Abstract
Background
Despite well‐documented immunomodulation by interferon γ (IFNγ), its role and mechanism of regulation of matrix metalloproteinase 13 (MMP13) gene expression in human chondrocytes is unknown.
Objective
To investigate the ability and mechanism of IFNγ to suppress interleukin 1 (IL1)‐induced MMP13 expression in articular chondrocytes.
Methods
Human chondrocytes were treated with IFNγ or IL1β alone or in combination. MMP13 mRNA was analysed by semiquantitative reverse transcriptase‐PCR. MMP13 protein, phospho‐signal transducer and activator of transcription 1 (STAT1) and p44/42 mitogen‐activated protein kinase levels were measured by western blotting. MMP13 promoter luciferase, cytomegalovirus cyclic AMP response element‐binding protein (CBP)/p300 plasmids and STAT1 small interfering RNA (siRNA) were transfected by the calcium phosphate method. IFNγ receptor was also neutralised. Activator protein (AP) 1 activity was monitored by the TransAM transcription factor kit. STAT1‐CBP/p300 interaction was studied by immunoprecipitation.
Results
IFNγ potently suppressed IL1‐induced expression of MMP13 and promoter activity. Blockade with neutralising IFNγ R1 antibody revealed that MMP13 inhibition by IFNγ is mediated by the IFN receptor. IFNγ‐stimulated activation of STAT1 was directly correlated with MMP13 suppression. Knockdown of the STAT1 gene by specific siRNA or its inhibition with fludarabine partially restored the IL1β induction of MMP13 expression and promoter activity. IFNγ did not alter AP1 binding ability but promoted physical interaction of STAT1 and CBP/p300 coactivator. p300 overexpression reversed IFNγ inhibition of endogenous MMP13 mRNA expression and exogenous MMP13 promoter activity.
Conclusion
IFNγ, through its receptor, activates STAT1, which binds with CBP/p300 coactivator, sequesters it from the cell system, and thus inhibits transcriptional induction of the MMP13 gene in chondrocytes. IFNγ and its signalling pathways could be targeted therapeutically for diminishing IL1‐induced cartilage degradation by MMP13 in patients with arthritis.
Synovial fluid and cartilage of patients with rheumatoid arthritis (RA) and osteoarthritis exhibit increased interleukin 1 (IL1) levels, a major cytokine implicated in arthritic inflammation and cartilage/bone destruction.1,2,3 IL1 induces matrix metalloproteinases (MMPs) and represses extracellular matrix (ECM) genes in chondrocytes and thus alters the physiology of joints.4,5 Blocking IL1 with specific antibodies, and its actions by IL1 receptor antagonists reduces cartilage/bone loss and invasion of cartilage by synovium in animal models and in patients.6,7,8 Thus, inhibition of IL1 action constitutes a valid treatment for arthritis.1,2
MMPs contribute to the physiological and pathological remodelling of ECM either by direct cleavage or by liberating or modifying ECM‐regulatory growth factors and cytokines.9,10 MMP13 cleaves the major cartilage type II collagen most efficiently.11 MMP13 levels are increased in cartilage and synovium of patients with arthritis.12,13,14 MMP13 expression is increased in ageing human chondrocytes and could contribute to cartilage catabolism in osteoarthritis.15 Cartilage‐specific overexpression of human MMP13 in mice mimics arthritic damage.16 MMP13 also preferentially cleaves fibromodulin at its N‐terminus.17 Thus, MMP13 is an important target for developing cartilage‐protective treatments.
Interferons (IFNs) have antiviral, antitumour and immunomodulatory activities.18 IFNs include type I (α, β), type II (γ) and IFNλ.19 IFNγ was considered pro‐inflammatory owing to its increase in the synovium of patients with RA.20 IFNγ inhibits aggrecan and biglycan synthesis and reduces chondrocyte proliferation.21,22 Here, we tested the hypothesis that IFNγ could antagonise IL1‐inducible MMP13 gene expression and investigated the mechanisms of such regulation in human articular chondrocytes. We show for the first time that IFNγ potently suppresses IL1β‐induced expression of MMP13 and promoter activity in chondrocytes through the specific receptor 1, activated STAT1 and its interaction with cyclic AMP response element‐binding protein (CBP)/p300.
Materials and methods
Chondrocytes and treatments
Normal human knee chondrocytes (Cambrex, Walkerville, Maryland, USA) were grown at high density in differentiation Bullekit medium where they maintain differentiated phenotype by expressing type II collagen until passage 3, as examined by northern blotting. Chondrocytes were grown in six‐well plates in Dulbecco's modified Eagle's medium (Invitrogen, Burlington, Canada) with 10% fetal calf serum, washed with phosphate‐buffered saline (PBS), kept in serum‐free Dulbecco's modified Eagle's medium for 24 h and exposed to IFNγ (300 U/ml) and IL1β (10 ng/ml; R&D Systems, Minneapolis, Minnesota, USA), alone or in combination for 24 h. In some experiments, chondrocytes were pretreated with Fludarabine (Sigma) in N,N‐dimethyl formamide.
Reverse transcriptase‐PCR
MMP13 and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) mRNA levels were analysed by reverse transcriptase ‐PCR with MMP13 specific primers23 as described previously12 yielding 491 and 226 bp cDNA bands.
Western blotting
Cells were lysed and centrifuged at 14 000 rpm for 10 min, and 20 μg of supernatant protein (Bio‐Rad Protein Assay) was resolved by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane by electroblotting. Membranes were blocked with 5% non‐fat milk in PBS, incubated with the primary antibodies diluted in 5% milk or bovine serum albumin overnight at 4°C. Antibodies against phospho‐STAT1‐tyr‐701, P‐STAT1‐ser‐727, total STAT1 and p44/42 (Cell Signalling Technology Inc, Danvers, Massachusetts, USA) were used at 1:1000 dilutions. The blots were washed four times with Tris‐buffered saline and incubated for 2 h with horseradish peroxidase (HRP)‐conjugated secondary antibody. Immunoreactive bands were developed with enhanced chemiluminescent substrate (Amersham, Biosciences, Piscataway, New Jersey, USA) and visualised.
For MMP13 blots, supernatant proteins were precipitated with cold 10% trichloroacetic acid and dissolved in 0.1 M NaOH. Sample‐loading buffer was added and boiled for 5 min, and proteins were separated by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis. Antibody against MMP13 (Sigma) was used at 1:500 dilution.
Plasmids, siRNA, transfections and luciferase assay
Chondrocytes were transfected with different vectors or siRNA by the calcium phosphate method for adherent cells in suspension as follows. Cells were detached by trypsinisation and the suspension was incubated with control and STAT1 siRNA (100 nM; Cell Signalling). Calcium phosphate was precipitated for 10 min and plated in a serum‐containing medium without antibiotics for 3 h. Cells were washed with PBS, allowed to recover for 30 h, maintained in serum‐free medium for 16 h and stimulated with IFNγ and IL1 for 24 h. An equal amount (20 μg) of protein was analysed for STAT1 levels. In some experiments, 4 μg of MMP13 promoter luciferase (−1000 to +71 region; p1000‐LUC), cytomegalovirus (CMV)–Renilla luciferase (4 μg, transfection control) and STAT1 siRNA (100 nM) were cotransfected and after recovery, treated with IFNγ and IL1, and luciferase activity measured with Promega Dual‐Luciferase Reporter Assay System (Promega Corporation, Madison, Wisconsin, USA) and Turner Designs Luminometer TD‐20/20 (Turner Designs, Sunnyvale, California, USA). Similarly, p300/CBP wild‐type cDNA‐expressing pCMVβ vectors (Upstate USA Inc, Charlottesville, Virginia, USA) were transfected alone or with MMP13 LUC vector.
Neutralisation of IFNγ receptor with antibody
Chondrocytes in serum‐free medium were incubated with IFNγ R1 monoclonal antibody and control‐matched isotype (mouse IgG1; R&D Systems) at 2 μg/ml for 1 h and then treated with IL1β or IFNγ. Alternatively, IFNγ was added 5 min before IL1β treatment for 24 h. Cells and media were analysed for MMP13 mRNA and protein, respectively. STAT1 protein was measured after 15 min of stimulation with IFNγ.
AP1 binding assay
For nuclear proteins, chondrocytes were resuspended in 1 ml of hypotonic buffer (20 mM HEPES, pH 7.5, 1.5 mM MgCl2, 20 mM KCl, 5 mM NaF, 0.2 mM EDTA, 0.5 mM dithiothreitol) for 20 min, centrifuged at 1200 rpm for 5 min and resuspended in hypotonic buffer containing 0.1% NP‐40. Cells were incubated on ice for 10 min, vortexed and centrifuged at 10 000 rpm for 10 min. Pellets were resuspended in 50 μl of Active Motif lysis buffer. After 30 min incubation on ice, samples were centrifuged and proteins measured. AP1 consensus nucleotide‐binding activity from nuclear extracts (10 μg) was measured with TransAM AP1 family (Active Motif, Carlsbad, California, USA) colorimetric system as recommended. Nuclear extract was added to the immobilised oligonucleotides, followed by primary transcription factor antibody, secondary HRP‐conjugated antibody and HRP substrate, and colorimetric values measured at 450 nm were plotted.
Immunoprecipitation
Cells were washed and harvested in lysis buffer with 10 μl/ml proteases inhibitors cocktail (Sigma), kept on ice for 30 min and then vortexed. Lysates were centrifuged at 14 000 rpm for 10 min and protein concentration in supernatants measured by Bio‐Rad kit and adjusted at 1 μg/μl with ice‐cold PBS. Total protein (1 mg) was cleared by incubating for 10 min with 50 μl of 50% bead slurry of protein A (Amersham) on a shaker. After centrifugation, supernatants were transferred to new pre‐chilled tubes. Primary antibody was added and incubated overnight with gentle shaking. Mouse or rabbit IgG was used as isotype‐matched antibody control. After incubation, protein A agarose beads (50 μl of 50% bead slurry) were added and incubated for 2 h on a shaker at 4°C. Tubes were centrifuged at 3000 rpm for 5 min. Beads with immunoprecipitated (IP) protein were washed with ice‐cold 1×radioimmunoprecipitation assay buffer. Bead pellets were resuspended in 2× sodium dodecyl sulphate loading buffer, vortexed, heated at 95°C for 5 min and supernatants analysed by western blotting.
All experiments were performed at least three times, and the reported results were reproducible.
Results
IFNγ inhibits IL1‐stimulated expression of MMP13 and promoter activity
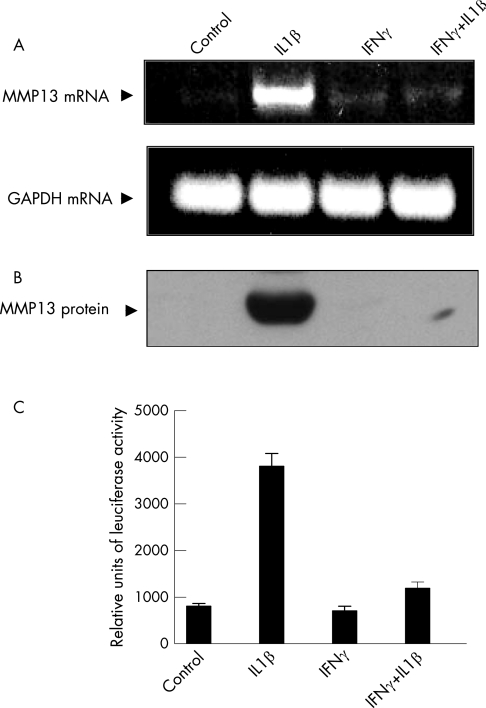
MMPs are critical modulators of cartilage degradation in arthritis. We studied the previously unexplored impact of IFNγ on IL1‐induced MMP13 expression in human articular chondrocytes. Serum‐free medium and IFNγ alone had no impact on MMP13 mRNA expression. As reported previously,24 IL1β potently induced MMP13 mRNA and protein expression within 24 h. IFNγ completely suppressed MMP13 mRNA induction without affecting the constitutive GAPDH mRNA expression (fig 1A). Western blotting of media showed comparable MMP13 protein inhibition (fig 1B). To investigate the mechanism of IFNγ‐mediated MMP13 suppression, chondrocytes were transfected with human MMP13 promoter (−1000 to +71)‐driven luciferase reporter plasmid.25 IL1β‐induced MMP13 promoter activity was completely abolished in IFNγ‐treated cells (fig 1C). Thus, IL1β‐inducible MMP13 expression can be completely suppressed by IFNγ at the levels of promoter, mRNA and protein expression.
Figure 1 Inhibition of interleukin (IL) 1β‐induced matrix metalloproteinase 13 (MMP13) expression and promoter activity by interferon γ (IFNγ). (A) Human chondrocytes in serum‐free medium were treated with vehicles only (0.1% bovine serum albumin; control) or exposed to IL1β/IFNγ for 24 h. MMP13 (upper panel) and glyceraldehyde‐3‐phosphate dehydrogenase (lower panel) mRNA was analysed by reverse transcriptase‐PCR. (B) MMP13 protein levels were determined by western blotting. (C) Chondrocytes were cotransfected with MMP13 promoter luciferase and Renilla luciferase vectors (4 μg). Following the recovery and indicated treatments in serum‐deficient medium, luciferase activity was measured in extracts and normalised with Renilla luciferase internal control. The values are mean (SD) of three separate experiments.
Anti‐IFNγR1 neutralising antibody reverses the IFNγ‐mediated suppression of MMP13
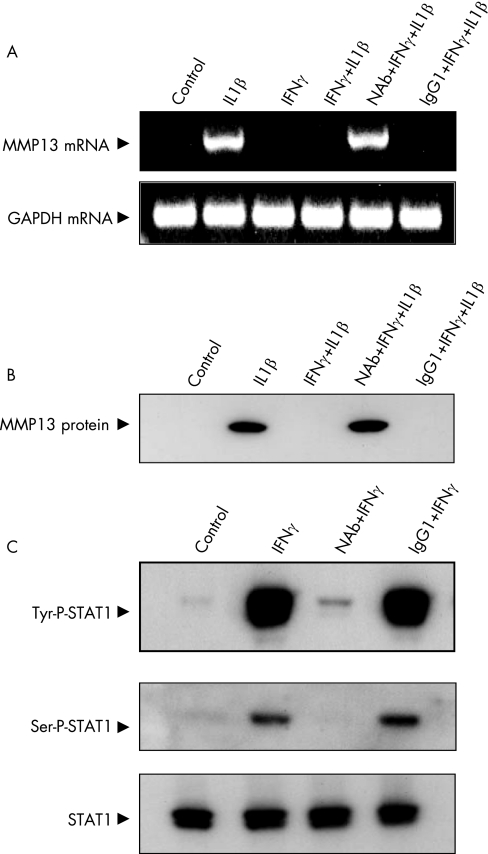
IFNγ exerts its effects by binding with IFNγR1, which is increased in rheumatoid synovium20 and is present in human chondrocytes (unpublished results). We investigated the role of IFNγR1 in the inhibition of MMP13 by blocking it with receptor antibody. Control mouse IgG1 isotype did not affect IFNγ‐mediated inhibition. However, IFNγR1 antibody blockade fully restored the IL1β‐induced upregulation of MMP13 mRNA (fig 2A) and protein (fig 2B) in the presence of IFNγ. To examine whether the neutralising antibody affected IFNγ signal transduction, we measured signal transducer and activator of transcription (STAT1) phosphorylation, a well‐documented IFN‐induced response.18 IFNγR1 antibody treatment blocked IFNγ‐stimulated STAT1 tyrosine phosphorylation and serine phosphorylation compared with the cells treated with control antibody. The total STAT1 levels were not affected by the treatments (fig 2C). Thus, interaction between IFNγ and IFNγR1 is essential for IFNγ‐driven inhibition of MMP13.
Figure 2 Blockade of interferon γ (IFNγ)‐mediated inhibition of matrix metalloproteinase 13 (MMP13) by anti‐IFNγ receptor 1 antibody. Chondrocytes were incubated with IFNγR1 monoclonal antibody and control‐matched isotype (mouse IgG1) at 2 μg/ml for 1 h. Cells were treated as indicated. (A) MMP13 mRNA and (B) protein was determined. (C) Chondrocytes incubated with neutralising antibody for IFN‐γR1 (NAb) and mouse IgG1 for 1 h were subjected to different treatments for 5 min and harvested. Western blots showing complete elimination of signal transducer and activator of transcription (STAT)‐1 tyrosine (tyr) and serine (ser) phosphorylation by NAb are depicted. Lower panel shows STAT1 blot as loading control.
IFNγ‐stimulated phosphorylation of STAT1 is directly associated with MMP13 inhibition
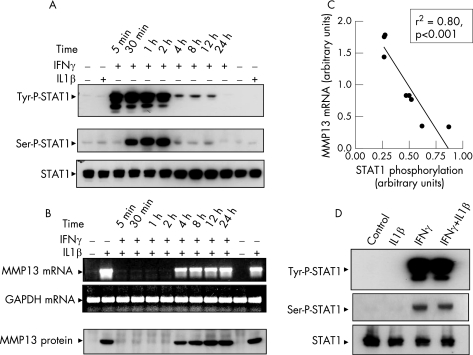
STAT1 is activated on binding of IFNγ to its receptor.18,19 To examine whether IFNγ‐activated STAT1 affected MMP13 expression, chondrocytes were incubated with IFNγ for different time periods. The maximal level of tyrosine‐phosphorylated STAT1 was detected within 5 min, was maintained at high level for at least 2 h and started to decline by 4 h, returning to the basal level within 24 h (fig 3A). A similar time course was observed with STAT1 serine phosphorylation, which increased at 30 min instead of at 5 min (fig 3A). Phosphorylation at specific tyrosine and serine residues is needed for full STAT1‐mediated transcriptional activity.19 IL1β did not induce STAT1 phosphorylation, and total STAT1 levels remained constant (fig 3A). To examine whether IFNγ‐activated STAT1 directly affects the IL1‐induced MMP13 expression, chondrocytes were pretreated with IFNγ for 5 min to 24 h, followed by additional stimulation with IL1β for 24 h. IL1β maximally induced MMP13 expression in 24 h (fig 3B, lane 2) and this induction remained similar at 48 h (fig 3B, last lane). Interestingly, during the 2 h of maximal STAT1 phosphorylation (fig 3A), IFNγ completely inhibited IL1‐induced MMP13 expression (fig 3B). Conversely, during the 4–24 h of minimal STAT1 phosphorylation (fig 3A), suppression of MMP13 mRNA and protein expression could be partially reversed (fig 3B). Thus, there was a significant direct correlation between STAT1 phosphorylation status and inhibition of MMP13 (fig 3C). The IL1β and IFNγ combination did not affect the IFNγ‐stimulated ser/tyr phosphorylation of STAT1 (fig 3D). These results provide strong evidence that IFNγ‐stimulated STAT1 phosphorylation is closely associated with the suppression of MMP13 expression.
Figure 3 Correlation of interferon γ (IFNγ)‐stimulated signal transducer and activator of transcription (STAT)1 phosphorylation with suppression of matrix metalloproteinase 13 (MMP13). (A) Chondrocytes were subjected to the indicated treatments with vehicle (control), interleukin (IL) 1 or IFNγ and extracts analysed for STAT1 tyrosine (tyr)‐701, serine (ser)‐727 phosphorylation and total STAT1. (B) Chondrocytes were pretreated with IFNγ for the indicated time points before additional stimulation with IL1β for 24 h. MMP13 mRNA and protein levels are shown. (C) Densitometric values of MMP13 reverse transcriptase‐PCR products in (B) and STAT1 phosphorylation in (A) quantified by NIH ImageJ 1.32j (National Institutes of Health, USA) software in arbitrary units were plotted against each other to depict the correlation between the two. (D) Western blots show that IL1β does not affect the IFNγ‐mediated activation (tyr/ser) of STAT1 in combined treatment.
STAT1 knockdown reverses inhibition of MMP13 expression and promoter activity by IFNγ
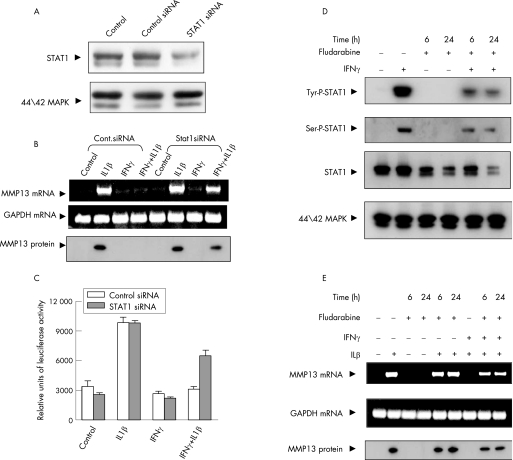
Owing to the observed association of STAT1 with MMP13 suppression, we investigated whether genetic and pharmacological modulation of STAT1 affected MMP13 expression. Transfection of chondrocytes with STAT1‐specific siRNA down regulated the endogenous STAT1 levels by 70% (determined by densitometry), whereas an equivalent amount of control siRNA had no effect. Additionally, the p42/44 mitogen‐activated protein kinase levels were not affected (fig 4A). STAT1‐specific siRNA significantly restored IL1‐mediated stimulation of MMP13 mRNA and protein, whereas control siRNA did not reverse the inhibition by IFNγ. The control GAPDH expression was not affected (fig 4B). To further confirm the involvement of STAT1, we analysed luciferase activity in chondrocytes cotransfected with STAT1 and control siRNAs, MMP13 promoter‐luciferase reporter plasmid and CMV–Renilla luciferase expression vector. STAT1‐specific siRNA significantly reversed the IFNγ‐mediated inhibition of MMP13 promoter‐driven luciferase activity, but STAT1‐negative control siRNA did not (fig 4C). Thus, STAT1 plays a major role in the IFNγ inhibition of MMP13 expression and promoter activity.
Figure 4 Reversal of interferon γ (IFNγ)‐mediated matrix metalloproteinase 13 (MMP13) suppression by signal transducer and activator of transcription (STAT)‐1 knockdown. (A) Cells were either mock transfected (control) or transfected with negative control and STAT1‐specific small interfering RNA (siRNA), incubated for 48 h and lysates subjected to western blotting with STAT1 and p44/42 mitogen‐activated protein kinase (MAPK) antibodies. (B) Chondrocytes transfected with STAT1 or control siRNA were analysed for MMP13 and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) gene expression by reverse transcriptase ‐PCR and MMP13 protein. (C) Chondrocytes were cotransfected with control or STAT1 siRNA, along with MMP13 promoter‐luciferase vector. Luciferase activity from cell lysates is depicted as bar graphs. (D) Chondrocytes were pretreated with fludarabine (15 μM) for 6 and 24 h, treated with IFNγ for 15 min and cell lysates used for STAT1 tyrosine (tyr)/serine (ser) phosphporylation or total STAT1 and p44/42 MAPK analysis. (E) Cells pretreated with fludarabine were incubated with IFNγ and ILβ for 24 h and MMP13 and GAPDH mRNA and MMP13 protein analysed.
Fludarabine is a specific pharmacological inhibitor of cytokine‐induced activation of STAT1 that does not affect other STATs.26 To investigate whether IFNγ signal transduction and STAT1 activation could be interrupted, we incubated the cells with or without fludarabine for 6 and 24 h before treatment with IFNγ. The drug significantly reduced IFNγ‐induced tyrosine/serine phosphorylation and total STAT1 levels. Fludarabine did not affect total p42/44 mitogen‐activated protein kinase levels (fig 4D). To further confirm the involvement of STAT1 in MMP13 down regulation, we analysed the IFNγ‐mediated inhibition of MMP13 in chondrocytes that underwent fludarabine‐driven inhibition of STAT1. Fludarabine treatment blocked IFNγ‐mediated suppression of MMP13 by restoring its mRNA and protein expression (fig 4E).
IFNγ‐stimulated phosphorylation of STAT1 does not alter AP1 binding ability but recruits CBP/p300
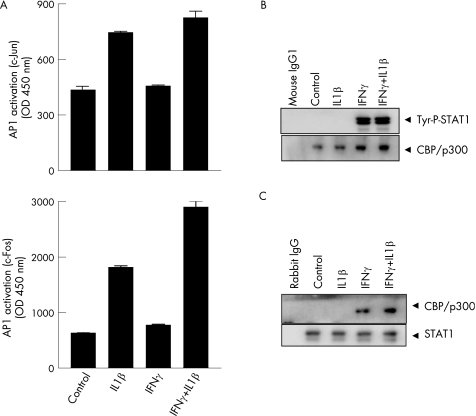
Human MMP13 gene contains AP1 and Ets1 transcription factor‐binding motifs in its promoter.25 As IL1 induces MMP13 gene in chondrocytes through the activation of AP1,27 we determined whether IFNγ inhibited the IL1β‐activated AP1‐binding ability. Nuclear extracts were analysed by AP1‐binding assay. IFNγ did not block the binding activity of c‐Fos and c‐Jun components of AP1, but rather increased c‐Fos binding (fig 5A). This suggested that IFNγ did not mediate MMP13 inhibition through AP1.
Figure 5 Analysis of activator protein (AP) 1 binding activity and phospho‐signal transducer and activator of transcription (STAT) 1‐cyclic AMP response element‐binding protein (CBP)/p300 interaction in response to interleukin (IL) 1β and interferon γ (IFNγ). (A) Chondrocytes were either treated with vehicles (control) or exposed to IL1β and IFNγ as shown, nuclear extracts were analysed by Active Motif AP1 c‐Jun (upper panel) and c‐Fos (lower panel) ELISA and values measured at 450 nm were plotted. (B) Proteins from treated chondrocytes were first immunoprecipitated (IP) with CBP/p300 antibody and then analysed by western blotting with anti‐phospho‐STAT1 antibody (upper panel). The protein from the first immunoprecipitation was also analysed by western blotting with CBP/p300 as loading control (lower panel). (C) Cellular extracts from (B) were first IP with STAT1 and then analysed by western blotting with anti‐CBP/p300. The protein from the first immunoprecipitation was also analysed by western blotting with STAT1 as loading control. Tyr, tyrosine.
CBP and p300 are critical transcriptional coactivators, which control many cellular functions through interactions with transcription factor IIB, RNA polymerase II and regulatory transcription factors.28,29 To determine whether STAT1 physically interacts with p300, chondrocytes were subjected to different treatments for 30 min and cell extracts immunoprecipitated with p300/CBP antibody and complexes assessed by western blotting with anti‐tyrosine‐phospho STAT1. Tyrosine‐phosphorylated STAT1 was detected in the IFNγ‐treated lysates and not in IL1‐stimulated cells, thus demonstrating CBP/p300‐STAT1 interaction. The levels of CBP/p300 were constant (fig 5B). By contrast, no interaction between serine‐phosphorylated STAT1 and CBP/p300 was observed (results not shown). To further confirm this interaction, the cell extracts were first immunoprecipitated with STAT1 antibody followed by detection with CBP/p300 antibody by western blotting. CBP/p300 was detected in IFNγ‐treated chondrocyte lysates, reaffirming the STAT1–CBP/p300 interaction. The levels of total STAT1 in the extracts remained unchanged (fig 5C).
P300 overexpression reverses IFNγ inhibition of MMP13 expression and promoter
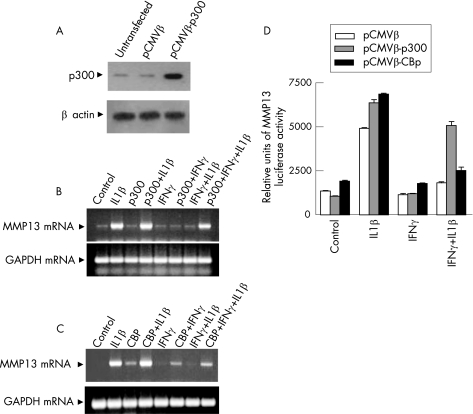
To further explore the role of CBP/p300 coactivators in MMP13 suppression, p300 was overexpressed under the control of CMV promoter by transfection (fig 6A), which reversed the IFNγ suppression of endogenous MMP13 mRNA (fig 6B, lane 8) and MMP13 promoter activity (fig 6D). CBP overexpression reversed the suppression to a much lesser extent (fig 6C, lane 8) or minimally (fig 6D).
Figure 6 Impact of p300 and cyclic AMP response element‐binding protein (CBP) overexpression on matrix metalloproteinase 13 (MMP13) mRNA and promoter activity. (A) Chondrocytes were either not transfected or transfected with pCMVβ or cytomegalovirus (CMV)‐p300 vectors (2 μg each), and total cellular protein (40 μg) was analysed after 48 h by western blotting for p300 and β actin levels. (B,C) Chondrocytes transfected with p300 or CBP expression vectors (2 μg) for 48 h were further treated for 24 h as indicated and MMP13 and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) mRNAs were analysed by reverse transcriptase‐PCR. (D) Chondrocytes were cotransfected with MMP13 luciferase promoter (4 μg), Renilla luciferase (4 μg) vectors and pCMVβ or pCMVβ‐p300 or pCMVβ‐CBP (2 μg each) and luciferase activity was measured. The values are mean (SD) of three separate experiments. IFN, interferon; IL, interleukin.
Discussion
IL1 is a key pro‐inflammatory stimulus for degradation of cartilage‐specific type II collagen by MMP13 in patients with arthritis. We have shown that induction of MMP13 expression by IL1 can be potently inhibited at all levels by IFNγ in cartilage cells. With multiple approaches such as receptor neutralisation, RNA interference, pharmacological inhibition, protein–protein interaction and CBP/p300 cotransfection, we demonstrated the pivotal role of STAT1 phosphorylation and CBP/p300 coactivator in the suppression. Similar suppression of MMP13 promoter, mRNA and protein by IFNγ suggests inhibition primarily at the level of transcription. Few studies to date have investigated the modulation of MMPs by IFNγ in chondrocytes. It has been shown that IFNγ inhibited induction of MMP‐3 and MMP‐1 by IL1 in human chondrocytes,30,31 but implicated mechanisms were not investigated. The previously unknown suppression of MMP13 by IFNγ in chondrocytes suggests a cartilage‐protective role in arthritis.
Coordinate reversal of MMP13 mRNA and protein suppression by neutralising antibody strongly support the requirement of IFNγR1 in IFNγ signal transduction including tyrosine/serine phosphorylation and downstream responses. Lack of inhibition of tyrosine/serine phosphorylation by the control antibody and clear inhibition by the IFNγR1 antibody demonstrates the specificity of the neutralising antibody response. Interestingly, these experiments also demonstrate the previously unknown expression of IFNγR1 on chondrocytes. Besides tyrosine‐701, serine‐727 phosphorylation in the transcription activation domain was also observed, which leads to maximal transcriptional activity of STAT1.32 Thus, IFNγ, its receptor, subsequent signal transduction pathways and their target genes may constitute an endogenous defence mechanism against proarthritic agents. Owing to altered metabolism in arthritic cartilage, such a mechanism may be impaired. The protective role is supported by the observation that IFNγRα knockout mice developed accelerated susceptibility to collagen‐induced arthritis.33
As STAT1 phosphorylation has been involved in both activation and repression of target genes, time‐course studies in chondrocytes revealed a strong correlation of STAT1 tyrosine/serine phosphorylation with suppression of MMP13 mRNA and protein expression. Additionally, the reversal of MMP13 suppression by STAT1 siRNA and by fludarabine strongly support STAT1 as the major mediator of IFNγ‐driven inhibition of MMP13 promoter activity and expression. Interestingly, STAT1 also mediated IFNγ suppression of type II collagen gene in a chondrocyte cell line34 and IL1‐induced responses in macrophages.35 STAT1 is persistently phosphorylated at tyrosine‐701 and serine‐727 in the synovial fluid and synoviocytes of patients with RA,36,37 which may be a failed defensive attempt to suppress cartilage‐degrading MMPs. STAT1 has both pathogenic and protective roles in arthritis.38
IFNγ did not directly affect the DNA‐binding activity of AP1 transcription factor complex. AP1‐dependent gene expression requires ubiquitous coactivators, CBP/p300.28,39 In most cells, CBP/p300 availability is the rate‐limiting step for STAT1 and AP1 transcription factor‐mediated gene expression. In response to IFNγ and IL1β cotreatment, STAT1 and AP1 could compete for limiting amounts of p300 to achieve transcriptional activation. When IFNγ treatment increases STAT1 affinity and binding to p300, AP1 still displays DNA‐binding activity but lacks transcriptional activation. Thus, IFNγ‐mediated inhibition of MMP13 and promoter activity can be explained by such a mechanism. Significant reversal of MMP13 mRNA and promoter suppression by p300 transfection compared with minimal inhibition by CBP suggests a dominant and distinct role of p300 relative to CBP.
The role of IFNγ in animal models of RA is contradictory. IFNγ receptor‐deficient mice have decreased40 or increased33,41 incidences of arthritis. However, our results support a protective role for IFNγ against arthritis. This view is reinforced by a report where IL1‐induced inflammatory mediators in macrophages, MMP‐1, MMP‐3 and MMP‐9 expression, tissue invasion of macrophages and bovine cartilage destruction was suppressed by type I and type II (IFNγ) IFNs, suggesting their homeostatic role in arthritis.35 IFNγ also decreased IL1‐stimulated glycosaminoglycan release, possibly by MMP‐3, from cartilage explants.42 These studies, however, did not investigate MMP13 regulation in chondrocytes, the major enzyme responsible for tissue destruction in arthritis. Besides suppression of MMP13 and cartilage degradation,43 IFNγ could also protect cartilage integrity by the reported inhibition of CD95‐induced apoptotic death in human chondrocytes.44 In summary, IFNγ potently suppresses the induction of major cartilage collagen‐degrading enzyme through IFNR1, STAT1 and recruitment of limiting CBP/p300 (predominantly p300) coactivator. Thus, the therapeutic potential of IFNγ and possibly other interferons45,46 for blocking cartilage‐destructive effects of IL1 should be explored further.
Acknowledgements
This research was supported by the Canadian Institutes of Health Research (CIHR), The Arthritis Society and CAN grants. RA and MEM acknowledge CIHR and Wyeth fellowships. We thank Dr Carlos Lopez‐Otin for the MMP‐13 promoter.
Abbreviations
AP - activator protein
CBP - cyclic AMP response element‐binding protein
CMV - cytomegalovirus
ECM - extracellular matrix
GAPDH - glyceraldehyde‐3‐phosphate dehydrogenase
HRP - horseradish peroxidase
IFNγ - interferon γ
MMP13 - matrix metalloproteinase 13
PBS - phosphate‐buffered saline
RA - rheumatoid arthritis
RT - reverse transcriptase
siRNA - small interfering RNA
STAT1 - signal transducer and activator of transcription 1
Footnotes
Competing interests: None declared.
References
- 1.Dayer J M, Bresnihan B. Targeting interleukin‐1 in the treatment of rheumatoid arthritis. Arthritis Rheum 200246574–578. [DOI] [PubMed] [Google Scholar]
- 2.Abramson S B, Amin A. Blocking the effects of IL‐1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford) 200241972–980. [DOI] [PubMed] [Google Scholar]
- 3.Tetlow L C, Adlam D J, Woolley D E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum 200144585–594. [DOI] [PubMed] [Google Scholar]
- 4.Goldring S R, Goldring M B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res 2004427(Suppl)S27–S36. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Squires G R, Mousa A, Tanzer M, Zukor D J, Antoniou J.et al Role of interleukin‐1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum 200552128–135. [DOI] [PubMed] [Google Scholar]
- 6.Joosten L A, Helsen M M, Saxne T, van De Loo F A, Heinegard D, van Den Berg W B. IL‐1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen‐induced arthritis, whereas TNF‐alpha blockade only ameliorates joint inflammation. J Immunol 19991635049–5055. [PubMed] [Google Scholar]
- 7.Evans C H, Gouze J N, Gouze E, Robbins P D, Ghivizzani S C. Osteoarthritis gene therapy. Gene Ther 200411379–389. [DOI] [PubMed] [Google Scholar]
- 8.Neidhart M, Gay R E, Gay S. Anti‐interleukin‐1 and anti‐CD44 interventions producing significant inhibition of cartilage destruction in an in vitro model of cartilage invasion by rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 2000431719–1728. [DOI] [PubMed] [Google Scholar]
- 9.Mott J D, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 200416558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 200669562–573. [DOI] [PubMed] [Google Scholar]
- 11.Dahlberg L, Billinghurst R C, Manner P, Nelson F, Webb G, Ionescu M.et al Selective enhancement of collagenase‐mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1). Arthritis Rheum 200043673–682. [DOI] [PubMed] [Google Scholar]
- 12.Liacini A, Sylvester J, Li W Q, Huang W, Dehnade F, Ahmad M.et al Induction of matrix metalloproteinase‐13 gene expression by TNF‐alpha is mediated by MAP kinases, AP‐1, and NF‐kappaB transcription factors in articular chondrocytes. Exp Cell Res 2003288208–217. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell P G, Magna H A, Reeves L M, Lopresti‐Morrow L L, Yocum S A, Rosner P J.et al Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase‐13 from human osteoarthritic cartilage. J Clin Invest 199697761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindy O, Konttinen Y T, Sorsa T, Ding Y, Santavirta S, Ceponis A.et al Matrix metalloproteinase 13 (collagenase 3) in human rheumatoid synovium. Arthritis Rheum 1997401391–1399. [DOI] [PubMed] [Google Scholar]
- 15.Forsyth C B, Cole A, Murphy G, Bienias J L, Im H J, Loeser R F., Jr Increased matrix metalloproteinase‐13 production with aging by human articular chondrocytes in response to catabolic stimuli. J Gerontol A Biol Sci Med Sci 2005601118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuhold L A, Killar L, Zhao W, Sung M L, Warner L, Kulik J.et al Postnatal expression in hyaline cartilage of constitutively active human collagenase‐3 (MMP‐13) induces osteoarthritis in mice. J Clin Invest 200110735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heathfield T F, Onnerfjord P, Dahlberg L, Heinegard D. Cleavage of fibromodulin in cartilage explants involves removal of the N‐terminal tyrosine sulfate‐rich region by proteolysis at a site that is sensitive to matrix metalloproteinase‐13. J Biol Chem 20042796286–6295. [DOI] [PubMed] [Google Scholar]
- 18.Chadha K C, Ambrus J L, Jr, Dembinski W, Ambrus J L., Sr Interferons and interferon inhibitory activity in disease and therapy. Exp Biol Med (Maywood) 2004229285–290. [DOI] [PubMed] [Google Scholar]
- 19.Vilcek J. Novel interferons. Nat Immunol 200348–9. [DOI] [PubMed] [Google Scholar]
- 20.Dolhain R J, ter Haar N T, Hoefakker S, Tak P P, de Ley M, Claassen E.et al Increased expression of interferon (IFN)‐gamma together with IFN‐gamma receptor in the rheumatoid synovial membrane compared with synovium of patients with osteoarthritis. Br J Rheumatol 19963524–32. [DOI] [PubMed] [Google Scholar]
- 21.Dodge G R, Diaz A, Sanz‐Rodriguez C, Reginato A M, Jimenez S A. Effects of interferon‐gamma and tumor necrosis factor alpha on the expression of the genes encoding aggrecan, biglycan, and decorin core proteins in cultured human chondrocytes. Arthritis Rheum 199841274–283. [DOI] [PubMed] [Google Scholar]
- 22.Verbruggen G, Malfait A M, Veys E M, Gyselbrecht L, Lambert J, Almqvist K F. Influence of interferon‐gamma on isolated chondrocytes from human articular cartilage. Dose dependent inhibition of cell proliferation and proteoglycan synthesis. J Rheumatol 1993201020–1026. [PubMed] [Google Scholar]
- 23.Shlopov B V, Lie W R, Mainardi C L, Cole A A, Chubinskaya S, Hasty K A. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum 1997402065–2074. [DOI] [PubMed] [Google Scholar]
- 24.Liacini A, Sylvester J, Li W Q, Zafarullah M. Inhibition of interleukin‐1‐stimulated MAP kinases, activating protein‐1 (AP‐1) and nuclear factor kappa B (NF‐kappa B) transcription factors down‐regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol 200221251–262. [DOI] [PubMed] [Google Scholar]
- 25.Pendas A M, Balbin M, Llano E, Jimenez M G, Lopez‐Otin C. Structural analysis and promoter characterization of the human collagenase‐3 gene (MMP13). Genomics 199740222–233. [DOI] [PubMed] [Google Scholar]
- 26.Frank D A, Mahajan S, Ritz J. Fludarabine‐induced immunosuppression is associated with inhibition of STAT1 signaling. Nat Med 19995444–447. [DOI] [PubMed] [Google Scholar]
- 27.Mengshol J A, Vincenti M P, Coon C I, Barchowsky A, Brinckerhoff C E. Interleukin‐1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c‐Jun N‐terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum 200043801–811. [DOI] [PubMed] [Google Scholar]
- 28.Kalkhoven E. CBP and p300: HATs for different occasions. Biochem Pharmacol 2004681145–1155. [DOI] [PubMed] [Google Scholar]
- 29.Korzus E, Torchia J, Rose D W, Xu L, Kurokawa R, McInerney E M.et al Transcription factor‐specific requirements for coactivators and their acetyltransferase functions. Science 1998279703–707. [DOI] [PubMed] [Google Scholar]
- 30.Henrotin Y E, Zheng S X, Labasse A H, Deby G P, Crielaard J M, Reginster J Y. Modulation of human chondrocyte metabolism by recombinant human interferon. Osteoarthritis Cartilage 20008474–482. [DOI] [PubMed] [Google Scholar]
- 31.Andrews H J, Bunning R A, Plumpton T A, Clark I M, Russell R G, Cawston T E. Inhibition of interleukin‐1‐induced collagenase production in human articular chondrocytes in vitro by recombinant human interferon‐gamma. Arthritis Rheum 1990331733–1738. [DOI] [PubMed] [Google Scholar]
- 32.Wen Z, Zhong Z, Darnell J E., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 199582241–250. [DOI] [PubMed] [Google Scholar]
- 33.Manoury‐Schwartz B, Chiocchia G, Bessis N, Abehsira‐Amar O, Batteux F, Muller S.et al High susceptibility to collagen‐induced arthritis in mice lacking IFN‐gamma receptors. J Immunol 19971585501–5506. [PubMed] [Google Scholar]
- 34.Osaki M, Tan L, Choy B K, Yoshida Y, Cheah K S, Auron P E.et al The TATA‐containing core promoter of the type II collagen gene (COL2A1) is the target of interferon‐gamma‐mediated inhibition in human chondrocytes: requirement for Stat1 alpha, Jak1 and Jak2. Biochem J 2003369103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu X, Ho H H, Lou O, Hidaka C, Ivashkiv L B. Homeostatic role of interferons conferred by inhibition of IL‐1‐mediated inflammation and tissue destruction. J Immunol 2005175131–138. [DOI] [PubMed] [Google Scholar]
- 36.Kasperkovitz P V, Verbeet N L, Smeets T J, van Rietschoten J G, Kraan M C, van der Pouw Kraan T C.et al Activation of the STAT1 pathway in rheumatoid arthritis. Ann Rheum Dis 200463233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokota A, Narazaki M, Shima Y, Murata N, Tanaka T, Suemura M.et al Preferential and persistent activation of the STAT1 pathway in rheumatoid synovial fluid cells. J Rheumatol 2001281952–1959. [PubMed] [Google Scholar]
- 38.Ivashkiv L B, Hu X. The JAK/STAT pathway in rheumatoid arthritis: pathogenic or protective? Arthritis Rheum 2003482092–2096. [DOI] [PubMed] [Google Scholar]
- 39.Arias J, Alberts A S, Brindle P, Claret F X, Smeal T, Karin M.et al Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature 1994370226–229. [DOI] [PubMed] [Google Scholar]
- 40.Kageyama Y, Koide Y, Yoshida A, Uchijima M, Arai T, Miyamoto S.et al Reduced susceptibility to collagen‐induced arthritis in mice deficient in IFN‐gamma receptor. J Immunol 19981611542–1548. [PubMed] [Google Scholar]
- 41.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen‐induced arthritis in IFN‐gamma receptor‐deficient mice. J Immunol 19971585507–5513. [PubMed] [Google Scholar]
- 42.Andrews H J, Bunning R A, Dinarello C A, Russell R G. Modulation of human chondrocyte metabolism by recombinant human interferon gamma: in‐vitro effects on basal and IL‐1‐stimulated proteinase production, cartilage degradation and DNA synthesis. Biochim Biophys Acta 19891012128–134. [DOI] [PubMed] [Google Scholar]
- 43.Bunning R A, Russell R G. The effect of tumor necrosis factor alpha and gamma‐interferon on the resorption of human articular cartilage and on the production of prostaglandin E and of caseinase activity by human articular chondrocytes. Arthritis Rheum 198932780–784. [DOI] [PubMed] [Google Scholar]
- 44.Grassi F, Piacentini A, Cristino S, Toneguzzi S, Facchini A, Lisignoli G. Inhibition of CD95 apoptotic signaling by interferon‐gamma in human osteoarthritic chondrocytes is associated with increased expression of FLICE inhibitory protein. Arthritis Rheum 200450498–506. [DOI] [PubMed] [Google Scholar]
- 45.Allen J B, Bansal G P, Feldman G M, Hand A O, Wahl L M, Wahl S M. Suppression of bacterial cell wall‐induced polyarthritis by recombinant gamma interferon. Cytokine 1991398–106. [DOI] [PubMed] [Google Scholar]
- 46.van Holten J, Plater‐Zyberk C, Tak P P. Interferon‐beta for treatment of rheumatoid arthritis? Arthritis Res 20024346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]