxib (n = 326) | Ibuprofen (n = 373) | Lumiracoxib (n = 394) | ||
| Age (years) | 62.3 | 62.3 | 65.6 | 65.3 | 64.6 | 64.3 | 67.6 | 68.0 | |
| Female sex (%) | 78.7 | 77.9 | 73.4 | 75.0 | 70.0 | 69.9 | 78.3 | 73.9 | |
| Body mass index (kg/m2) | 29.5 | 29.7 | 29.6 | 29.8 | 32.0 | 32.0 | 30.9 | 30.4 | |
| Hypertension (%) | 36.5 | 38.7 | 57.2 | 56.1 | 68.8 | 66.6 | 78.3 | 73.9 | |
| Diabetes mellitus (%) | 0.8 | 1.2 | 1.2 | 0.9 | 64.8 | 63.8 | 36.7 | 36.0 | |
| Dyslipidaemia (%) | 18.7 | 18.8 | 30.0 | 28.7 | 34.4 | 31.9 | 44.8 | 45.9 | |
| Current smoker (%) | 9.6 | 10.6 | 7.1 | 6.5 | 13.2 | 16.0 | 13.9 | 11.9 | |
| History of angina pectoris (%) | 0.0 | 0.0 | 0.0 | 0.0 | 4.8 | 6.4 | 11.8 | 14.7 | |
| Prior myocardial infarction (%) | 0.0 | 0.0 | 0.0 | 0.0 | 5.6 | 4.0 | 13.7 | 11.2 | |
| Prior cardiac revascularisation (%) | 0.0 | 0.0 | 0.0 | 0.0 | 3.2 | 1.5 | 7.5 | 6.9 | |
| Prior cardiac catheterisation (%) | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 | 0.3 | 0.8 | 1.5 | |
| History of cerebrovascular disease (%) | 0.0 | 0.0 | 0.0 | 0.0 | 4.8 | 4.3 | 14.2 | 14.0 | |
| High CV risk (Framingham) (%) | 0.0 | 0.0 | 0.0 | 0.0 | 17.2 | 16.0 | 10.7 | 9.9 | |
| Prior CCV history (%) | 0.0 | 0.0 | 0.0 | 0.0 | 29.2 | 31.0 | 71.6 | 74.1 | |
| CCV history or high CV risk (%) | 0.0 | 0.0 | 0.0 | 0.0 | 46.4 | 46.9 | 82.3 | 84.0 | |
| Total cholesterol (mg/l) | 217.9 | 217.5 | 211.0 | 212.3 | 217.1 | 216.3 | 209.4 | 209.5 | |
| Baseline systolic BP (mm Hg) | 130.0 | 129.9 | 131.4 | 131.8 | 134.3 | 134.5 | 137.8 | 135.8 | |
| Baseline diastolic BP (mm Hg) | 79.0 | 79.3 | 78.5 | 78.7 | 79.2 | 79.5 | 80.4 | 79.0 | |
| Low‐dose aspirin (%) | 0.0 | 0.0 | 100.0 | 100.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Study completed (%) | 56.9 | 61.6 | 58.0 | 60.9 | 48.4 | 54.6 | 53.6 | 56.6 | |
| Naproxen (n = 3202) | Lumiracoxib (n = 3231) | Naproxen (n = 688) | Lumiracoxib (n = 651) | Naproxen (n = 335) | Lumiracoxib (n = 318) | Naproxen (n = 505) | Lumiracoxib (n = 541) | ||
| Age (years) | 62.5 | 62.6 | 65.0 | 64.9 | 65.6 | 65.5 | 66.8 | 67.0 | |
| Female sex (%) | 78.5 | 78.3 | 76.3 | 77.6 | 72.5 | 77.7 | 67.7 | 70.6 | |
| Body mass index (kg/m2) | 28.9 | 29.0 | 29.0 | 29.6 | 30.8 | 30.3 | 30.1 | 30.4 | |
| Hypertension (%) | 34.7 | 36.5 | 60.0 | 61.4 | 66.3 | 66.4 | 69.5 | 74.5 | |
| Diabetes mellitus (%) | 1.1 | 0.8 | 1.2 | 0.9 | 54.3 | 52.5 | 23.4 | 28.1 | |
| Dyslipidaemia (%) | 12.3 | 13.4 | 24.3 | 22.1 | 26.9 | 21.7 | 31.5 | 28.5 | |
| Current smoker (%) | 9.4 | 10.2 | 8.7 | 7.8 | 12.5 | 10.7 | 10.1 | 9.1 | |
| History of angina pectoris (%) | 0.0 | 0.0 | 0.0 | 0.0 | 8.1 | 9.1 | 23.8 | 25.7 | |
| Prior myocardial infarction (%) | 0.0 | 0.0 | 0.0 | 0.0 | 4.2 | 4.1 | 11.7 | 14.8 | |
| Prior cardiac revascularisation (%) | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.9 | 5.9 | 5.2 | |
| Prior cardiac catheterisation (%) | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.9 | 0.4 | 0.4 | |
| History of cerebrovascular disease (%) | 0.0 | 0.0 | 0.0 | 0.0 | 8.1 | 7.5 | 15.8 | 15.5 | |
| High CV risk (Framingham) (%) | 0.0 | 0.0 | 0.0 | 0.0 | 15.5 | 12.9 | 6.3 | 5.2 | |
| Prior CCV history (%) | 0.0 | 0.0 | 0.0 | 0.0 | 41.8 | 45.3 | 83.0 | 82.1 | |
| CCV history or high CV risk (%) | 0.0 | 0.0 | 0.0 | 0.0 | 57.3 | 58.2 | 89.3 | 87.2 | |
| Total cholesterol (mg/l) | 221.9 | 221.1 | 216.7 | 214.5 | 218.1 | 218.8 | 216.5 | 219.9 | |
| Baseline systolic BP (mm Hg) | 132.7 | 132.7 | 134.8 | 134.1 | 138.4 | 135.6 | 138.5 | 138.5 | |
| Baseline diastolic BP (mm Hg) | 80.1 | 80.3 | 80.8 | 80.7 | 81.2 | 80.6 | 81.6 | 81.5 | |
| Low‐dose aspirin (%) | 0.0 | 0.0 | 100.0 | 100.0 | 0.0 | 0.0 | 100.0 | 100.0 | |
| Study completed (%) | 63.2 | 65.6 | 63.8 | 61.1 | 55.2 | 61.9 | 62.6 | 60.1 | |
CV, cardiovascular; CCV, cerebrovascular; BP, blood pressure.
Composite cardiovascular end point
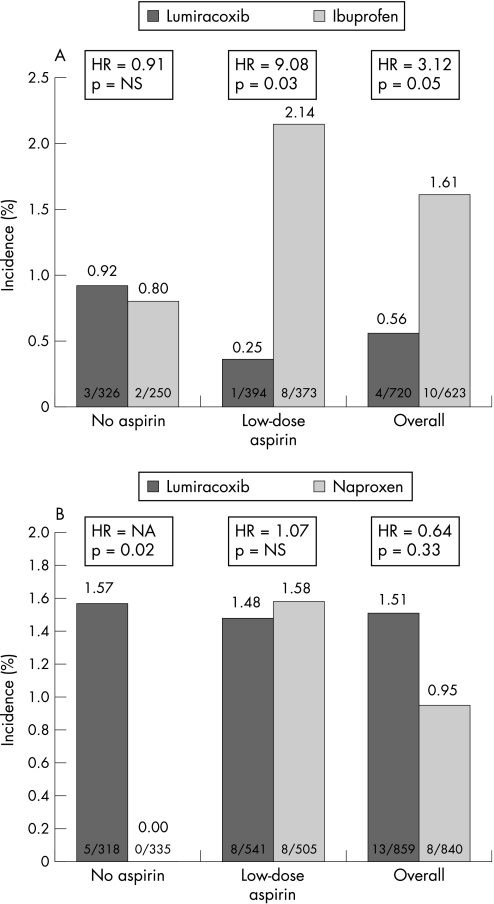
In the ibuprofen substudy, in patients at high cardiovascular risk receiving low‐dose aspirin, ibuprofen was associated with a higher 1 year rate of composite cardiovascular events than lumiracoxib (2.14% vs 0.25%; HR = 9.08, 95% CI 1.13 to 72.8; p = 0.038) (fig 1A). However, event rates were similar between naproxen and lumiracoxib (1.58% vs 1.48%; HR = 1.07, 95% CI 0.40 to 2.84; p = 0.90) (fig 1B). In patients at high cardiovascular risk without low‐dose aspirin treatment, there was no difference between ibuprofen and lumiracoxib (0.92% vs 0.80%; HR = 0.91, 95% CI 0.15 to 5.47; p = 0.92) (fig 1A); in contrast, there were no composite cardiovascular events in the naproxen group, whereas the incidence was 1.57% in the lumiracoxib group (p = 0.027) (fig 1B and table 2).
Figure 1 (A) Composite cardiovascular outcomes in the ibuprofen (A) and naproxen (B) substudies for high risk patients.
Table 2 Incidence of the composite cardiovascular outcome by baseline risk.
| Without aspirin | HR (95% CI) | p Value | With aspirin | HR (95% CI) | p Value | |||
|---|---|---|---|---|---|---|---|---|
| Ibuprofen No (%) | Lumiracoxib No (%) | Ibuprofen No (%) | Lumiracoxib No (%) | |||||
| Ibuprofen substudy | ||||||||
| Overall | 13/3431 (0.38) | 13/3401 (0.38) | 1.06 (0.49 to 2.28) | 0.88 | 10/966 (1.04) | 6/975 (0.62) | 1.79 (0.65 to 4.93) | 0.26 |
| Low CV risk | 11/3181 (0.35) | 10/3075 (0.33) | 1.13 (0.48 to 2.66) | 0.77 | 2/593 (0.34) | 5/581 (0.86) | 0.40 (0.08 to 2.08) | 0.27 |
| High CV risk | 2/250 (0.80) | 3/326 (0.92) | 0.91 (0.15 to 5.47) | 0.92 | 8/373 (2.14) | 1/394 (0.25) | 9.08 (1.13 to 72.76) | 0.038 |
| Naproxen No (%) | Lumiracoxib No (%) | Naproxen No (%) | Lumiracoxib No (%) | |||||
| Naproxen substudy | ||||||||
| Overall | 14/3537 (0.40) | 22/3549 (0.62) | 0.67 (0.34 to 1.31) | 0.242 | 13/1193 (1.09) | 18/1192 (1.51) | 0.70 (0.35 to 1.44) | 0.337 |
| Low CV risk | 14/3202 (0.44) | 17/3231 (0.53) | 0.88 (0.43 to 1.78) | 0.714 | 5/688 (0.73) | 10/651 (1.54) | 0.45 (0.15 to 1.32) | 0.149 |
| High CV risk | 0/335 (0.00) | 5/318 (1.57) | Not applicable | 0.027* | 8/505 (1.58) | 8/541 (1.48) | 1.07 (0.40 to 2.84) | 0.899 |
Regarding the incidence of composite cardiovascular events in the overall population (both aspirin and non‐aspirin users), there was a significant treatment by substudy interaction (p = 0.038), indicating that, relative to lumiracoxib, naproxen and ibuprofen have different hazards. Hazard ratios were also found to be heterogeneous across the four substudies by aspirin strata (p = 0.023), predominantly because patients receiving aspirin and ibuprofen had a higher risk than patients receiving lumiracoxib (2.14% vs 0.25%, respectively), while patients not receiving aspirin had a lower risk when receiving naproxen than lumiracoxib (0% vs 1.57%, respectively.)
In the low cardiovascular risk subgroups, no significant differences between lumiracoxib versus either ibuprofen or naproxen were seen (table 2). All composite cardiovascular events occurred either during treatment with the study drug or within 60 days after discontinuation.
Congestive heart failure
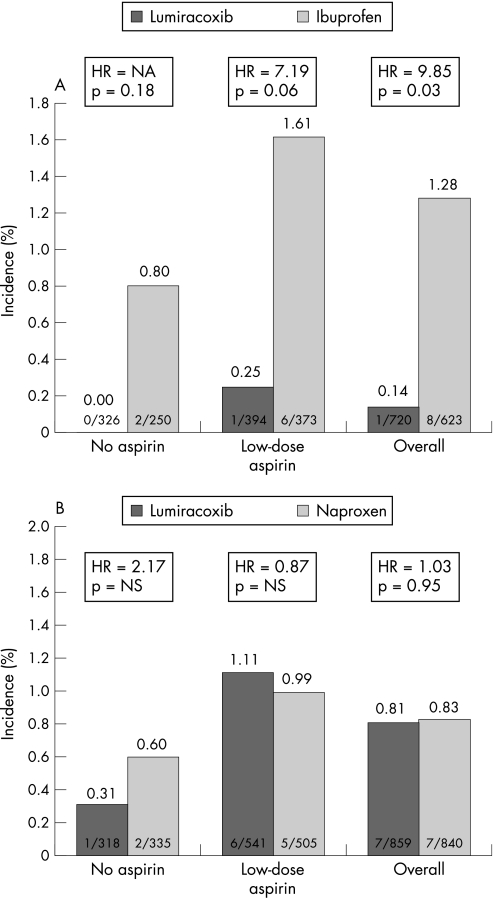
In the ibuprofen substudy congestive heart failure developed more often with ibuprofen in patients at high cardiovascular risk than with lumiracoxib (1.28% vs 0.14%; p = 0.031), whereas there was no difference between naproxen and lumiracoxib in the naproxen substudy (0.83% vs 0.81%; p = 0.95; table 3).
Table 3 Rates of development of congestive heart failure by cardiovascular risk.
| Without aspirin | HR | p Value | With aspirin | HR | p Value | |||
|---|---|---|---|---|---|---|---|---|
| Ibuprofen No (%) | Lumiracoxib No (%) | Ibuprofen No (%) | Lumiracoxib No (%) | |||||
| Ibuprofen substudy | ||||||||
| Overall | 6/3431 (0.17) | 9/3401 (0.26) | 0.92 (0.31 to 2.74) | 0.879 | 9/966 (0.93) | 3/975 (0.31) | 3.26 (0.88 to 12.05) | 0.077 |
| Low CV risk | 4/3181 (0.13) | 9/3075 (0.29) | 0.60 (0.18 to 2.05) | 0.414 | 3/593 (0.51) | 2/581 (0.34) | 1.48 (0.25 to 8.87) | 0.666 |
| High CV risk | 2/250 (0.80) | 0/326 (0.00) | Not applicable | 0.188 * | 6/373 (1.61) | 1/394 (0.25) | 7.19 (0.86 to 59.94) | 0.068 |
| Naproxen No (%) | Lumiracoxib No (%) | Naproxen No (%) | Lumiracoxib No (%) | |||||
| Naproxen substudy | ||||||||
| Overall | 9/3537 (0.25) | 4/3549 (0.11) | 1.86 (0.54 to 6.36) | 0.322 | 7/1193 (0.59) | 6/1192 (0.50) | 1.14 (0.38 to 3.38) | 0.818 |
| Low CV risk | 7/3202 (0.22) | 3/3231 (0.09) | 1.77 (0.42 to 7.40) | 0.435 | 2/688 (0.29) | 0/651 (0.00) | Not applicable | 0.50* |
| High CV risk | 2/335 (0.60) | 1/318 (0.31) | 2.17 (0.20 to 24.19) | 0.528 | 5/505 (0.99) | 6/541 (1.11) | 0.87 (0.26 to 2.84) | 0.814 |
*p Values from Fisher exact tests; †p values based on Wald χ2 statistics derived from Cox proportional hazards models with factors age and treatment.
‡Composite cardiovascular end point = APTC end point of confirmed or probable MI (clinical and silent), stroke or cardiovascular death.
§Hazard ratios use lumiracoxib as reference group—that is, HRs >1 favour lumiracoxib.
Stratification by aspirin use disclosed no significant differences (figs 2A and B), in high‐risk patients taking aspirin, there was a trend towards more heart failure events in the ibuprofen group compared with the lumiracoxib group (0.25% vs 1.61%; p = 0.06). In the low cardiovascular risk subgroups, no significant differences between lumiracoxib versus either ibuprofen or naproxen were seen (table 3).
Figure 2 (A) Development of congestive heart failure in high risk patients in the ibuprofen (A) and naproxen (B) substudies.
Discussion
In this study we found that the outcome of a large cohort of cardiovascular high risk patients with osteoarthritis receiving non‐selective NSAIDs or selective COX‐2 inhibitors largely depends on the specific analgesic agent, as well as the presence or absence of low‐dose aspirin treatment. We found that high risk patients not taking aspirin did have a higher cardiovascular event rate with lumiracoxib than with naproxen, but not ibuprofen. In contrast, high risk patients treated with ibuprofen who were taking low‐dose aspirin had a higher incidence of cardiovascular events than patients treated with lumiracoxib, a finding consistent with the hypothesis that ibuprofen interferes with the antiplatelet effects of aspirin. In addition, an increased risk of congestive heart failure events for high risk patients was observed for patients treated with ibuprofen compared with lumiracoxib. These findings, though limited by the post hoc design of the study, and the small number of events in the subgroups of interest suggest that caution is warranted in prescribing ibuprofen to high risk patients.
Evidence from randomised clinical trials to determine the safety of prescribing non‐selective NSAIDs and COX‐2 inhibitors in high risk cardiovascular patients is extremely limited. Two randomised trials of parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery raised concern owing to an excess number of cardiovascular adverse events.14,15 In the second parecoxib/valdecoxib trial, patients randomised to intravenous parecoxib/oral valdecoxib had a higher incidence of cardiovascular events than patients given placebo (2.0% vs 0.5%, p = 0.03).15 It should be noted that all patients in these studies received low‐dose aspirin treatment, and, therefore, any observed increase in cardiovascular events can not be attributed to unopposed, “selective” COX‐2 inhibition. These are the only studies aside from ours in which aspirin use was encouraged in a high risk population. Aside from short term clinical studies of the effect of COX‐2 inhibitors on endothelial function,28,29 no other clinical trials of “hard” cardiovascular outcomes have been conducted in the high cardiovascular risk population for commonly prescribed non‐selective NSAIDs or COX‐2 inhibitors.
Limitations of previously published COX‐2 inhibitor clinical trials have been the exclusion of aspirin users and patients at high cardiovascular risk, the short term study duration and the non‐blinded adjudication of cardiovascular end points. The absence of prospective, long term cardiovascular safety data on ibuprofen and naproxen has led to a reliance on retrospective observational studies, frequently from administrative data subject to confounding by both measured and unmeasured variables. In contrast, TARGET included a prespecified recruitment of aspirin users explicitly to employ patients at high cardiovascular risk, which allowed conclusions to be drawn on cardiovascular safety and toxicity relative to aspirin use. Moreover, TARGET was a 12 month study with prespecified cardiovascular end points and independent adjudication.
Three important cardiovascular findings regarding ibuprofen and naproxen should be highlighted from this subanalysis. First, our data suggest that ibuprofen treatment among low‐dose aspirin users is associated with an increased incidence of composite cardiovascular events in patients at high cardiovascular risk in comparison with lumiracoxib. This finding has significant public health implications. Our findings are consistent with previous evidence that ibuprofen can interfere with aspirin acetylation of the COX‐1 binding site on platelets, thereby blocking aspirin‐mediated inhibition of platelet aggregation.13 Further evidence of this interaction was observed by MacDonald and Wei in a retrospective study demonstrating an increase in all‐cause and cardiovascular mortality among patients taking aspirin plus ibuprofen as compared with those taking aspirin alone.11 Similarly, Kurth et al conducted a subanalysis of the Physicians Health Study and concluded that regular use of non‐selective NSAIDs in combination with low‐dose aspirin is associated with an increased risk of MI relative to low‐dose aspirin users not taking non‐selective NSAIDs.30 However, other studies examining the aspirin interaction with non‐selective NSAIDs found no significant difference in aspirin efficacy in the presence of non‐selective NSAID co‐therapy.6,15
The second principal finding was the relative cardiovascular safety of naproxen 500 mg twice daily relative to supratherapeutic lumiracoxib doses of 400 mg once daily among non‐aspirin users in patients with increased cardiovascular risk. In the overall TARGET population, the risk of the composite cardiovascular end point for lumiracoxib versus naproxen among non‐aspirin users was similar (HR = 0.67, 95% CI 0.34 to 1.31, p = 0.24).20 Our high risk analysis, however, observed a significant difference in composite cardiovascular events (p = 0.02) for naproxen compared with lumiracoxib among patients not receiving low‐dose aspirin. In contrast, no significant difference in the rate of composite cardiovascular events was noted in the subanalysis of ibuprofen versus lumiracoxib among non‐aspirin users. In high risk patients not receiving aspirin, naproxen 500 mg twice daily appears to be associated with the lowest cardiovascular risk. This finding is consistent with the ability of naproxen to inhibit platelet aggregation in a similar way to aspirin. However, unlike aspirin, persistent blood levels of naproxen are needed to maintain inhibition of platelet aggregation.18,31
Whether or not naproxen is truly cardioprotective has been of great interest since the VIGOR trial demonstrated higher rates of cardiovascular events for rofecoxib 50 mg daily versus naproxen 500 mg twice daily.2 Recently, Juni et al conducted a meta‐analysis of 11 observational studies of naproxen compared with control and concluded that naproxen was associated with a small, but statistically significant reduction in risk of MI (combined estimate 0.86, 95% CI 0.75 to 0.99).7 However, many of these studies individually failed to demonstrate a cardioprotective effect for naproxen, and one of the studies reported an increased risk of MI among current naproxen users relative to past users of NSAIDs. Subsequent to this meta‐analysis, Graham et al published a nested case–control study of patients in the Kaiser Permanente health system and found that naproxen was associated with an increased risk of MI or sudden cardiac death relative to past NSAID users.32
Explanations for the conflicting evidence for naproxen may be related to the naproxen dosage prescribed and whether or not aspirin was co‐administered. Among healthy volunteers, it has been demonstrated that naproxen 500 mg twice daily suppresses thromboxane B2 production, a marker of platelet COX‐1 activity, to a similar level as low‐dose aspirin 100 mg daily.18 However, lower doses of naproxen are frequently prescribed for analgesic treatment that may not provide comparable inhibition of platelet aggregation. In addition, the administration of naproxen to aspirin users has recently been shown to interfere with the effects of aspirin on platelet COX‐1 activity in healthy volunteers.33 Thus, the conflicting nature of the evidence on the effects of naproxen may be due to inadequate platelet inhibition with low‐dose naproxen and/or competition with aspirin for the platelet binding site. Alternatively, depending on the study duration, naproxen may increase cardiovascular risk owing to a sustained rise in blood pressure. Based on our findings, in high risk patients not receiving aspirin, high‐dose naproxen appears to have the lowest cardiovascular risk among the three agents studied in TARGET, although the confidence intervals around these estimates are wide.
The third principal finding from this study was that patients at high cardiovascular risk given ibuprofen (but not naproxen) had a significantly higher incidence of congestive heart failure events than patients administered lumiracoxib. This was primarily owing to events that occurred in patients at high cardiovascular risk receiving aspirin. One possible explanation for this finding may be differences in fluid retention and blood pressure effects among the three agents. In TARGET, Farkouh et al previously reported that lumiracoxib induced a smaller effect on blood pressure than either ibuprofen or naproxen, although the absolute differences were more pronounced between lumiracoxib and ibuprofen than between lumiracoxib and naproxen.20 However, the mechanism underlying these differences in observed haemodynamic effects remains speculative and requires further study.
Limitations
As a post hoc subgroup analysis, this study is subject to inherent limitations, and therefore should be interpreted as a hypothesis‐generating study. The risk of bias may be minimal; however, the risk of a type I error due to multiple comparisons is certainly increased. Moreover, the incidence of cardiovascular events was low in both the low and high risk subgroups, thereby affecting the power of the analyses. Although low‐dose aspirin use was recorded at study entry, further information about aspirin use was not collected, and the reason for not using aspirin in high risk patients was not specified. Also, the 1 year duration of the trial may not reflect the longer term risks of non‐selective NSAIDs and selective COX‐2 inhibitors in stable high risk patients. However, clinical trials of NSAIDs and COX‐2 inhibitors typically last for 6–12 weeks, rendering even pooled analyses difficult to interpret owing to low event rates. In contrast, the TARGET trial, along with the VIGOR trial2 and the Celecoxib Long‐term Arthritis Safety Study (CLASS)34 trials, represent the longest duration of arthritis clinical trials to date.
Conclusions
In patients with osteoarthritis at high cardiovascular risk not receiving low‐dose aspirin treatment, naproxen, but not ibuprofen, was found to have a lower cardiovascular risk than lumiracoxib. In contrast, among patients at high cardiovascular risk taking low‐dose aspirin, ibuprofen was associated with a higher incidence of cardiovascular events than lumiracoxib and naproxen. These results are consistent with the ability of ibuprofen to interfere with the effects of aspirin on platelet aggregation.
Owing to the over‐the‐counter availability of ibuprofen and naproxen, coupled with the scarcity of long term NSAID clinical trials in high risk patients, the findings of this study have immediate relevance to patients with arthritis at increased cardiovascular risk. Given the continuing debate on the cardiovascular safety of non‐selective NSAIDs and COX‐2 inhibitors, further research concentrating on patients at high cardiovascular risk and on the aspirin–non‐steroidal drug interaction is warranted. A long term, placebo controlled, randomised trial in the high cardiovascular risk population is required to evaluate further the cardiovascular safety of both selective and non‐selective NSAIDs and to balance these risks with the associated gastrointestinal effects of these agents. In the meantime, caution is advised for high risk patients prescribed COX‐2 inhibitors and non‐selective NSAIDs, particularly ibuprofen.
Abbreviations
CCSC - Cardiovascular and Cerebrovascular Safety Committee
CI - confidence interval
COX - cyclooxygenase
HOPE - Heart Outcomes Prevention Evaluation
HR - hazard ratio
MI - myocardial infarction
NSAIDs - non‐steroidal anti‐inflammatory drugs
TARGET - Therapeutic Arthritis Research and Gastrointestinal Event Trial
VIGOR - Vioxx Gastrointestinal Outcomes Research
Footnotes
Funding: Source of support: Novartis AG, Basel, Switzerland.
Conflict of interest: MEF has received speaking and consulting honoraria from Novartis, Bristol Myers Squibb and Sanofi. JDG has received speaking and consulting honoraria from Pfizer and was supported by a Physician Scientist Development Award from the American College of Rheumatology/Arthritis Foundation. RVJ was supported by the AstraZeneca Scholarship of the Swiss Society of Hypertension; the Freiwillige Akademische Gesellschaft Basel, Switzerland; and the Fund for the Promotion of a new Generation of Academics at the University of Basel, Switzerland. FWAV has received educational and research grants from Bayer, Roche, Eli Lilly, and Boehringer Ingelheim; and has received consulting honoraria from Pharmacia Upjohn, Eli Lilly, Merck, and Bayer. HK has acted as a consultant for Novartis, Wyeth, Sanofi‐Synthelabo, Bristol Myers Squibb, and AstraZeneca; as a study investigator for Pfizer, Bristol Myers Squibb, Sanofi‐Synthelabo, Boehringer Ingelheim, ONO, Fujisawa and AstraZeneca; and is a member of the speakers' bureau for Pfizer, Novartis, Janssen, Forest, Boehringer Ingelheim, Sanofi Synthelabo, Bristol Myers Squibb, AstraZeneca and Wyeth. JSH has received clinical trial research support from Arginox and has consulted for GlaxoSmithKline, Sanofi and Datascope. CLL has received speaking and consulting honoraria from Ortho‐McNeil, Pfizer, Allergan, and GlaxoSmithKline. SR has received consulting honoraria from Novartis, Boehringer Ingelheim, Bristol Myers Squibb, Solvay and ESP Pharma. BM and PTM are employees of Novartis. VF has acted as a consultant to GlaxoSmithKline, Kereos and Vasogen. SBA has received speaking and consulting honoraria from Pfizer, GlaxoSmithKline, Merck, NicOX, Eli Lilly and Novartis.
References
- 1.Griffin M R. Epidemiology of nonsteroidal anti‐inflammatory drug‐associated gastrointestinal injury. Am J Med 199810423–9S discussion 412S. [DOI] [PubMed] [Google Scholar]
- 2.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos Vargas R, Davis B.et al Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 20003431520–1528. [DOI] [PubMed] [Google Scholar]
- 3.Bresalier R S, Sandler R S, Quan H, Bolognese J A, Oxenius B, Horgan K.et al Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 20053521092–1102. [DOI] [PubMed] [Google Scholar]
- 4.Nussmeier N A, Whelton A A, Brown M T, Langford R M, Hoeft A, Parlow J L.et al Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 20053521081–1091. [DOI] [PubMed] [Google Scholar]
- 5.Solomon S D, Zelenkofske S, McMurray J J, Finn P V, Velazquez E, Ertl G.et al Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 20053521071–1080. [DOI] [PubMed] [Google Scholar]
- 6.Curtis J P, Krumholz H M. The case for an adverse interaction between aspirin and non‐steroidal anti‐inflammatory drugs: is it time to believe the hype? J Am Coll Cardiol 200443991–993. [DOI] [PubMed] [Google Scholar]
- 7.Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe P A, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta‐analysis. Lancet 20043642021–2029. [DOI] [PubMed] [Google Scholar]
- 8.Kimmel S E, Berlin J A, Reilly M, Jaskowiak J, Kishel L, Chittams J.et al Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann Intern Med 2005142157–164. [DOI] [PubMed] [Google Scholar]
- 9.Patel T N, Goldberg K C. Use of aspirin and ibuprofen compared with aspirin alone and the risk of myocardial infarction. Arch Intern Med 2004164852–856. [DOI] [PubMed] [Google Scholar]
- 10.Kimmel S E, Berlin J A, Reilly M, Jaskowiak J, Kishel L, Chittams J.et al The effects of nonselective non‐aspirin non‐steroidal anti‐inflammatory medications on the risk of nonfatal myocardial infarction and their interaction with aspirin. J Am Coll Cardiol 200443985–990. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald T M, Wei L. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet 2003361573–574. [DOI] [PubMed] [Google Scholar]
- 12.Brochier M L. Evaluation of flurbiprofen for prevention of reinfarction and reocclusion after successful thrombolysis or angioplasty in acute myocardial infarction. The Flurbiprofen French Trial. Eur Heart J 199314951–957. [DOI] [PubMed] [Google Scholar]
- 13.Altman R, Luciardi H L, Muntaner J, Del Rio F, Berman S G, Lopez R.et al Efficacy assessment of meloxicam, a preferential cyclooxygenase‐2 inhibitor, in acute coronary syndromes without ST‐segment elevation: the Nonsteroidal Anti‐Inflammatory Drugs in Unstable Angina Treatment‐2 (NUT‐2) pilot study. Circulation 2002106191–195. [DOI] [PubMed] [Google Scholar]
- 14.Ott E, Nussmeier N A, Duke P C, Feneck R O, Alston R P, Snabes M C.et al Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg 20031251481–1492. [DOI] [PubMed] [Google Scholar]
- 15.Nussmeier N A, Whelton A A, Brown M T, Langford R M, Hoeft A, Parlow J L.et al Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 20053521081–1091. [DOI] [PubMed] [Google Scholar]
- 16.FitzGerald G A, Patrono C. The coxibs, selective inhibitors of cyclooxygenase‐2. N Engl J Med 2001345433–442. [DOI] [PubMed] [Google Scholar]
- 17.Catella‐Lawson F, Reilly M P, Kapoor S C, Cucchiara A J, DeMarco S, Tournier B.et al Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 20013451809–1817. [DOI] [PubMed] [Google Scholar]
- 18.Capone M L, Tacconelli S, Sciulli M G, Grana M, Ricciotti E, Minuz P.et al Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low‐dose aspirin in healthy subjects. Circulation 20041091468–1471. [DOI] [PubMed] [Google Scholar]
- 19.Schnitzer T J, Burmester G R, Mysler E, Hochberg M C, Doherty M, Ehrsam E.et al Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004364665–674. [DOI] [PubMed] [Google Scholar]
- 20.Farkouh M E, Kirshner H, Harrington R A, Ruland S, Verheugt F W, Schnitzer T J.et al Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 2004364675–684. [DOI] [PubMed] [Google Scholar]
- 21.Hawkey C J, Farkouh M, Gitton X, Ehrsam E, Huels J, Richardson P. Therapeutic arthritis research and gastrointestinal event trial of lumiracoxib ‐ study design and patient demographics. Aliment Pharmacol Ther 20042051–63. [DOI] [PubMed] [Google Scholar]
- 22.Grundy S M, Pasternak R, Greenland P, Smith S, Jr, Fuster V. AHA/ACC scientific statement: assessment of cardiovascular risk by use of multiple‐risk‐factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol 1999341348–1359. [DOI] [PubMed] [Google Scholar]
- 23.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000342145–153. [DOI] [PubMed] [Google Scholar]
- 24.Pearson T A, Blair S N, Daniels S R, Eckel R H, Fair J M, Fortmann S P.et al AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002106388–391. [DOI] [PubMed] [Google Scholar]
- 25.Harrington R A, Becker R C, Ezekowitz M, Meade T W, O'Connor C M, Vorchheimer D A.et al Antithrombotic therapy for coronary artery disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004126(Suppl)513–48S. [DOI] [PubMed] [Google Scholar]
- 26.Anonymous Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ 199430881–106. [PMC free article] [PubMed] [Google Scholar]
- 27.Alpert J S, Thygesen K, Antman E, Bassand J P. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 200036959–969. [DOI] [PubMed] [Google Scholar]
- 28.Chenevard R, Hurlimann D, Bechir M, Enseleit F, Spieker L, Hermann M.et al Selective COX‐2 inhibition improves endothelial function in coronary artery disease. Circulation 2003107405–409. [DOI] [PubMed] [Google Scholar]
- 29.Bogaty P, Brophy J M, Noel M, Boyer L, Simard S, Bertrand F.et al Impact of prolonged cyclooxygenase‐2 inhibition on inflammatory markers and endothelial function in patients with ischemic heart disease and raised C‐reactive protein: a randomized placebo‐controlled study. Circulation 2004110934–939. [DOI] [PubMed] [Google Scholar]
- 30.Kurth T, Glynn R J, Walker A M, Chan K A, Buring J E, Hennekens C H.et al Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal antiinflammatory drugs. Circulation 20031081191–1195. [DOI] [PubMed] [Google Scholar]
- 31.Rothschild B M. Comparative antiplatelet activity of COX1 NSAIDs versus aspirin, encompassing regimen simplification and gastroprotection: a call for a controlled study. Reumatismo 20045689–93. [DOI] [PubMed] [Google Scholar]
- 32.Graham D J, Campen D, Hui R, Spence M, Cheetham C, Levy G.et al Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo‐oxygenase 2 selective and non‐selective non‐steroidal anti‐inflammatory drugs: nested case‐control study. Lancet 2005365475–481. [DOI] [PubMed] [Google Scholar]
- 33.Capone M L, Sciulli M G, Tacconelli S, Grana M, Ricciotti E, Renda G.et al Pharmacodynamic interaction of naproxen with low‐dose aspirin in healthy subjects. J Am Coll Cardiol 2005451295–1301. [DOI] [PubMed] [Google Scholar]
- 34.Silverstein F E, Faich G, Goldstein J L, Simon L S, Pincus T, Whelton A.et al Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study. JAMA 20002841247–1255. [DOI] [PubMed] [Google Scholar]