Abstract
Objectives
To compare utility and disease‐specific direct costs between patients with ankylosing spondylitis (AS) and patients with rheumatoid arthritis (RA) in the Netherlands.
Methods
Patients with AS and those with RA completed questions on disease characteristics, the EuroQol‐5D (EQ‐5D) to assess utility, and questionnaire resource utilisation. Resource utilisation was assessed prospectively in AS, but retrospectively in RA. True cost estimates (2003) were used to calculate the costs. Differences in disease characteristics between AS and RA were described, and determinants of EQ‐5D utility and costs were explored by Cox proportional hazard regressions.
Results
576 patients with RA and 132 with AS completed the questionnaires. EQ‐5D utility (0.63 vs 0.7) was lower, and annual direct costs higher in RA (€5167 vs €2574). In multivariate Cox proportional hazard regressions, there was no difference in utility between the diagnostic groups, but patients with RA incurred higher direct costs after controlling for age, gender and disease duration.
Conclusions
In patients with RA and patients with AS, who are under the care of a rheumatologist, utility is equally reduced, but healthcare costs are higher in RA after controlling for age, gender and disease duration. These data can be helpful to provide insights into the differences and similarities between the healthcare needs of both patient groups and to identify issues for further research and for policy in healthcare organisations.
Ankylosing spondylitis (AS) and rheumatoid arthritis (RA) are the most common inflammatory diseases dealt with by rheumatologists. Although their pathophysiology, clinical signs and symptoms differ, it has been shown that the impact on physical functioning is similar for both diseases.1 As yet, there are only few studies directly comparing the socioeconomic impact of these two diseases with regard to utility and healthcare utilisation. It is important to compare socioeconomic consequences of different rheumatic diseases in one study, because limited healthcare resources and funding policies for research could then be applied more reliably than when using a similar approach for all rheumatic diseases. As yet, no study has compared the utility and healthcare utilisation of different rheumatic diseases in one report. A review of cost‐of‐illness (COI) studies on AS showed that direct costs in AS varied between US$1913 (£990, €1450) and US$4478 (£2318, €3396),2 whereas reviews in RA reported that direct costs varied between US$1503 (£778, €1140) and US$16 514 (£8550, €12 525).3,4 This suggests that the costs of AS are within the range of costs for RA. However, because of differences in sampling of patients (including different countries), timing of the survey, methods used to collect resources and choice of unit costs per resource, it is difficult to reliably compare total direct costs between various studies.
The aim of this study was to compare utility and resource utilisation in Dutch patients with AS and RA using an identical utility score and methods to value healthcare utilisation.
Methods
Patients and questionnaires
For this comparison study, data were obtained from two Dutch studies, one performed on patients with AS and the other on patients with RA. Patients with AS were part of a longitudinal study that was started in 1996. All patients who were registered with AS in the diagnostic register of the academic hospital of Maastricht and the affiliated non‐academic regional hospital were invited. Patients with RA were part of the Utrecht Rheumatoid Arthritis Cohort study group, which is an ongoing inception cohort since 1990 at the academic hospital Utrecht and affiliated non‐academic hospitals. These patients were invited to participate in a COI study in 1999–2000. To ensure the entire spectrum of disease duration, a random sample of patients with RA with a disease duration of >10 years was also invited to participate in the COI survey. In the Netherlands, all patients with inflammatory disease are referred to the rheumatology department.
Disease characteristics
At the time of survey (baseline measurement in the AS cohort), patients from both samples completed a questionnaire including demographic and disease characteristics. Demographics included age, educational level and working status. Clinical characteristics included duration of morning stiffness (0–120 min), visual analogue scale (VAS) general well‐being (0–100 mm, 100 being the worst general well‐being) and level of pain. For RA, pain was assessed using a VAS pain (0–100 mm, 100 denoting the most severe pain), whereas for AS the two pain scales of the Bath Ankylosing Spondylitis Disease Activity Index were used.5 Question 2 asks about pain in the neck, back and hips on a VAS (0–100 mm, 100 denoting the worst pain), and question 3 asks about pain in joints other than neck, back and hips (VAS 0–100 mm, 100 denoting the worst pain). Erythrocyte sedimentation rate expressed in mm/hr1st was available for both groups. To assess utility, the EuroQol‐5D (EQ‐5D) was used.6 The EuroQol instrument consists of two parts. For this study, we used the part comprising five questions each addressing a different attribute (domain) of health status (EQ‐5 dimension) covering mobility, self‐care, daily activities, pain and mood. Each of the five questions can be answered on a three‐point categorical scale. Utility values were derived in choice experiments in the general population. These values were then used to transform the results in an equation that provides utility values ranging from −0.594 to 1, 1 denoting the best health status.
Socioeconomic consequences
During the first two years of the cohort study (1997–9), patients with AS completed a cost questionnaire on AS‐related resource utilisation and a questionnaire on working status every 2 months.7,8 Resources comprised the number of contacts with all kinds of healthcare providers (eg, general practitioner, rheumatologists, other specialists, rheumatology research nurse, physiotherapist and paid home help), laboratory tests, type and dose of drugs used, number of days in hospital (including surgical procedures) and admissions to a rehabilitation centre. In addition, six‐monthly questionnaires collected information on aids and appliances purchased, adaptations at home, and travel distances from and to healthcare providers.
In 1999–2000, patients with RA completed a retrospective questionnaire on RA‐related resource utilisation9,10 that included the same socioeconomic items as in the study among patients with AS. In the RA study, recall was at 3 months for visits to healthcare providers and at 1 year for admissions to the hospital and the rehabilitation centre, and for the purchase of aids and for adaptations at home. Drug use was determined at the time of filling out the questionnaire, and the number and kind of laboratory tests was estimated based on number of visits to the rheumatologist and types of drug used.
Cost estimates to value resource use
First, resource utilisation was annualised. For AS, the utilisation numbers were summed for all 12 questionnaires and divided by two. For RA, the questions with a recall of 3 months were multiplied by four. Subsequently, the annual costs per resource utilisation rate were multiplied by the costs per resource to calculate the annual costs for each resource. Costs per resource for contacts with healthcare providers, admissions to the hospital and rehabilitation centre and travel expenses were derived from the Dutch guidelines for pharmacoeconomic studies, which provide true cost estimates based on activity‐based costing and including overhead costs.11 Table 1 shows an overview of the most important costs. Costs for devices and adaptations were reported by the patients in the original questionnaires and adjusted to 2003 prices by applying the healthcare consumer price index.12 Costs for medication, laboratory assessments and surgical procedures were obtained from the original year of study and also adjusted to 2003 prices using the consumer price index. The year 2003 was chosen because costs estimates for this year were the last updated costs published in the Dutch guidelines.
Table 1 Overview of the most important costs reported in the Dutch guidelines for pharmacoeconomic studies11.
| Admissions to the hospital | |
| Academic hospital | 476 |
| Non‐academic hospital | 337 |
| Admissions to the rehabilitation centre | 336 |
| Contacts with | |
| Rheumatologist | |
| Academic hospital | 100 |
| Non‐academic hospital | 56 |
| Physiotherapist | 22.75 |
| General practitioner | 20.20 |
Costs are 2003 tariffs in Euros.
Statistical analyses
Demographic and clinical differences between both groups were analysed using descriptive statistics and tested by using χ2, independent Student's t tests or Mann–Whitney U, whichever appropriate. All tests were two sided and a p value of <0.05 was considered to indicate a statistically significant difference between the two disease groups. Data on employment and work disability are presented as rate ratios after adjusting for age and gender with the general population. Differences in costs between the two patient populations were assessed by difference in mean costs and their 95th centile bootstrapped CIs after 1000 replications. To assess the contribution of diagnosis (AS vs RA) on the total direct costs, Cox proportional regression analyses were applied, controlling for age, gender and disease duration. A similar analysis was performed to assess the contribution of diagnostic group to utility.
Results
Data were available for 132 patients with AS and 576 patients with RA. Table 2 shows that 30% of the population with AS and 72% of the population with RA were women; their mean age at diagnosis was 34 vs 52 years, respectively. With respect to pain, patients with AS had significantly more back pain than the global pain in patients with RA (44 vs 25 mm), but pain was comparable in the peripheral joints for the two groups. Reported morning stiffness was longer in AS (37 vs 30 min), but erythrocyte sedimentation rate values were significantly lower (15 vs 22 mm/hr1st); global well‐being was comparable between the two patient groups. Employment ratios were reduced to a similar extent in AS and RA, but (partial) work disability was more pronounced in AS.
Table 2 Patient characteristics for the total population with ankylosing spondylitis and for the total population with rheumatoid arthritis, and for women and men separately.
| Patients with AS | Patients with RA | p Value | |
|---|---|---|---|
| n = 132 | n = 576 | ||
| Gender, female (%) | 39 (30%) | 417 (72%) | <0.001 |
| Age in years, total group | 45 (12) | 59 (14) | <0.001 |
| Women | 47 (12) | 58 (14) | |
| Men | 45 (12) | 62 (12) | |
| Educational level (>high vocational), total group | 19% | 13% | 0.084 |
| Women | 18% | 12% | |
| Men | 18% | 14% | |
| Marital status (% married), total group | 80% | 76% | 0.322 |
| Women | 82% | 71% | |
| Men | 78% | 88% | |
| Employed in paid work; adjusted rate ratio compared with that in the general population (95% CI) | 0.6 (0.5 to 0.8) | 0.7 (0.6 to 0.8) | |
| Official work disabled; adjusted rate ratio compared with that in the general population (95% CI) | 4.9 (4.0 to 5.8) | 2.7 (2.3 to 3.1) | |
| Age at onset, total group | 34 (11) | 52 (14) | <0.001 |
| Women | 34 (11) | 51 (15) | |
| Men | 33 (11) | 55 (12) | |
| Disease duration in years, total group | 12 (9) | 7 (7) | <0.001 |
| Women | 13 (10) | 7 (7) | |
| Men | 11 (9) | 6 (5) | |
| VAS pain, total group* | 44 (27) | 25 (26) | <0.001 |
| Women | 50 (22) | 27 (27) | |
| Men | 42 (28) | 21 (25) | |
| VAS pain in the joints other than back, neck and hip† | 26 (23) | ||
| VAS general well‐being, total group | 36 (27) | 34 (26) | 0.584 |
| Women | 41 (26) | 35 (27) | |
| Men | 33 (27) | 31 (25) | |
| Morning stiffness, total group | 37 (29) | 30 (39) | 0.018 |
| Women | 44 (32) | 30 (39) | |
| Men | 34 (28) | 30 (39) | |
| ESR, total group | 15 (15) | 22 (18) | <0.001 |
| Women | 17 (20) | 23 (19) | |
| Men | 15 (13) | 20 (17) | |
| Utility (EQ‐5D), total group | 0.70 (0.2) | 0.63 (0.3) | 0.001 |
| Women | 0.67 (0.2) | 0.62 (0.3) | |
| Men | 0.71 (0.2) | 0.67 (0.3) |
AS, ankylosing spondylitis; EQ‐5D, EuroQol 5D; ESR, erythrocyte sedimentation rate; RA, rheumatoid arthritis; VAS, visual analogue scale.
*Global pain for patients with RA and back pain for patients with AS.
†For the entire group of patients with AS, of whom 28% had peripheral arthritis. p Values were assessed by χ2 test for dichotomous variables, by independent t test for continuous variables with normal distribution and by Mann–Whitney U test for continuous variables with a skewed distribution (EQ‐5D). Higher educational level was defined as higher vocational education or university. VAS scale (range 0–100 mm, 100 denoting the worst score), morning stiffness (range 0–120 min), EQ (range from −0.594 to 1, 1 denoting the best health state).
EQ‐5D utility assessed by EQ‐5D was better for patients with AS than for those with RA (p = 0.001). The latter could be attributed to the lower proportion of patients with AS who reported moderate or severe scores on the domains mobility (50% vs 70%, p<0.001), self‐care (16% vs 39%, p<0.001) and daily functioning (61% vs 75%, p = 0.002). There was no difference in proportions of patients reporting moderate‐to‐severe pain (86% vs 82%, p = 0.334) and mood disturbance (25% vs 27%, p = 0.709). In multivariate Cox proportional regression analyses, diagnosis (RA vs AS) did not contribute significantly to utility and was therefore not included in the final model. Lower utility—that is, worse health status—was explained by longer disease duration (hazard ratio (HR) 1.02, 95% CI 1.01 to 1.03) and female gender (HR 1.32, 95% CI 1.11 to 1.57). Compared with the general Dutch population, the percentage of patients with moderate‐to‐severe problems on each EQ‐5D dimension was higher for patients with AS and with RA. The proportion of subjects from the general Dutch population with moderate‐to‐severe limitations were 18% for mobility, 4% for self‐care, 15% for usual activity, 34% for pain and 12% for anxiety.13
Direct costs
Table 3 presents the average annual resource utilisation rates of the most important healthcare resources and percentage of patients using each resource. A higher proportion of patients with RA visited the rheumatologist, and also the average number of contacts was higher for patients with RA than for patients with AS. By contrast, more patients with AS had contacts with the physiotherapist and hydrotherapy than patients with RA. Also, those patients with AS visited a physiotherapist more frequently than patients with RA. A higher proportion of patients with AS was admitted to a hospital or a rehabilitation centre than patients with RA, but the average duration of days admitted to a hospital or rehabilitation centre was longer for patients with RA than for patients with AS.
Table 3 Number of patients with at least one contact with a healthcare provider, number of admissions to the hospital or rehabilitation centre, and mean (SD) annual number of contacts and admissions for both disease groups.
| Number (%) of patients with at least one contact or admission | Mean (SD) number of contacts or admissions | Mean (SD) number of contacts or admissions | p Value | ||||
|---|---|---|---|---|---|---|---|
| Patients with AS | Patients with RA | Patients with AS with a contact | Patients with RA with a contact | All patients with AS (n = 132) | All patients with RA (n = 576) | ||
| Rheumatologist | 90 (68%) | 517 (91%) | 2.5 (1.9) | 6.3 (5.3) | 1.7 (1.9) | 5.7 (5.4) | <0.001 |
| Rheumatology nurse | 30 (23%) | 172 (30%) | 1.1 (1.5) | 7.0 (7.5) | 0.2 (0.7) | 2.1 (5.2) | 0.002 |
| Physiotherapist/hydrotherapy | 106 (80%) | 149 (26%) | 42.4 (36.3) | 62.1 (37.3) | 34.0 (36.6) | 16.1 (33.2) | <0.001 |
| Home help | 6 (5%) | 73 (13%) | 93.2 (60.7) | 83.2 (95.5) | 4.2 (22.8) | 10.5 (43.7) | 0.009 |
| General practitioner | 78 (59%) | 138 (24%) | 2.4 (3.0) | 11.3 (10.8) | 1.4 (2.6) | 2.7 (7.1) | <0.001 |
| Admission | |||||||
| Hospital | 27 (20%) | 61 (11%) | 7.8 (11.0) | 12.5 (12.4) | 1.6 (5.8) | 1.3 (5.5) | 0.029 |
| Rehabilitation centre | 7 (5%) | 11 (2%) | 17.9 (12.4) | 66.2 (61.9) | 1.0 (4.8) | 1.3 (12.2) | 0.005 |
AS, ankylosing spondylitis; RA, rheumatoid arthritis.
p Values were assessed by the Mann–Whitney U test to test statistically significant differences in number of contacts between the total population with AS and the total population with RA. A p value of <0.05 indicates a statistically significant difference between the two disease populations with respect to contacts and admissions.
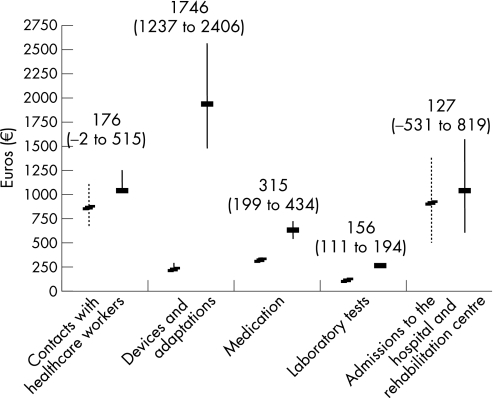
Overall, the mean (SD, IQ0.25–0.75 range) total direct costs were €2574 (3537, 532–3056) for patients with AS and €5167 (10 392, 1238–4637) for patients with RA, difference €2592 (95% CI 1586 to 3699) after bootstrapping. Figure 1 shows the mean (95% CI) annual expenses of each predefined cost category except for travel expenses. Mean differences in costs were statistically significantly higher for medication, laboratory tests, and for devices and adaptations in the RA‐ group compared with the AS group. In the multivariate Cox proportional regression analyses for total direct costs, the diagnosis of RA (HR 1.9, 95% CI 1.51 to 2.39) and longer disease duration (HR 0.98, 95% CI 0.97 to 0.99) were associated with higher costs after adjusting for gender and age.
Figure 1 Mean (95% CI estimated with bootstrapping) annual costs in Euros for different predefined cost categories. Patients with rheumatoid arthritis (solid line) and patients with ankylosing spondylitis (dotted line).
Discussion
In this study, we compared several aspects of the burden of illness, with emphasis on the utility and direct costs of two rheumatic diseases, AS and RA. Comparing disease characteristics such as pain and morning stiffness between different rheumatological conditions was challenging. Patients with AS reported more (back and neck) pain than the overall joint pain reported by patients with RA. When combining VAS score of pain of the back and neck, and the score of pain in places other than the back and neck, the average VAS score for both was 32 (SD 18), which is lower than the score for back pain alone but still higher than the VAS score for joint pain reported by patients with RA. In the same line, the duration of morning stiffness was longer for patients with AS than for patients with RA. The differences in pain and morning stiffness did not result in a lower mean overall well‐being of patients with AS, which was similar to that in patients with RA.
On the other hand, utility assessed by EQ‐5D was significantly lower—that is, worse—for patients with RA compared with patients with AS in univariate analysis. Although the proportion of patients with moderate‐to‐severe pain or mood disturbances were similar between the diagnostic groups, a higher proportion of patients with RA had moderate‐to‐severe problems on the physical related quality of life domains (mobility, self‐care and daily functioning), resulting in lower attributed utility. These results confirm previous findings showing that patients with RA had lower rating of the physical component but not of the mental component of the Short Form‐36, compared with patients with AS.14 The results of the multivariate regression analyses showed that age and gender could largely explain these differences in utility which was also observed in another study.13
The higher annual direct costs for patients with RA than those for patients with AS in our study mainly reflect the differences in treatment approaches between both diseases in the Netherlands. At the time of the survey, treatment options for patients with AS were limited to exercise (including physiotherapy) and non‐steroidal anti‐inflammatory drugs to preserve functional ability, and few indications for disease‐modifying antirheumatic drugs, resulting in 6% of patients in this study taking a disease‐modifying antirheumatic drugs as opposed to 81% of patients with RA. As a result, the number of visits to medical specialists, laboratory examinations and use of medication in patients with AS were much lower than in patients with RA. It can, however, be expected that costs for drug use, nowadays and in the coming years, will be higher in both disease populations, because of an increased use of biological agents. For patients with AS, this will probably result in an increase of costs for laboratory assessments. The costs for aids and adaptations were remarkably higher in patients with RA. The proportion of patients with RA and patients with AS who purchased an aid or adapted their house were 50% and 23%, respectively. The aids purchased were mainly adapted taps for patients with RA (23% of all patients), and mattresses and pillows for patients with AS (8% over 2 years for all patients). However, the results of this study cannot answer the question whether this is due to worse physical functioning in RA or due to the better availability of aids for patients with functional problems of hands and feet. This could be the subject of further investigation.
The difference in the direct costs might have consequences when modelling cost‐effectiveness ratios for expensive rheumatic drugs. Several studies have already shown the beneficial effect of expensive biologicals on disease activity in both patient populations,15,16,17,18,19,20 which may possibly result in less use of healthcare. It should be noted that in AS, however, the possible cost savings will be lower in view of the lower total costs, despite the similar impact of the disease on utility.
The literature directly comparing the COI of RA or AS with other diseases is scarce. One study showed that patients with fibromyalgia and chronic low back pain incurred higher costs than patients with AS, even though they reported worse well‐being.21 Another study compared costs between patients with RA, patients with osteoarthritis and people with high blood pressure. The annual direct costs were $9300 (£4824.59, €7063.82), $5700 (£2956.75, €4329.24) and $3900 (£2023.04, €2962.11), respectively, and patients with RA had five times higher indirect costs than those of patients with either osteoarthritis or high blood pressure.22 To our knowledge, there are no reports comparing utilities.
Our study has some limitations. First, patients were selected using a slightly different approach and were sampled in different regions in the Netherlands. Second, although we used similar utilisation categories and resources to value these utilisation rates, there were still some differences with respect to data collection. Patients with AS had to fill out a questionnaire prospectively during 2 years, whereas, those with RA had to fill only one retrospective questionnaire. Despite these differences, both samples are probably representative for patients with RA and patients are seen by AS Dutch rheumatologists outpatient clinics. In the Netherlands, all patients with an inflammatory condition are typically referred to a rheumatologist. However, there is a longstanding focus on early diagnosis of patients with inflammatory arthritis but not of patients with inflammatory back pain. AS is probably underdiagnosed and hence usually more severe cases are seen by rheumatologists.
In conclusion, among Dutch patients under the care of a rheumatologist, utility was equally affected in RA and AS, but direct healthcare costs were higher for RA after controlling for age, gender and disease duration. These data can be helpful to provide insight into the differences and similarities between the healthcare needs of both patient groups, and to identify issues for research and for healthcare organisations.
Acknowledgements
We thank all participating rheumatologists of the Utrecht Rheumatoid Arthritis Cohort study group (SRU) and the OASIS study group. The economic study among patients with rheumatoid arthritis was supported by the Dutch Arthritis Association.
Abbreviations
AS - ankylosing spondylitis
COI - cost of illness
EQ‐5D - EuroQol‐5D
RA - rheumatoid arthritis
VAS - visual analogue scale
Footnotes
Competing interests: None declared.
References
- 1.Zink A, Braun J, Listing J, Wollenhaupt J. Disability and handicap in rheumatoid arthritis and ankylosing spondylitis: results from the German rheumatological database. German Collaborative Arthritis Centers. J Rheumatol 200027613–622. [PubMed] [Google Scholar]
- 2.Boonen A, van der Heijde D. Review of the costs of illness of ankylosing spondylitis and methodologic notes. Expert Rev Pharmacoecon Outcomes Res 20055163–181. [DOI] [PubMed] [Google Scholar]
- 3.Cooper N J. Economic burden of rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 20003928–33. [DOI] [PubMed] [Google Scholar]
- 4.Rosery H, Bergemann R, Maxion‐Bergemann S. International variation in resource utilisation and treatment costs for rheumatoid arthritis: a systematic literature review. Pharmacoeconomics 200523243–257. [DOI] [PubMed] [Google Scholar]
- 5.Garrett S, Jenkinson T, Kennedy L G, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994212286–2291. [PubMed] [Google Scholar]
- 6.Busschbach J J, McDonnell J, Essink‐Bot M L, van Hout B A. Estimating parametric relationships between health description and health valuation with an application to the EuroQol EQ‐5D. J Health Econ 199918551–571. [DOI] [PubMed] [Google Scholar]
- 7.Boonen A, van der Heijde D, Landewe R, Spoorenberg A, Schouten H, Rutten‐van Mölken M.et al Work status and productivity costs due to ankylosing spondylitis: comparison of three European countries. Ann Rheum Dis 200261429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boonen A, van der Heijde D, Landewe R, Guillemin F, Rutten‐van Mölken M, Dougados M.et al Direct costs of ankylosing spondylitis and its determinants: an analysis among three European countries. Ann Rheum Dis 200362732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verstappen S M, Verkleij H, Bijlsma J W, Buskens E, Kruize A A, Heurkens A H M.et al Determinants of direct costs in Dutch rheumatoid arthritis patients. Ann Rheum Dis 200463817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verstappen S M, Boonen A, Bijlsma J W, Buskens E, Verkleij H, Schenk Y.et al Working status among Dutch patients with rheumatoid arthritis: work disability and working conditions. Rheumatology (Oxford) 200544202–206. [DOI] [PubMed] [Google Scholar]
- 11.Oostenbrink J B, Koopmanschap M A, Rutten F F H.Guideline for cost‐of‐illness study; methods and guideline‐rates for economic evaluations in health care. Diemen, The Netherlands: College voor Zorgverzekeringen, 2004
- 12.The Dutch Bureau of Statistics (Centraal Bureau voor de Statistiek) Voorburg/Heerlen, The Netherlands: The Dutch Bureau of Statistics, 1999
- 13.Hoeymans N, van Lindert H, Westert G P. The health status of the Dutch population as assessed by the EQ‐6D. Qual Life Res 200514655–663. [DOI] [PubMed] [Google Scholar]
- 14.Chorus A M, Miedema H S, Boonen A, van der Linden S. Quality of life and work in patients with rheumatoid arthritis and ankylosing spondylitis of working age. Ann Rheum Dis 2003621178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkham N, Kong K O, Tennant A, Fraser A, Hensor E, Keenan A M.et al The unmet need for anti‐tumour necrosis factor (anti‐TNF) therapy in ankylosing spondylitis. Rheumatology (Oxford) 2005441277–1281. [DOI] [PubMed] [Google Scholar]
- 16.Heiberg M S, Nordvag B Y, Mikkelsen K, Rodevand E, Kaufman C, Mowinckel P.et al The comparative effectiveness of tumor necrosis factor‐blocking agents in patients with rheumatoid arthritis and patients with ankylosing spondylitis: a six‐month, longitudinal, observational, multicenter study. Arthritis Rheum 2005522506–2512. [DOI] [PubMed] [Google Scholar]
- 17.Baraliakos X, Listing J, Brandt J, Rudwaleit M, Sieper J, Braun J. Clinical response to discontinuation of anti‐TNF therapy in patients with ankylosing spondylitis after 3 years of continuous treatment with infliximab. Arthritis Res Ther 20057R439–R444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smolen J S, Han C, Bala M, Maini R N, Kalden J R, van der Heijde D.et al Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: a detailed subanalysis of data from the anti‐tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum 2005521020–1030. [DOI] [PubMed] [Google Scholar]
- 19.Maini R N, Breedveld F C, Kalden J R, Smolen J S, Furst D, Weisman M H.et al Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum 2004501051–1065. [DOI] [PubMed] [Google Scholar]
- 20.Keystone E C, Kavanaugh A F, Sharp J T, Tannenbaum H, Hua Y, Teoh L S.et al Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti‐tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo‐controlled, 52‐week trial. Arthritis Rheum 2004501400–1411. [DOI] [PubMed] [Google Scholar]
- 21.Boonen A, van den Heuvel R, van Tubergen A, Goossens M, Severens J L, van der Heijde D.et al Large differences in cost of illness and wellbeing between patients with fibromyalgia, chronic low back pain, or ankylosing spondylitis. Ann Rheum Dis 200564396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maetzel A, Li L C, Pencharz J, Tomlinson G, Bombardier C. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: a comparative study. Ann Rheum Dis 200463395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]