Abstract
Background
Systemic sclerosis (SSc) is a multisystem autoimmune disease, which is classified into a diffuse cutaneous (dcSSc) and a limited cutaneous (lcSSc) subset according to the skin involvement. In order to better understand the vascular, immunological and fibrotic processes of SSc and to guide its treatment, the EULAR Scleroderma Trials And Research (EUSTAR) group was formed in June 2004.
Aims and methods
EUSTAR collects prospectively the Minimal Essential Data Set (MEDS) on all sequential patients fulfilling the American College of Rheumatology diagnostic criteria in participating centres. We aimed to characterise demographic, clinical and laboratory characteristics of disease presentation in SSc and analysed EUSTAR baseline visits.
Results
In April 2006, a total of 3656 patients (1349 with dcSSc and 2101 with lcSSc) were enrolled in 102 centres and 30 countries. 1330 individuals had autoantibodies against Scl70 and 1106 against anticentromere antibodies. 87% of patients were women. On multivariate analysis, scleroderma subsets (dcSSc vs lcSSc), antibody status and age at onset of Raynaud's phenomenon, but not gender, were found to be independently associated with the prevalence of organ manifestations. Autoantibody status in this analysis was more closely associated with clinical manifestations than were SSc subsets.
Conclusion
dcSSc and lcSSc subsets are associated with particular organ manifestations, but in this analysis the clinical distinction seemed to be superseded by an antibody‐based classification in predicting some scleroderma complications. The EUSTAR MEDS database facilitates the analysis of clinical patterns in SSc, and contributes to the standardised assessment and monitoring of SSc internationally.
Systemic sclerosis (SSc) is a multisystem disease with prevalence rate of around 5/105 and an incidence of 1/105.1 Higher rates are reported in the US, Australia and Eastern Europe, and lower rates in Northern Europe and Japan.2,3,4,5,6,7 SSc may be rapidly fatal in its severe form, but may also have a prolonged course, with patients being compromised only by distal vasospasm, sclerodactyly and dysphagia.8,9,10,11 Predicting outcome early in the course of the disease is critical in deciding on the appropriate treatment, but is not yet sufficiently reliable in many patients. The diagnosis is generally established with high specificity, according to the criteria of the American College of Rheumatology (ACR, formerly called American Rheumatism Association).12 Early SSc can be further divided into diffuse cutaneous (dcSSc) and limited cutaneous (lcSSc), with a part of those manifestations previously called CREST (calcinosis raynaud phenomenon esophageal dysmotility sclerodactyly and telangiectasia) syndrome.13 Other forms are characterised by features of scleroderma combined with features of a second connective tissue disease.14
SSc subsets are also associated with the presence of autoantibodies: dcSSc has been associated with Scl70 autoantibodies (also called topoisomerase I autoantibodies), whereas anticentromere autoantibodies (ACA) are typically detected in lcSSc. However, autoantibody profiles do not completely predict disease presentation. For example, a Japanese study showed that 31% of patients with SSc with Scl70 antibodies had lcSSc.15 Conversely, 18% of patients with lcSSc were positive for Scl70 antibodies in a US report.16 Autoantibodies may even disappear during the course of the disease, which then predicted a more favourable outcome.17
Genetic factors also seem to have an influence on SSc, as the disease occurs more frequently within families than in the general population.18 A relatively high concordance rate between monozygotic twins for antinuclear antibodies also supports the influence of genetic factors on autoantibody production, although the low overall concordance between monozygotic twins demonstrates the importance of environmental factors.19
The low incidence of SSc and the clinical variability result in difficulties in understanding the pathogenesis and evolution of the disease, and in selecting appropriate patients for clinical trials.20,21,22
In order “to foster the awareness, understanding and research of scleroderma and its care and management throughout Europe”, the EULAR Scleroderma Trials And Research (EUSTAR) group (www.eustar.org) was inaugurated, and, under the auspices of the EULAR Standing Committee on International Clinical Studies Including Therapeutic Trials, has established a prospective multicentre scleroderma cohort.
In this paper, we report the cross‐sectional prevalence of clinical and laboratory characteristics in SSc, and present a multivariate analysis in order to gain insight into factors that are associated with particular organ manifestations and therefore possibly also with the disease process. By focusing on age at onset of Raynaud's phenomenon, gender and autoantibodies, we also examined whether the dichotomy into limited and diffuse subsets is the best way to capture the disease and its organ manifestations, or whether other variables may be more appropriate.
Patients and methods
The EUSTAR database
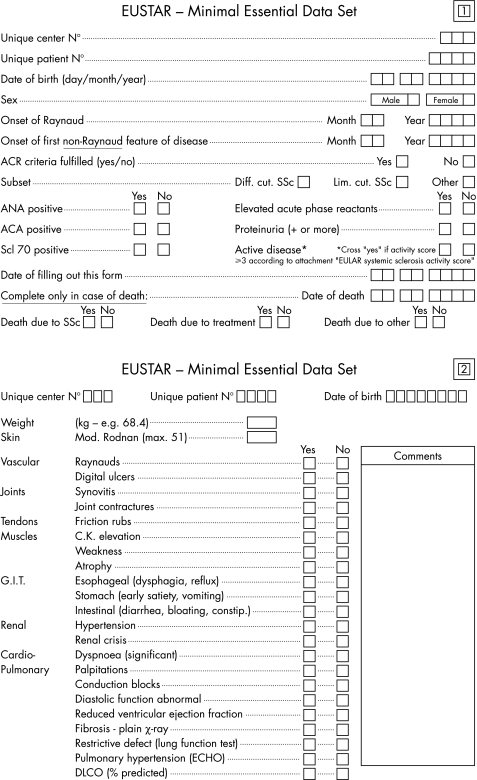
The EUSTAR database was inaugurated in June 2004 and documents a multinational, prospective and open scleroderma cohort. Participating centres seek ethics committee approval, followed by the entry of the Minimal Essential Data set (MEDS) for all consecutive consenting patients most of whom fulfil the ACR classification criteria for SSc.12 Scleroderma subsets are classified as “diffuse SSc” if skin thickening extends proximal to the elbows and knees or includes the trunk. The SSc subset is classified as “limited SSc” if skin thickening is confined to the elbows and knees, or the face.13 Patients who fulfil the ACR criteria for scleroderma but who had simultaneous overlap syndromes with typical features of one or more of other connective tissue diseases (mixed connective tissue disease, systemic lupus erythematosus, Sjögren's syndrome, dermatomyositis, polymyositis or rheumatoid arthritis), are classified as “other”. Cases of localised scleroderma (morphea and linear disease) are not included. The MEDS (fig 1) was constructed in consensus by the EUSTAR members, and covers demographic aspects, disease duration, organ involvement and laboratory data. Disease activity was calculated as a composite score from MEDS features according to the preliminary index for SSc as a whole, proposed by the European Scleroderma Study Group and detailed elsewhere.23 Annual follow‐up examinations are carried out. The centres were coached several times on how to fill out the forms. Coaching sessions included ACR classification of SSc, and definitions of the subgroups and the activity score. Standardised teaching sessions included the documentation of the modified Rodnan skin score at the bedside, following two “teach the teachers” sessions held in 2004 and 2005. Pseudonymised paper entry forms are faxed or mailed to the EUSTAR registry in Florence, Italy. Data monitoring includes suspect double entries, missing data and plausability checks. The definitions of the MEDS parameters and video coaching material are also available on the EUSTAR website (http://www.eustar.org).
Figure 1 Items of the Minimal Essential Data Set.
Data analysis
SSc presentations were analysed cross‐sectionally for differences in demographic and clinical features. For each patient, only the baseline data from the first visit were used. The dataset was analysed using the SPSS V.13.0 statistical package. Group means and percentages within dichotomised groups were compared by t test.
Significant differences in disease presentation on univariate comparisons were then retested by forward multivariate logistic regression. The following variables were entered in the model: presence or absence of dcSSc, lcSSc, antinuclear antibodies, ACA, Scl70 autoantibodies and gender. Further variables included early versus late onset of first Raynaud's phenomenon (dichotomised at the mean onset of Raynaud's phenomenon among all patients), and the time interval between the first Raynaud's phenomenon and first non‐Raynaud's event (dichotomised at the mean interval among all patients). Variables with quantitatively minor explanatory power (contributing <0.01 to the overall Nagelkerkes‐R2) were removed from the model even if their effect on the model was statistically significant.
Results
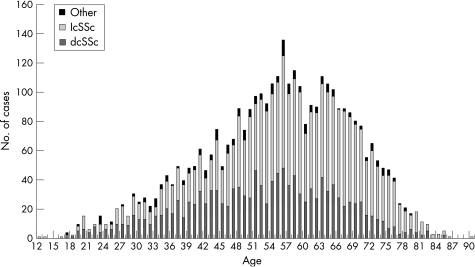
As of April 2006, a total of 3656 patients had been enrolled from 102 participating centres in 24 European and 6 non‐European countries. There were very little missing data (table 1), apart from parameters relating to the onset of Raynaud's phenomenon, onset of first non‐Raynaud's event and diffusing capacity of the lung for carbon monoxide, as these three parameters were included only after the first year of data collection. A total of 1349 (36.9%) patients had dcSSc, 2101 (57.5%) patients had lcSSc and 206 (5.6%) had scleroderma in combination with another connective tissue disease (table 1). Compared with patients with lcSSc, patients with dcSSC were on average 5.1 years younger. In all SSc subsets, the age of patients was normally distributed (fig 2).
Table 1 Prevalence of disease presentation among clinical scleroderma subsets.
| dcSSc | lcSSc | p (dcSSc vs lcSSC) | Other | Missing data (%) | |
|---|---|---|---|---|---|
| ACR criteria fulfilled | 100% | 100% | NA | 100% | 0 |
| Number of patients | 1349 (36.9%) | 2101 (57.5%) | <0.001 | 206 (5.6%) | 0 |
| Women | 81.1% | 90.9% | <0.001 | 86.9% | 0.4 |
| Age (years), mean (SD) | 52.3 (13.7) | 57.4 (13.1) | <0.001 | 52.7 (13.9) | 0.4 |
| Age at RO (years), mean (SD) | 42.9 (14.7) | 42.9 (14.5) | 0.98 | 40.6 (14.3) | 11.2 |
| Age at first non‐RO (years), mean (SD) | 44.8 (14.2) | 47.9 (13.4) | <0.001 | 43.8 (14.0) | 10.4 |
| Disease duration* (years), mean (SD) | 7.4 (6.9) | 9.6 (8.1) | <0.001 | 9.0 (7.5) | 10.7 |
| Time between RO and first non‐RO (years), mean (SD) | 1.9 (5.4) | 4.8 (8.5) | <0.001 | 3.2 (7.3) | 12.2 |
| ANA positive | 92.1% | 91.3% | 0.19 | 89.3% | 0.8 |
| Scl70 positive | 60.8% | 23.4% | <0.001 | 26.1% | 3.4 |
| ACA positive | 6.0% | 46.7% | <0.001 | 21.4% | 4.4 |
| mRSS, mean (SD) | 19.0 (10.0) | 8.1 (5.3) | <0.001 | 6.4 (6.6) | 3.0 |
| Active disease | 49.8% | 21.5% | <0.001 | 28.2% | 3.5 |
| Elevated acute phase reactants | 41.8% | 24.6% | <0.001 | 34.5% | 1.8 |
| Raynaud's phenomenon | 96.1% | 95.9% | 0.58 | 92.7% | 0.1 |
| Digital ulcers | 42.7% | 32.9% | <0.001 | 22.3% | 0.3 |
| Synovitis | 20.8% | 13.7% | <0.001 | 21.4% | 0.4 |
| Joint contractures (any joint) | 47.1% | 24.4% | <0.001 | 29.1% | 0.6 |
| Tendon friction rubs | 22.1% | 7.4% | <0.001 | 8.3% | 0.9 |
| Muscle weakness | 37.1% | 22.8% | <0.001 | 36.4% | 0.4 |
| Muscle atrophy | 21.1% | 10.8% | <0.001 | 20.9% | 1.1 |
| CK elevation | 11.3% | 4.4% | <0.001 | 12.1% | 2.8 |
| Oesophagus | 68.2% | 66.8% | 0.38 | 68.0% | 0.3 |
| Stomach | 26.6% | 22.8% | 0.04 | 21.8% | 0.7 |
| Intestine | 22.5% | 21.7% | 0.68 | 19.4% | 0.7 |
| Pulmonary fibrosis | 53.4% | 34.7% | <0.001 | 44.2% | 2.2 |
| Lung restrictive defect | 49.3% | 26.7% | <0.001 | 32.0% | 2.4 |
| % of predicted DLCO, mean (SD) | 64.0 (20.7) | 71.8 (21.0) | <0.001 | 71.6 (19.5) | 62.5 |
| PAH | 22.3% | 20.5% | 0.32 | 18.9% | 2.5 |
| PAH without fibrosis | 5.9% | 9.2% | <0.001 | 5.8% | 2.5 |
| PAH with fibrosis | 15.8% | 11.0% | <0.001 | 12.6% | 3.9 |
| Dyspnoea | 44.9% | 34.0% | <0.001 | 37.4% | 0.2 |
| Palpitations | 27.3% | 22.6% | 0.003 | 31.6% | 0.5 |
| Conduction block | 12.7% | 10.4% | 0.12 | 9.7% | 1.9 |
| Diastolic dysfunction | 16.6% | 15.4% | 0.42 | 15.0% | 2.3 |
| LVEF | 7.2% | 5.0% | 0.59 | 2.4% | 3.2 |
| Hypertension | 19.3% | 18.6% | 0.46 | 15.5% | 0.3 |
| Hypertensive renal crisis | 4.2% | 1.1% | <0.001 | 1.9% | 0.4 |
| Proteinuria | 9.2% | 3.7% | <0.001 | 10.2% | 1.5 |
ACA, anticentromere autoantibody; ACR, American College of Rheumatology; ANA, antinuclear antibodies; CK, creatine kinase; DLCO, diffusion capacity of the lung for carbon monoxide; dcSSc, diffuse cutaneous systemic sclerosis; lcSSc, limited cutaneous systemic scerosis; LVEF, left ventricular ejection fraction; mRSS, modified Rodnan Skin Score; NA, not applicable; PAH, pulmonary artery hypertension (assessed by echocardiography); RO, onset of Raynaud's phenomenon.
* Disease duration was calculated on the basis of the onset of the first non‐Raynaud's feature.
Figure 2 Age distribution of scleroderma subsets. dcSSc, diffuse cutaneous systemic sclerosis; lcSSc, limited cutaneous systemic scerosis.
Disease manifestations
Patients with dcSSc and lcSSc had an identical mean age of onset (42.9 years) of Raynaud's phenomenon. However, the age at onset of first non‐Raynaud's manifestation differed between dcSSc and lcSSc, being 44.8 (SD 14.2) years on average in the former and 47.9 (SD 13.4) years in the latter (p<0.001). Consequently, there was a significantly longer lag period between the onset of Raynaud's phenomenon and the next non‐Raynaud's clinical feature of disease in the lcSSc (mean (SD) 4.8 (8.5) years), as opposed to the dcSSc (mean (SD) 1.9 (5.4) years). In total, 148 (4.0%) patients fulfilled the ACR criteria for scleroderma but had no Raynaud's phenomenon.
The mean skin score (modified Rodnan's skin score) was higher (19.0 (SD 10.0)) in dcSSC than in lcSSc (8.1 (SD 5.3)) or in other scleroderma presentations (6.4 (SD 6.6)), as expected. Overlapping skin scores between dcSSc and lcSSc emphasise that the numerical value of the score is not just determined not only by distribution but also by the severity of skin involvement.
Disease activity was scored as “active” in 49.8% of dcSSc, 21.5% of lcSSc and 28.2% of “other”. Acute‐phase reactants were more frequently elevated in dcSSc (table 1).
Musculoskeletal manifestations (joint contractures, tendon friction rubs, muscle weakness, muscle atrophy and raised creatine kinase (CK)) were almost twice as common in dcSSc as in lcSSc. Joint contractures were reported most commonly. A substantial number of patients had muscle weakness and atrophy, but only a few had simultaneous CK elevation.
Gastrointestinal involvement was most common in the oesophagus, but, with the exception of a slightly more predominant gastric involvement in the dcSSC (26.6% in dcSSc vs 22.8% in lcSSc), was observed in similar frequencies among the scleroderma subsets.
Pulmonary fibrosis was more common in dcSSc (53.4%) than in lcSSc (34.7%), whereas the frequency of pulmonary artery hypertension (PAH) diagnosed by echocardiography was similar between the two subsets (in 22.3% of patients with dcSSc and in 20.5% of patients with lcSSc). Isolated PAH (in the absence of lung fibrosis) was found in 26% of patients with dcSSc with PAH and in 45% of patients with lcSSc with PAH.
Objective cardiac complications (conduction block, diastolic dysfunction and left ventricular ejection failure) were reported with a similar frequency among scleroderma subsets. Subjective manifestations (palpitations) were slightly more common in dcSSc, than in lcSSc (27.3% vs 22.6%). Reduced left ventricular ejection fraction was associated with PAH in only 3.2% of patients with dcSSc. This prevalence was similar in patients with lcSSc (2.8%, p = 0.52).
Renal complications (hypertensive renal crisis and proteinuria) were more frequent in the dcSSc subset.
Differences in disease presentation according to gender
Among all scleroderma patients, 87% were women; the women‐to‐men ratio was 6:1. Women were slightly older than men (mean (SD) age 55.5 (13.6) vs 53.9 (13.3) years; p = 0.02). Women had an earlier onset of Raynaud's phenomenon than men (mean (SD) age 42.2(14.5) vs 46.4 (14.3) years; p<0.001). Similarly, the onset of non‐Raynaud's manifestations was reported at a slightly younger age in women than in men (46.4 (13.8) vs 47.9 (13.8) years; p = 0.04).
Within the dcSSc subset, 1094 patients were women and 254 patients were men (women:men ratio 4:1). Within the lcSSc subset, 1910 patients were women and 180 patients were men (women:men ratio 11:1). Men were more commonly affected by dcSSc than by lcSSc (p<0.001). The mean age of patients did not differ between sexes when compared among individual SSc subsets (table 2). Women, however had an earlier onset of Raynaud's phenomenon in both SSc (by a mean of 4.3 years earlier) and lcSSC (by a mean of 4.6 years earlier) compared with men. In absolute numbers, ACA were rarely positive in men. Among the lcSSc subset, women had more frequently ACA and men more frequently Scl‐70 autoantibodies (table 2).
Table 2 Gender‐specific variations among SSc subsets.
| dcSSc | p (♂ vs ♀) | lcSSc | p (♂ vs ♀) | |||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||
| Number of patients | 254 | 1094 | NA | 180 | 1910 | NA |
| Age (years), mean (SD) | 52.7 (12.6) | 52.3 (14.0) | 0.66 | 56.2 (13.2) | 57.5 (13.0) | 0.21 |
| Age at RO (years), mean (SD) | 46.4 (13.4) | 42.1 (14.9) | <0.001 | 47.1 (14.9) | 42.5 (14.4) | <0.001 |
| Age at first non‐RO (years), mean (SD) | 47.6 (13.1) | 44.1 (14.3) | 0.001 | 49.0 (14.1) | 47.8 (13.3) | 0.26 |
| Disease duration, years mean (SD) | 5.1 (5.0) | 7.9 (7.2) | <0.001 | 6.7 (5.7) | 9.8 (8.2) | <0.001 |
| Time between RO and first non‐RO (years), mean (SD) | 1.4 (4.7) | 2.0 (5.6) | 0.10 | 2.0 (5.2) | 5.1 (8.7) | <0.001 |
| ANA positive | 93.7% | 93.0% | 0.71 | 92.7% | 91.8% | 0.67 |
| Scl‐70 positive | 62.7% | 60.4% | 0.51 | 31.3% | 22.8% | 0.02 |
| ACA positive | 4.3% | 7.0% | 0.08 | 26.3% | 50.3% | <0.001 |
ACA, anticentromere autoantibody; ANA, antinuclear antibodies; dcSSc, diffuse cutaneous systemic sclerosis; lcSSc, limited cutaneous systemic scerosis; SSC, Systemic sclerosis; RO, onset of Raynaud's phenomenon; ♂, male; ♀, female.
Differences in disease presentation according to age at disease onset
In order to analyse the possible differences in organ manifestations according to the patient's age at disease onset (defined as the first onset of Raynaud's phenomenon), we categorised patients according to their mean age at the onset of Raynaud's phenomenon into two groups: one below and the other above the mean. The former group of “early” onset had an average age of 42.8 years and the latter group of “late” onset of Raynaud's phenomenon had an average age of 60.9 years (table 3). Although the groups exhibiting early and late onset of Raynaud's manifestation had no or only slight differences in their autoantibody profile within the individual SSc subsets (table 3), they differed in the prevalence of clinical manifestations. In both subsets, people with an earlier onset of Raynaud's phenomenon had digital ulcers more often than those with a late onset. However, patients with an early onset of Raynaud's phenomenon had significantly less pulmonary fibrosis, pulmonary hypertension, diastolic dysfunction and arterial hypertension (table 3).
Table 3 Prevalence of disease presentation according to the onset of Raynaud's phenomenon.
| DcSSc | p (early vs late) | lcSSc | p (early vs late) | |||
|---|---|---|---|---|---|---|
| Early Raynaud | Late Raynaud | Early Raynaud | Late Raynaud | |||
| Number of patients | 553 | 594 | NA | 914 | 1003 | NA |
| Age (years), mean (SD) | 42.8 (11.9) | 60.9 (8.5) | <0.001 | 49.9 (12.9) | 64.1 (8.6) | <0.001 |
| Women | 84.6% | 77.9% | 0.004 | 93.1% | 89.4% | 0.004 |
| ANA positive | 93.8% | 93.4% | 0.76 | 92.5% | 91.6% | 0.46 |
| Scl70 positive | 63.2% | 60.0% | 0.26 | 25.5% | 21.5% | 0.04 |
| ACA positive | 5.5% | 6.6% | 0.45 | 46.5% | 49.6% | 0.18 |
| mRSS (years), mean (SD) | 18.7 (9.4) | 19.5 (10.4) | 0.18 | 8.1 (5.2) | 8.0 (5.2) | 0.66 |
| Active disease | 43.8% | 52.7% | 0.005 | 18.1% | 21.9% | 0.05 |
| Elevated acute‐phase reactants | 37.3% | 44.3% | 0.02 | 21.8% | 26.3% | 0.03 |
| Digital ulcers | 50.8% | 35.2% | <0.001 | 38.8% | 27.9% | <0.001 |
| Muscle weakness | 32.7% | 39.2% | 0.02 | 21.0% | 22.5% | 0.43 |
| Pulmonary fibrosis | 47.4% | 59.4% | <0.001 | 31.8% | 37.2% | 0.02 |
| Lung restrictive defect | 47.9% | 50.3% | 0.26 | 24.1% | 29.2% | 0.009 |
| PAH | 17.7% | 26.3% | <0.001 | 16.8% | 23.4% | <0.001 |
| Dyspnoea | 37.8% | 52.2% | <0.001 | 31.3% | 37.0% | 0.008 |
| Palpitations | 23.5% | 30.3% | 0.006 | 20.6% | 23.6% | 0.07 |
| Conduction block | 11.4% | 13.5% | 0.18 | 9.0% | 12.2% | 0.01 |
| Diastolic dysfunction | 11.9% | 20.7% | <0.001 | 12.2% | 18.6% | <0.001 |
| Hypertension | 11.6% | 23.9% | <0.001 | 12.9% | 22.0% | <0.001 |
ACA, anticentromere autoantibody; ANA, antinuclear antibodies; dcSSc, diffuse cutaneous systemic sclerosis; lcSSc, limited cutaneous systemic sclerosis; mRSS, modified Rodnan Skin Score; NA, not applicable; PAH, pulmonary artery hypertension (assessed by echocardiography).
Manifestations with statistically similar prevalence between early and late onset are not shown.
Differences in disease presentation according to autoantibodies
Patients positive for ACA mostly (88.7%) had lcSSc (table 4), whereas only 60% of those carrying Scl70 autoantibodies had dcSSc; 36.1% of Scl70‐positive patients were classified as lcSSc. Patients with ACA were slightly older than those with anti‐Scl70 autoantibodies. Although there was no significant difference in the mean age at onset of Raynaud's phenomenon within people carrying the two different autoantibodies (42.2 years in anti‐Scl70 autoantibody positive individuals vs 43.3 years in ACA positive patients), those with ACA had a significantly longer lag period (mean (SD) 6.5 (10.0) years) until the onset of first non‐Raynaud's manifestations compared with those with anti‐Scl70 autoantibodies (mean (SD) 2.4 (5.6) years).
Table 4 Prevalence of disease presentation according to autoantibody serology.
| ANA positive | Scl70 positive | ACA positive | p (Scl70 vs ACA) | |
|---|---|---|---|---|
| Number of patients | 3346 | 1330 | 1106 | <0.001 |
| Presenting as dcSSC | 37.1% | 60.0% | 7.3% | <0.001 |
| Presenting as lcSSC | 57.4% | 36.1% | 88.7% | <0.001 |
| Presenting as “other” | 5.5% | 3.9% | 4.0% | 0.88 |
| Women | 87.3% | 83.7% | 94.4% | <0.001 |
| Age (years), mean (SD) | 55.1 (13.6) | 52.6 (13.7) | 59.6 (11.8) | <0.001 |
| Age at RO (years), mean (SD) | 42.7 (14.6) | 42.2 (14.4) | 43.4 (14.7) | 0.28 |
| Age at first non‐RO (years), mean (SD) | 46.4 (13.8) | 44.5 (14.0) | 50.0 (12.6) | <0.001 |
| Time between RO and non‐RO (years), mean (SD) | 3.7 (7.6) | 2.4 (5.6) | 6.5 (10.0) | <0.001 |
| mRSS (years), mean (SD) | 12.0 (9.1) | 15.1 (9.9) | 8.2 (5.9) | <0.001 |
| Active disease | 32.7% | 45.2% | 18.9% | <0.001 |
| Elevated acute‐phase reactants | 31.9% | 42.6% | 20.7% | <0.001 |
| Raynaud's phenomenon | 96.3% | 97.4% | 96.7% | 0.45 |
| Digital ulcers | 36.7% | 44.8% | 31.2% | <0.001 |
| Synovitis | 16.7% | 21.4% | 11.9% | <0.001 |
| Joint contractures (any joint) | 33.7% | 44.5% | 17.6% | <0.001 |
| Tendon friction rubs | 13.1% | 18.9% | 6.0% | <0.001 |
| Muscle weakness | 28.4% | 32.2% | 22.7% | <0.001 |
| Muscle atrophy | 14.6% | 16.1% | 9.5% | <0.001 |
| CK elevation | 7.6% | 8.7% | 2.9% | <0.001 |
| Oesophagus | 67.9% | 68.0% | 70.7% | 0.18 |
| Stomach | 24.5% | 24.1% | 26.9% | 0.11 |
| Intestine | 22.5% | 20.7% | 25.1% | 0.01 |
| Pulmonary fibrosis | 42.6% | 60.2% | 21.3% | <0.001 |
| Lung restrictive defect | 35.8% | 50.3% | 17.4% | <0.001 |
| % of predicted DLCO (years), mean (SD) | 68.9 (21.6) | 65.1 (20.9) | 75.0 (20.9) | <0.001 |
| PAH | 21.1% | 23.2% | 22.0% | 0.36 |
| PAH without fibrosis | 8.0% | 5.0% | 13.0% | <0.001 |
| PAH with fibrosis | 12.7% | 17.2% | 8.0% | <0.001 |
| Dyspnoea | 38.6% | 44.5% | 29.4% | <0.001 |
| Palpitations | 24.8% | 27.2% | 23.2% | 0.01 |
| Conduction block | 11.2% | 13.6% | 9.1% | <0.001 |
| Diastolic dysfunction | 15.7% | 17.7% | 12.7% | 0.001 |
| Reduced LVEF | 5.7% | 5.9% | 5.2% | 0.29 |
| Hypertension | 18.5% | 14.4% | 20.0% | <0.001 |
| Hypertensive renal crisis | 2.3% | 2.0% | 1.3% | 0.15 |
| Proteinuria | 6.0% | 7.8% | 2.7% | <0.001 |
ACA, anticentromere autoantibody; ANA, antinuclear antibodies; CK, creatine kinase; DLCO, diffusion capacity of the lung for carbon monoxide; dcSSc, diffuse cutaneous systemic sclerosis; lcSSc, limited cutaneous systemic sclerosis; LVEF, left ventricular ejection fraction; PAH, pulmonary artery hypertension (assessed by echocardiography); RO, onset of Raynaud's phenomenon.
Autoantibody associations with particular clinical complications are shown in table 4. The presence of autoantibodies (Scl70 and ACA on the one hand) distinguished the frequency of clinical manifestations very similarly to the distinction of dcSSc and lcSSc subsets on the other hand (table 1). However, there were some differences. Most notably, Scl70 positivity, unlike diffuse skin involvement, was associated with significant differences in the prevalence of intestinal symptoms, myocardial conduction block, diastolic dysfunction and renal hypertension. On the other hand, a positive history of gastric complications and hypertensive renal crisis was associated with skin involvement, but not with autoantibody status.
Multivariate analysis of disease determinants
The multivariate analysis confirmed the results of most univariate comparisons (table 5). The ranking of the variables according to their overall explanatory effect on the model shows that, for some disease manifestations, the contributory effect of antibody status exceeds that of the clinical dichotomy into lcSSc and dcSSc. For many other disease manifestations, antibody status also contributed as an independent variable. In accord with the univariate analysis, late onset of Raynaud's phenomenon was negatively associated with digital ulcers and positively associated with pulmonary fibrosis, PAH and renal hypertension. On multivariate analysis, gender was only significantly associated with a few disease manifestations—for example, association of raised CK with male gender. However, gender was removed from all models because it did not have a quantitatively pronounced explanatory effect, as it contributed <0.01 to the overall Nagelkerkes' R2 in the model.
Table 5 Independent predictors of disease presentation.
| 1 | 2 | 3 | |
|---|---|---|---|
| mRSS above mean | DcSSc | ||
| Active disease | DcSSc | ACA negative | |
| Elevated acute‐phase reactants | Not lcSSc | Scl70 positive | |
| Digital ulcers | Scl70 positive | Early RO | |
| Synovitis | ACA negative | ||
| Joint contractures (any joint) | DcSSc | ACA negative | |
| Tendon friction rubs | DcSSc | ACA negative | |
| Muscle weakness | Not lcSSc | ||
| Muscle atrophy | Not lcSSc | ||
| CK elevation | Not lcSSc | ACA negative | |
| Oesophagus | None | ||
| Stomach | None | ||
| Intestine | None | ||
| Pulmonary fibrosis | Scl70 positive | ACA negative | Late RO |
| Lung restrictive defect | DcSSc | Scl70 positive | ACA negative |
| DLCO above mean | ACA positive | ||
| PAH | Late RO | ||
| PAH without fibrosis | ACA | ||
| PAH with fibrosis | Scl70‐positive | ACA‐negative | |
| Dyspnoea | ACA negative | Late RO | |
| Palpitations | None | ||
| Conduction block | None | ||
| Diastolic dysfunction | Late RO | ||
| LVEF | None | ||
| Hypertension | Scl70 negative | Late RO | |
| Hypertensive renal crisis | DcSSc | Scl70 negative | |
| Proteinuria | Not lcSSc |
ACA, anticentromere autoantibody; CK, creatine kinase; DLCO, diffusion capacity of the lung for carbon monoxide; dcSSc, diffuse cutaneous systemic sclerosis; late and early RO, age at onset of Raynaud's phenomenon above and below the mean age of all patients; lcSSc, limited cutaneous systemic sclerosis; LVEF, left ventricular ejection fraction; mRSS, modified Rodnan Skin Score; PAH, pulmonary artery hypertension (assessed by echocardiography).
The variables are calculated by multivariate logistic regression and ranked in columns 1, 2 and 3 according to the magnitude of their explanatory effect (“1” being the strongest predictor). Variables discarded from the model are not listed. Details are described in patients and methods section.
Discussion
In this large EUSTAR cohort of predominantly Caucasian patients with scleroderma, 57% of individuals were classified as lcSSc and 36.9% as dcSSc. Other investigators also found that limited disease was more common than diffuse disease among prevalent cases (65.1% vs 34.9%).7
Women were six times more frequent than men in our cohort. This sex ratio is between the numbers reported in smaller cohorts for the UK6 (women:men ratio 3:1) and Japan (women:men ratio 14:1), and similar to those from Iceland (8:1).3,5 Differences may be partly explained by the proportion of lcSSc within the cohorts, because our data suggest that the women: men ratio may be higher in lcSSc than in dcSSc. In the UK study, however, the women:men ratio was lower in the lcSSc subset (3.2:1) than in the dcSSc subset (4.6:1).6 In lcSSc, we found a higher prevalence of Scl70 autoantibodies and a lower prevalence of ACA among men than in women, whereas in dcSSc there were no differences in autoantibodies between sexes. Other investigators also suggest that ACA are less common among men.7
In previous studies, the mean age at diagnosis was not different between sexes.7 In our cohort, patients with dcSSc experienced the first non‐Raynaud's feature of their disease at a slightly younger age than patients with lcSSc. Previous incidence calculations suggested that the difference in prevalence between diffuse and limited disease was not attributable to the survival advantage of patients with limited disease.7
Our analysis found no differences between the two SSc subsets with regard to the age at onset of Raynaud's phenomenon, but in patients with diffuse disease, the first non‐Raynaud's manifestation developed sooner than in those with limited disease. These findings fit well with the observation that ACA positivity was associated with longer duration of Raynaud's phenomenon before the diagnosis of SSc was made.24 The onset of disease, whether based on first Raynaud's phenomenon or on first non‐Raynaud's event, was earlier in women. Furthermore, an early onset of disease was associated with a reduced prevalence of the more severe complications of scleroderma, such as lung fibrosis and PAH, in our cohort. This is in accordance with the observation that being a woman positively affects survival.7 The gender‐specific differences of the disease features indicate a modifying influence of sex hormones or reproduction. They could also point to gender‐specific environmental exposure.
In the multivariate analysis, however, gender was not associated with disease manifestations. This suggests that any effect of gender may be better explained by other variables such as age of onset of Raynaud's phenomenon and/or autoantibody status.
In both SSc subsets, individuals with an early onset of Raynaud's phenomenon had digital ulcers more commonly than those with a late onset, whereas an onset of Raynaud's phenomenon later in life was associated with a higher prevalence of more severe disease manifestations such as pulmonary fibrosis and PAH. The independent contribution of the time of onset of Raynaud's phenomenon to the prevalence of the above‐mentioned complications despite a similar prevalence of autoantibodies was confirmed in the multivariate analysis, and is in accord with the finding of others that older age at diagnosis negatively affects survival.7 It should be noted, however, that the time of onset of Raynaud's phenomenon does not discriminate between the two disease subsets. The first non‐Raynaud's feature does follow the onset of Raynaud's phenomenon more rapidly in dcSSc than in lcSSc; the relatively small difference however may not be helpful in the assessment of an individual patient.
Scl70 autoantibodies are associated with the more severe diffuse form of SSc, but 36.1% of patients were classified as lcSSc. Another study found that 31% of patients with SSc with this autoantibody had limited disease.15 Conversely, 23.4% of patients with lcSSc in our cohort and 18% in other investigations were positive for anti‐Scl70,16 and serum levels of anti‐Scl70 autoantibody levels also appear to be correlated with disease activity in some studies.25 Disappearance of anti‐Scl70 autoantibodies has been noted in patients with a more favourable outcome.17 The multivariate analysis shows that autoantibody status contributes to 15 of the organ complications, whereas the clinical SSc subtype serves as an explanatory variable to 11 of the organ complications. This could imply that autoantibody status is more closely related to organ involvement than SSc subsets in the LeRoy classification.
Of note, the MEDS does not capture the status of anti‐RNA‐polymerase antibodies which are associated with dcSSc and renal involvement.26 The presence of anti‐RNA‐polymerase antibodies may explain the finding that hypertensive renal crisis was not more frequent in individuals carrying anti‐Scl‐70 autoantibodies (table 4), but on the other hand was associated with the absence of Scl70 autoantibodies (table 5), despite the link between renal complications and dcSSc (table 1).
Our analysis nevertheless confirms the importance of dcSSc and lcSSc scleroderma subdivision in their association with particular organ manifestations. The age at onset of Raynaud's phenomenon may also contribute in the assessment of the likelihood of some organ complications. Clearly, both clinical and laboratory parameters must be combined and evaluated longitudinally in the prognostication of SSc. The EUSTAR MEDS database contributes to the critical assessment of the current diagnostic and prognostic dogma. The long‐term prospective data on this large and still growing number of patients will continue to facilitate the analysis of clinical patterns in SSc and allow rapid evaluation of new diagnostic tests and therapeutic strategies. Large‐scale co‐operation is a necessary and powerful tool in the study of a rare disease like SSc.
Acknowledgements
EUSTAR is supported by a research grant from EULAR, and is under the auspices of the Standing Committee for International Studies Including Clinical Trials (ESCISIT). We thank M Enters (Statsolutions, Freiburg, Germany) for statistical assistance.
Abbreviations
ACA - anticentromere autoantibody
ACR - American College of Rheumatology
CK - creatine kinase
dcSSc - diffuse cutaneous systemic sclerosis
EUSTAR - EULAR Scleroderma Trials And Research
lcSSc - limited cutaneous systemic scerosis
PAH - pulmonary artery hypertension (assessed by echocardiography)
SSc - sytemic sclerosis
Appendix
CO‐AUTHOR LIST
Gabriela Riemekasten1, Claudia Brückner1, Paolo Airo'2, Mirko Scarsi2, Raffaella Scorza3, Lorenzo Beretta3, Franco Cozzi4, Francesco Tiso4, MC Vonk5, FHJ van den Hoogen5, Fredrick M Wigley6, Laura Hummers6, Tatjana Nevskaya7, Lidia Ananieva7, Irene Miniati8, Nicoletta Tartaglia9, Claudia Lomater9, Alexandra Balbir‐Gurman10, Yolanda Braun‐Moscovici10, Lisa Maria Bambara11, Paola Caramaschi11, Gabriele Valentini12, Luigia Ruocco12, Thomas Krieg13, Nicolas Hunzelmann13, Cecília Varjú14, Patricia E Carriera15, Beatriz Joven15, Florenzo Iannone16, Giovanni Lapadula16, André Kahan17, Yannick Allanore17, Armando Gabrielli18, Michele Imperatore18, Agneta Scheja19, Frank Wollheim19, Nemanja Damjanov20, Predrag Ostojic20, Petra Saar21, Ingo H. Tarner21, Ina Kötter22, Stefano Bombardieri23, Laura Bazzichi23, Nicoletta Del Papa24, Denise P Comina24, Andrea Lo Monaco25, Renato La Corte25, Eric Hachulla26, David Launay26, Oliver Distler27, Adrian Ciurea27, Stanislaw Sierakowski28, Holly Mitchell29, Richard M Silver29, Dorota Krasowska30 Malgorzata Michalska‐Jakubus30, Mohammed Tikly31, Nazrana Aboo31, Margitta Worm32, Pascal Klaus32, Jozef Rovenský33, Olga Lukáčová33, Blaz Rozman34, Alenka Sipek34, Paulo Clemente‐Coelho35, Yehuda Shoenfeld36, Pnina Langewitch36, Da Silva José A P37, Salvador MJ37, Annegret Kuhn38, Gunilla Erdmann38, Radim Bečvář39, Elke Friedl40, Winfried Graninger40, Valeria Riccieri41, Roberto Caporali42, Carlomaurizio Montecucco42, P Vlachoyiannopoulos43, Meike Distler44, Kristian Reich44, Maria Majdan45, Ewa Wielosz45, Simona Rednic46, Jacob M van Laar47, Stefan Heitmann48, Andreas Bruckner48, Andrea Himsel49, Julia Riemann49, Rotraud Meyringer50, Adelheid Müller50, Duska Martinovic51, Mislav Radic51, Michael Sticherling52, Zoltan Szekanecz53, Gabriella Szücs53, Roberto Giacomelli54, Alessandra Marrelli54, Bojana Stamenkovic55, Aleksandra Stankovic55, Martin Aringer56, Josef S Smolen56, Eugene J Kucharz57, Anna T Kotulska57, Stefania Jablonska58, Maria Blasczik58, Jae‐Bum Jun59, Carmel Mallia60, Bernard Coleiro60, Vera Ortiz Santamaria61, Ralf Hinrichs62, Henrik Nielsen63, Roberta Cossutta64, Ruxandra Ionescu65, Daniela Opris65, Kerstin Steinbrink66, Boris Grundt66, Gianluigi Bajocchi67, Štork Jiří68, Paloma García de la Peña Lefebvre69, Antonio C Zea Mendoza69, Camillo Ribi70, Carlo Chizzolini70, Margaret Wisłowska71, Srdan Novak72, Francesco Indiveri73, Søren Jacobsen74, Per Brown Frandsen74, I Zimmermann Gorska75, Jan Tore Gran76, Øyvind Midtvedt76, Filipa Oliveira Ramos77, Ljubinka Damjanovska Rajcevska78, Georgi Bozinovski78 Dieter Schöffel79, Cord Sunderkötter80, Markus Böhm80, Jadranka Morović‐Vergles81, Melanie‐Ivana Čulo81, Maurizio Cutolo82, Alberto Sulli82, Chris T Derk83, Sergio A Jimenez83, Panagiota Siakka84, Klaus Søndergaard85, Kristian Stengaard‐Pedersen85, Jean Cabane86, TIEV Kiet Phong86, Carina Mihai87, Roxana Sfrent‐Cornateanu87, Michael Jendro88, Piia Tuvik89, Marco Antivalle90, Giovanna Randisi90, Matthias Seidel91, Ricarda Clarenbach91, Ismail Simsek92, Ayhan Dinc92, Murat Inanc93, Monica Sinziana Capraru94, Dorin Capraru94, Inmaculada Bañegil95, Jutta Richter96, Saad Alhasani97, Ivan Földvari98, Sandra Pinto99, Filipe Brandão99, Antonio Juan Mas100
1. Department of Rheumatology‐Charitè University Hospital, Berlin, Germany
2. Servizio di Reumatologia Allergologia e Immunologia Clinica Spedali Civili di Brescia, Brescia, Italy
3. UO Immunologia Clinica‐Centro di Riferimento per le Malattie Autoimmuni Sistemiche, Milano, Italy
4. Division of Rheumatology, Department of Medical and Surgical Sciences, University of Padova, Padova, Italy
5. Radboud University Medical Centre, Nijmegen, The Netherlands
6. Johns Hopkins University Division of Rheumatology, Baltimore, Maryland, USA
7. Institute of Rheumatology, Russian Academy of Medical Science, Moscow, Russia
8. Department of Medicine, Division of Rheumatology, University of Florence, Florence, Italy
9. Ospedale Mauriziano Centro di Reumatologia, Torino, Italy
10. Rambam Medical Center, Haifa, Israel
11. Dipartimento di Medicina Clinica e Sperimentale, Università degli Studi di Verona, Verona, Italy
12. Dipartimento Medicina Clinica e Sperimentale II Policlinico UO Reumatologia, Napoli, Italy
13. Universitätshautklinik Köln, Köln, Germany
14. Department of Immunology and Rheumatology, Faculty of Medicine, University of Pécs, Pécs, Hungary
15. Hospital Universitario 12 de Octubre, Servicio de Reumatología, Madrid, Spain
16. UO Reumatologia Università degli studi di Bari, Bari, Italy
17. Paris Cochin Hospital, Groupe Hospitalier Cochin, Paris, France
18. Istituto di Clinica Medica Generale, Ematologia ed Immunologia Clinica Università di Ancona, Ancona, Italy
19. Department of Rheumatology, University Hospital Lund, Lund, Sweden
20. Institute of Rheumatology, Belgrade, Serbia
21. Kerckhoff‐Klinik Bad Nauheim Universität Giessen, Bad‐Nauheim, Germany
22. Medizinische Klinik und Poliklinik Internal Medicine, Rheumatology, Tübingen, Germany
23. Department of Internal Medicine, Rheumatology Unit, University of Pisa, Pisa, Italy
24. Day Hospital Rheumatology, “Gaetano Pini”, Milano, Italy
25. Department of Clinical and Experimental Medicine, Section of Rheumatology, University of Ferrara, Ferrara, Italy
26. Department of Internal Medicine, Hôpital Claude Huriez, Lille, France
27. Department of Rheumatology, University Hospital Zurich, Zurich, Switzerland
28. Department of Rheumatology and Internal Diseases, Medical University of Bialystok, Bialystok, Poland
29. Division of Rheumatology & Immunology, Charleston, South Carolina, USA
30. Department of Dermatology, Medical University of Lublin, Lubin, Poland
31. Rheumatology Unit Hospital and University of the Witwatersrand, Johannesburg, South Africa
32. Department of Dermatology and Allergy, Charité – Universitätsmedizin Berlin, Berlin, Germany
33. Institute of Rheumatic Diseases, Pieštány, Slovak Republic
34. Division of Internal Medicine, Department of Rheumatology, University of Ljublijana, Ljublijana, Slovenia
35. Instituto portugues de Reumatologia, Lisbon, Portugal
36. Center for Autoimmune Diseases, Department of Medicine B, Sakler Tel‐Aviv University, Tel Aviv, Israel
37. Reumatologia, Hospitais da Universidade, Coimbra, Portugal
38. Department of Dermatology, University of Düsseldorf, Düsseldorf, Germany
39. Institute of Rheumatology, 1st Medical School, Charles University of Prague, Prague, Czech Republic
40. Medizinische Universitätsklinik – Abteilung für Rheumatologie, University of Graz, Graz, Austria
41. Divisione di Reumatologia – Università “La Sapienza” Roma, Italy
42. Unità Operativa e Cattedra di Reumatologia, Policlinico S. Matteo, Pavia, Italy
43. Department of Pathopysiology Medical School, National University of Athens, Athens, Greece
44. Department of Dermatology, Georg‐August‐University of Göttingen, Göttingen, Germany
45. Department of Rheumatology and Connective Tissue Diseases, University of Lublin, Lublin, Poland
46. Clinica Reumatologie – Medicală II University of Medicine & Pharmacy, Cluj‐Napoca, Romania
47. Department of Rheumatology University Medical Center of Leiden, Leiden, The Netherlands
48. Department of Rheumatology Marienhospital, Stuttgart, Germany
49. Klinikum der Johan Wolfgang Goethe – Universität Medizinische Klinik III, Rheumatologische Ambulanz, Frankfurt am Main, Germany
50. Department of Internal Medicine‐I, University of Regensburg, Regensburg, Germany
51. Rheumatology Department of Internal Clinic Clinical Hospital of Split, Split, Croatia
52. Klinik für Dermatologie, Venerologie und Allergologie University of Leipzig, Leipzig, Germany
53. Rheumatology Division University of Debrecen, Debrecen, Hungary
54. Dipartimento di Medicina Interna e Sanità Pubblica, Insegnamento di Reumatologia University of L'Aquila, Aquila, Italy
55. Institute for prevention, treatment and rehabilitation rheumatic and cardiovascular disease Niska Banja, Serbia
56. Department of Rheumatology, Internal Medicin III, University of Vienna, Vienna, Austria
57. Department of Internal Medicine and Rheumatology, Medical University of Silesia, Katowice, Poland
58. Department of Dermatology, University of Warsaw, Warsaw, Poland
59. Hanyang University, Seoul, Korea
60. St Luke's Hospital, Guardamangia, Balzan, Malta
61. Rheumatology Granollers General Hospital, Granollers (Barcelona), Spain
62. Klinik für Dermatologie und Allergologie University of Ulm, Ulm, Germany
63. Department of Rheumatology and Endocrinology, Herlev, Denmark
64. Rheumatology Unit, Humanitas Clinical Institute, Rozzano Milano, Italy
65. Department of Rheumatology‐St Maria Hospital, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania
66. Department of Dermatology, University of Mainz, Mainz, Germany
67. Arcispedale Santa Maria Nuova UO di Reumatologia, Pad Spallanzani, Reggio Emilia, Italy
68. Department of Dermatology the 1st Faculty of Medicine, Charles University, Prague, Czech Republic
69. Servicio de Reumatología, Hospital Ramon Y Cajal, Madrid, Spain
70. Immunology and Allergy, University Hospital of Genève, Genève, Switzerland
71. Department of Rheumatology, Warsaw, Poland
72. Department of Rheumatology and Clinical Immunology, KBC, Rijeka, Croatia
73. Clinica di medicina interna ad orientamento immunologico Università di Genova, Genova, Italy
74. Department of Rheumatology Rigshospitalet, Copenhagen, Denmark
75. Department of Rheumatology and Rehabilitation, University of Poznan, Poznan, Poland
76. Department of Rheumatology, Rikshospitalet, Oslo, Norway
77. Department of Rheumatology, Hospital Santa Maria, Lisbon, Portugal
78. Rheumatology Clinic, Clinical Center Skopje, FYR Macedonia
79. Department of Rheumatology Westpfalz‐KliniKum, Kusel, Germany
80. Department of Dermatology, University of Münster, Münster, Germany
81. Division of Clinical Immunology and Rheumatology, Dubrava University Hospital of Zagreb, Zagreb, Croatia
82. Research Laboratory and Division of Rheumatology, Department of Internal Medicine, University of Genova, Genova, Italy
83. Thomas Jefferson University of Philadelphia, Philadelphia, PA, USA
84. Department of Rheumatology, Thessaloniki, Greece
85. Department of Rheumatology, University Hospital of Aarhus, Aarhus, Denmark
86. Service de Médecine Interne 2° Hopital Saint Antoine, Paris, France
87. Clinic of Internal Medicine and Rheumatology, Dr I Cantacuzino Hospital, Bucharest, Romania
88. Rheumatologische Ambulanz, Medizinische Klinik I, Universitaetskliniken Saarlandes, Homburg, Germany
89. North‐Estonian Regional Hospital, Tallin, Estonia
90. Unità Operativa di Reumatologia, Azienda Ospedaliera‐Polo Universitario, Ospedale L Sacco, Milano, Italy
91. Department of Rheumatology, Medizinische Univesitäts‐Poliklinik, Bonn, Germany
92. Division of Rheumatology, Gulhane Military Medical Academy, Ankara, Turkey
93. Department of Internal Medicine, Division of Rheumatology, Medical Faculty of Istanbul, Turkey
94. Department of Rheumatology, “Professor Dr D Gerota” Emergency Hospital, Bucharest, Romania
95. Consulta Reumatologia, Hospital de Mendaro, Mendaro, Spain
96. Department of Rheumatology Heinrich‐Heine University of Düsseldorf, Düsseldorf, Germany
97. Rheumatology and Rehabilitation Department of Mosul, Mosul, Iraq
98. Pediatric Rheumatology Clinic, Hamburg, Germany
99. Hospital São João Serviço de Reumatologia, Porto, Portugal
100. Hospital son Llàtzer, Palma de Mallorca, Spain
Footnotes
Competing interests: None declared.
References
- 1.Alamanos Y, Tsifetaki N, Voulgari P V, Siozos C, Tsamandouraki K, Alexiou G A.et al Epidemiology of systemic sclerosis in northwest Greece 1981 to 2002. Semin Arthritis Rheum 200534714–720. [DOI] [PubMed] [Google Scholar]
- 2.Roberts‐Thomson P J, Jones M, Hakendorf P, Kencana Dharmapatni A A, Walker J G, MacFarlane J G.et al Scleroderma in South Australia: epidemiological observations of possible pathogenic significance. Intern Med J 200131220–229. [DOI] [PubMed] [Google Scholar]
- 3.Tamaki T, Mori S, Takehara K. Epidemiological study of patients with systemic sclerosis in Tokyo. Arch Dermatol Res 1991283366–371. [DOI] [PubMed] [Google Scholar]
- 4.Czirjak L, Kiss C G, Lovei C, Suto G, Varju C, Fuzesi Z.et al Survey of Raynaud's phenomenon and systemic sclerosis based on a representative study of 10 000 south‐Transdanubian Hungarian inhabitants. Clin Exp Rheumatol 200523801–808. [PubMed] [Google Scholar]
- 5.Geirsson A J, Steinsson K, Guthmundsson S, Sigurthsson V. Systemic sclerosis in Iceland. A nationwide epidemiological study. Ann Rheum Dis 199453502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silman A, Jannini S, Symmons D, Bacon P. An epidemiological study of scleroderma in the West Midlands. Br J Rheumatol 198827286–290. [DOI] [PubMed] [Google Scholar]
- 7.Mayes M D, Lacey J V, Jr, Beebe‐Dimmer J, Gillespie B W, Cooper B, Laing T J.et al Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum 2003482246–2255. [DOI] [PubMed] [Google Scholar]
- 8.Bryan C, Knight C, Black C M, Silman A J. Prediction of five‐year survival following presentation with scleroderma: development of a simple model using three disease factors at first visit. Arthritis Rheum 1999422660–2665. [DOI] [PubMed] [Google Scholar]
- 9.Cox S R, Walker J G, Coleman M, Rischmueller M, Proudman S, Smith M D.et al Isolated pulmonary hypertension in scleroderma. Intern Med J 20053528–33. [DOI] [PubMed] [Google Scholar]
- 10.Mayes M D. Race, scleroderma, and survival: why is there a difference? J Rheumatol 2005321873–1874. [PubMed] [Google Scholar]
- 11.Ioannidis J P, Vlachoyiannopoulos P G, Haidich A B, Medsger T A, Jr, Lucas M, Michet C J.et al Mortality in systemic sclerosis: an international meta‐analysis of individual patient data. Am J Med 20051182–10. [DOI] [PubMed] [Google Scholar]
- 12.Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum 198023581–590. [DOI] [PubMed] [Google Scholar]
- 13.LeRoy E C, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger T A., Jret al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis, J Rheumatol 198815202–205. [PubMed] [Google Scholar]
- 14.Wollheim F A. Classification of systemic sclerosis. Visions and reality. Rheumatology 2005441212–1216. [DOI] [PubMed] [Google Scholar]
- 15.Kuwana M, Kaburaki J, Okano Y, Tojo T, Homma M. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthritis Rheum 19943775–83. [DOI] [PubMed] [Google Scholar]
- 16.Steen V D, Powell D L, Medsger T A., Jr Clinical correlation and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum 198831196–203. [DOI] [PubMed] [Google Scholar]
- 17.Kuwana M, Kaburaki J, Mimori T, Kawakami Y, Tojo T. Longitudinal analysis of autoantibody response to topoisomerase I in systemic sclerosis. Arthritis Rheum 2000431074–1084. [DOI] [PubMed] [Google Scholar]
- 18.Arnett F C, Cho M, Chatterjee S, Aguilar M B, Reveille J D, Mayes M D. Familial occurrence frequencies and relative risks for systemic sclerosis (scleroderma) in three United States cohorts. Arthritis Rheum 2001441359–1362. [DOI] [PubMed] [Google Scholar]
- 19.Feghali‐Bostwick C, Medsger T A, Jr, Wright T M. Analysis of systemic sclerosis in twins reveals low concordance for disease and high concordance for the presence of antinuclear antibodies. Arthritis Rheum 2003481956–1963. [DOI] [PubMed] [Google Scholar]
- 20.Lin A T, Clements P J, Furst D E. Update on disease‐modifying antirheumatic drugs in the treatment of systemic sclerosis. Rheum Dis Clin North Am 200329409–426. [DOI] [PubMed] [Google Scholar]
- 21.van Laar J M, Farge D, Tyndall A. Autologous Stem cell Transplantation International Scleroderma (ASTIS) trial: hope on the horizon for patients with severe systemic sclerosis. Ann Rheum Dis 2005641515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hachulla E, Coghlan J G. A new era in the management of pulmonary arterial hypertension related to scleroderma: endothelin receptor antagonism. Ann Rheum Dis 2004631009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valentini G, Bencivelli W, Bombardieri S, D'Angelo S, Della R A, Silman A J.et al European Scleroderma Study Group to define disease activity criteria for systemic sclerosis. III. Assessment of the construct validity of the preliminary activity criteria. Ann Rheum Dis 200362901–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cutolo M, Pizzorni C, Tuccio M, Burroni A, Craviotto C, Basso M.et al Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatology 200443719–726. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, Hamaguchi Y, Hasegawa M, Takehara K. Clinical significance of anti‐topoisomerase I antibody levels determined by ELISA in systemic sclerosis. Rheumatology 2001401135–1140. [DOI] [PubMed] [Google Scholar]
- 26.Bunn C C, Denton C P, Shi‐Wen X, Knight C, Black C M. Anti‐RNA polymerases and other autoantibody specificities in systemic sclerosis. Br J Rheumatol 19983715–20. [DOI] [PubMed] [Google Scholar]