Abstract
Objective
To assess changes in macrophage phenotype and function after rituximab‐induced B cell depletion in patients with rheumatoid arthritis (RA).
Methods
10 patients with RA were treated with rituximab, achieving significant B cell depletion 4 months later. Clinical improvement, rheumatoid factor (RF), anti‐cyclic citrullinated peptide (anti‐CCP) antibodies, mRNA of B cell activating factor (BAFF), interleukin (IL) 10 and CD86 in human monocyte‐derived macrophages (HMDMs) and tumour necrosis factor α (TNFα) secretion from cultured HMDMs were assessed at baseline and after the depletion.
Results
A clinical response of American College of Rheumatology (ACR) 50% improvement was noted in six patients, and another two patients responded with moderate improvement, equivalent to ACR 20–50% improvements. RF and anti‐CCP antibodies were positive at baseline in seven of ten patients. RF disappeared or declined in six patients 4 months after treatment, correlating with clinical improvement. By contrast, anti‐CCP remained unchanged in six patients. After rituximab treatment, and in association with clinical improvement, BAFF, IL10 and CD86 mRNA expression in HMDM were significantly upregulated compared with values at baseline. A significant decrease in TNFα in the supernatant of cultured HMDM was also noted.
Conclusions
In addition to B cell depletion and attenuation in some of the specific autoantibodies, clinical improvement in rituximab‐treated patients with RA occurred in association with changes in macrophage function.
In their seminal study, Shlomchik et al1 showed that systemic lupus erythematosus (SLE)‐prone MRL‐lpr/lpr mice lacking B cells do not develop SLE‐nephritis or autoantibodies, thus suggesting B cells to be potential targets in the treatment of autoimmune diseases. Apart from autoantibody production, B cells are potential regulators of other effector cells, produce pro‐inflammatory cytokines, such as tumour necrosis factor α (TNFα), and act as efficient antigen presenting cells (APCs).2
Approval of the anti‐CD20 chimeric monoclonal antibody rituximab for treatment of B cell lymphomas in 1997 set the stage for its wider use in the treatment of SLE and rheumatoid arthritis (RA),3 aiming at B cell depletion, especially plasma cell precursors or memory B cells becoming antibody producers. Indeed, with rituximab treatment, such alterations in memory and in autoreactive B cells have been reported.4
Simple quantitative depletion of B cells is inadequate to explain the results of rituximab treatment in RA, with effects on B cell function as efficient APCs, their promotion of extra‐follicular dendritic cells and their ability to produce pro‐inflammatory cytokines to be considered.5,6 The status of B cell activating factor (BAFF) during rituximab‐induced B cell depletion, which—although associated with clinical benefit—is “unphysiological”, also requires a better definition.7 The present study also investigated the behavioural and functional changes of human monocyte‐derived macrophages (HMDMs) in patients with RA, on therapeutic B cell depletion.
Patients and methods
Patients
Ten patients with active RA, unresponsive to methotrexate, were treated with a single course of rituximab, two infusions of 1000 mg, 2 weeks apart. An American College of Rheumatology (ACR) 50% response8 was considered positive. At baseline and 4 months after rituximab treatment, peripheral blood CD19 B cell counts, serum rheumatoid factor (RF), anti‐cyclic citrullinated peptide (anti‐CCP) antibodies and total immunoglobulins were assessed; peripheral blood monocyte‐derived macrophages were analysed for mRNA of BAFF, CD86 and interleukin (IL) 10, and supernatants of macrophage cultures were tested for TNFα.
The study was approved by the Bnai Zion Medical Center Human Research Committee, Haifa, Israel, and informed consent was obtained from patients.
HMDM isolation
Peripheral blood mononuclear cells were isolated from anticoagulated blood through Ficoll density gradient and plated at 107 cells/ml (Primaria Brand, Falcon Labware, Temse, Belgium). After 2 h of adherence, the medium was replaced with RPMI supplemented with 20% autologous serum and antibiotics, changed every 48–72 h and tested after 7 days. Cell viability, judged by trypan blue assay, was >95% under all conditions.
TNFα in culture supernatants
A commercial sandwich ELISA Kit (R&D Systems, Minneapolis, USA) was used to measure TNFα in supernatants, and all samples were assayed simultaneously to avoid interbatch variations, expressed as picograms of TNFα per milligram of cell protein. HMDM protein was determined by the Lowry method.9
mRNA expression by semiquantitative reverse transcriptase‐PCR analysis
Total RNA from HMDM cells was isolated with MasterPure (Epicentre, Madison, Wisconsin, USA). cDNAs were generated from 1 μg of total RNA using reverse transcriptase (RT) (Reverse‐iT ABgene, Surrey, UK) and random decamers (ABgene). RT products were subjected to PCR amplification with GoTaq Green Master Mix (Promega, Rockland, Maine, USA), all primers were obtained from Genosys, Sigma, Israel. The cDNA products were separated on 2% agarose gel containing ethidium bromide with bands analysed by Tina software. β‐Actin cDNA product was used as a standard to equivalent levels of total RNA subjected to RT‐PCR and used to normalise the band intensities of BAFF, CD86 and IL10.
Statistical methods
Results were expressed as mean (SEM). Student's paired t test was used for comparison of data obtained before and after rituximab treatment. For parameters without Gaussian distribution—that is, TNFα—values were transformed to logarithms for statistical analysis; p<0.05 was considered significant.
Results
A clinical response of ACR 50 or better was noted in six patients, with normalisation of C reactive protein levels. Two patients responded with moderate improvement, equivalent to ACR 20–50, with almost no improvement in the remainder.
B cell depletion
The mean (SD) absolute B cell count at baseline was 199 (158) cells/mm3, range 96–526 cells/mm3, depleted to mean absolute count of 16 (9.6) cells/mm3, range 5–35 cells/mm3 (p<0.001) 4 months after treatment. In three patients, depletion was even below 5 cells/mm3, with no correlation between clinical improvement and extent of depletion.
Autoantibodies
RF and anti‐CCP antibodies were positive at baseline in seven patients. After therapy, RF disappeared/decreased in six, remaining unchanged in one patient. RF disappearance/decrease correlated positively with clinical remission, persisting in the patient who failed to improve while taking rituximab. Anti‐CCP antibodies remained unchanged in six patients despite documented clinical response (table 1).
Table 1 Documented clinical response in patients.
| Patient | Age/sex | Clinical response | RF(IgM) | Anti‐CCP | % of change | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | BAFF | IL10 | CD86 | TNFα | |||
| VA | 40/F | Excellent | ++ | – | Pos | Pos | 304 | 71 | −85 | |
| KS | 68/F | Excellent | + | – | Pos | Pos | 118 | 112 | 85 | −94 |
| GL | 66/F | Excellent | – | – | Neg | Neg | 172 | −1 | −77 | |
| BR | 72/F | Moderate | ++ | + | Pos | Pos | −27 | |||
| IR | 68/F | Excellent | +++ | ++ | Pos | Neg | 165 | 105 | 91 | −83 |
| KZ | 48/F | Failure | ++ | ++ | Pos | Pos | 32 | 347 | 137 | 45 |
| AF | 48/F | Moderate | ++ | + | Pos | Pos | 373 | 37 | ||
| BY | 69/F | Excellent | ++ | – | Pos | Pos | −5 | −60 | 18 | −51 |
| LN | 65/F | Failure | – | – | Neg | Neg | 154 | 308 | 41 | 15 |
| LH | 62/F | Excellent | – | – | Neg | Neg | 194 | 187 | 179 | −90 |
Blank spaces indicate that the tests were not carried out.
Immunoglobulins at baseline were: mean (SD) IgG 12.0 (7.6) mg/ml; IgM 1.59 (0.89) mg/ml; and IgA 3.87 (1.84) mg/ml. Values were 10–15% lower after treatment, remaining within normal limits.
mRNA expression in HMDM
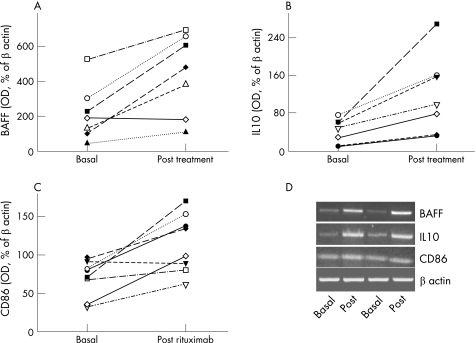
For technical reasons, complete data are unavailable for all patients. After treatment, BAFF mRNA expression in HMDM (n = 7) was significantly upregulated 2.5‐fold (p = 0.01), and IL10 mRNA (n = 7) was increased 2.8‐fold (p = 0.006). Expression of CD86 mRNA in HMDM (n = 8) was also increased 1.7‐fold after treatment (p = 0.007; fig 1).
Figure 1 B cell activating factor, interleukin (IL) 10 and CD86 mRNA expression (presented as percentage of optical density (OD) of β‐actin at baseline and 4 months after rituximab treatment). (A) BAFF expression was increased in six patients and remained unchanged in one (p = 0.01). (B) IL10 expression was increased in all studied patients (p = 0.006). (C) CD86 expression was increased in eight studied patients (p = 0.007). (D) Two randomly chosen reverse transcriptase‐PCR mRNA experiments of BAFF, IL10 and CD86 expressions (each at baseline and after treatment). A control of β‐actin expression is shown.
The increases in mRNA of BAFF, IL10 and CD86 correlated with improvement but, owing to the small numbers involved, no statistically significant correlations could be made with individual clinical response.
TNFα in supernatant of cultured HMDM
In seven patients, a significant decrease in TNFα was measured after treatment (mean 3.24 (0.5) vs 2.7 (0.6) pg/mg cell protein, p = 0.02), while remaining unchanged in three.
Discussion
In the current study, changes in the function of HMDM, major producers of TNFα in RA, after rituximab‐induced B cell depletion, are reported.
Initially, rituximab's effects on B cells—a decline in number, only moderate reduction in immunoglobulin levels and specific effects on antibodies associated with RA—were confirmed. Similar to findings of others, the reduction of RF correlated with improvement in patients whereas anti‐CCP antibodies persisted despite clinical benefit.10 To the extent that anti‐CCP is a more specific and hence “truer” reflection of the RA disease process, discordance between the B cell depletion documented in the present study and the continued presence of anti‐CCP, also a B cell product, is consistent with the understanding that B cell depletion by itself is not responsible for the clinical improvement in patients treated with rituximab. Further, the general observation that many patients with RA experience relapse of their clinical disease between 6 and 12 months after B cell depletion, when the B cell number is still quite low, may suggest that the disease process persists with survival of some pathogenic memory B cells, possibly producers of pathogenic autoantibodies such as anti‐CCP antibodies, that had escaped depletion and kept expanding in secondary lymphoid tissues.11 The inadequacy of viewing rituximab's beneficial clinical effects as the product of simple B cell number reduction had motivated the present study, with a focus on macrophage function before and after this therapeutic modality.
The principal finding in the present study relates to the functional change in HMDM after B cell depletion, associated with clinical improvement in RA. A significant reduction in TNFα was observed in the supernatant of cultured HMDMs. As immature macrophages are generally major TNF producers, dominant during active RA, possibly the HMDM population post B cell depletion is replaced by different, possibly more mature, macrophages of less pro‐inflammatory character.
Another important finding in this regard is the increased expression of IL10 (previously shown to play an important role in BAFF homeostasis12) mRNA in post‐depletion HMDMs. IL10‐activated macrophages were found to produce markedly higher levels of BAFF and to enhance B cell survival through a BAFF‐dependent component, with BAFF produced at high levels by positive feedback mechanisms after treatment with rituximab.10 The association between increased BAFF mRNA and IL10 mRNA in HMDM in the present study strengthens the assumption that increased BAFF after B cell depletion is related to compensatory increases in IL10 expression. These mechanisms still require better clarification.
The finding of increased IL10 mRNA in HMDM of patients with RA after rituximab‐induced B cell depletion may be part of a wider immunomodulation, including anti‐inflammatory changes, in macrophage function. With rituximab‐induced clinical improvement, HMDMs change their cytokine production, with IL10 upregulated and production of pro‐inflammatory cytokines such as TNFα reduced, suggesting that a change in macrophage function may contribute to the beneficial effect in patients with RA.13
In a recent study, serum BAFF levels were reported to be increased in patients with RA after rituximab‐induced B cell depletion.10 The authors speculated that the increase could be attributed either to lack of BAFF‐binding B cells in peripheral blood or possibly to its increased production owing to a homeostatic negative feedback signal from the depleted B cell compartment.14
In the current study, B cell depletion was followed by increased BAFF mRNA expression in HMDMs of patients treated for RA, suggesting it to be the main mechanism for the previously reported increase in serum BAFF, possibly representing homeostatic attempts to replenish B cells.
Depletion of B APCs with rituximab may drive the need for mature macrophages that also function as APCs, to maintain a normal protective immune response. In the current study, increased mRNA CD86 expression in HMDM was documented, compatible with such a response of mature macrophage APCs, thereby contributing by maintenance of a protective immune response and relative lack of infections, despite B cell depletion.15
These preliminary findings encourage the impression that changes in the function of macrophages should be considered when treatments of RA are evaluated, but, owing to the small number of patients studied, further study is required.
Abbreviations
APC - antigen‐presenting cell
anti‐CCP - anti‐cyclic citrullinated peptide
BAFF - B cell activating factor
HMDM - human monocyte‐derived macrophage
RA - rheumatoid arthritis
RF - rheumatoid factor
RT - reverse transcriptase
SLE - systemic lupus erythematosus
TNFα - tumour necrosis factor α
Footnotes
Competing interests: None declared.
References
- 1.Shlomchik M J, Madaio M P, Ni D, Trounslein M, Huszar D. The role of B cells in lpr/lpr induced autoimmunity. J Exp Med 19941801295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipsky P E. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol 20012764–766. [DOI] [PubMed] [Google Scholar]
- 3.Sfikakis P P, Boletis J N, Tsokos G C. Rituximab anti‐B‐cell therapy in systemic lupus erythematosus: pointing to the future. Curr Opin Rheumatol 200517550–557. [DOI] [PubMed] [Google Scholar]
- 4.Edwards J C, Szczepansky L, Szechinski J, Filipowicz‐Sosnowska A, Emery P, Close D R.et al Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 20043502572–2581. [DOI] [PubMed] [Google Scholar]
- 5.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol 20061763498–3506. [DOI] [PubMed] [Google Scholar]
- 6.Reinhardt R L, Hong S, Kang S J, Wang Z E, Locksley R M. Visualization of IL‐12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation. J Immunol 20061771618–1627. [DOI] [PubMed] [Google Scholar]
- 7.Thein M, Phan T G, Gardam S, Amesbury C, Basten A, Mackey F.et al Excess BAFF rescues self‐reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 200420785–798. [DOI] [PubMed] [Google Scholar]
- 8.Pincus T, Amara I, Koch G G. Continuous indices of core data set measures in rheumatoid arthritis clinical trials: lower responses to placebo than seen with categorical responses with the ACR 20% criteria. Arthritis Rheum 2005521031–1036. [DOI] [PubMed] [Google Scholar]
- 9.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin reagent. J Biol Chem 1951193265–275. [PubMed] [Google Scholar]
- 10.Cambridge G, Stohl W, Leandro M J, Migone T S, Hilbert D M, Edwards J C W. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment. Arthritis Rheum 200654723–732. [DOI] [PubMed] [Google Scholar]
- 11.Cambridge G, Leandro M J, Edwards J C, Ehrenstein M R, Salden M, Bodman‐Smith M.et al Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum 2003482146–2154. [DOI] [PubMed] [Google Scholar]
- 12.Ogden C A, Pound J D, Batth B K, Owens S, Johannessen I, Wood K, Gregory C D. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL‐10‐activated macrophages: implications for Burkitt's lymphoma. J Immunol 20051743015–3023. [DOI] [PubMed] [Google Scholar]
- 13.Brennan F M, Foey A D, Feldmann M. The importance of T cell interactions with macrophages in rheumatoid cytokine production. Curr Top Microbiol Immunol 2006305177–194. [DOI] [PubMed] [Google Scholar]
- 14.Scapini P, Carletto A, Nardelli B, Calzetti F, Roschke V, Merigo F.et al Pro‐inflammatory mediators elicit the secretion of the intracellular B‐lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood 2005105830–837. [DOI] [PubMed] [Google Scholar]
- 15.Park H J, Park O J, Shin J. Receptor activator of NF‐kappa ligand enhances the activity of macrophages as antigen presenting cells. Exp Mol Med 200537524–532. [DOI] [PubMed] [Google Scholar]